Abstract
After myocardial injury, the cardiac muscle does not regenerate and heals by forming a scar. This process results in loss of heart function and ultimately heart failure. Recent application of reprogramming technology, where forced expression of master regulators convert scar-forming cells to become cardiovascular cells in vivo, has fueled new hope for the development of therapies targeting heart disease.
Keywords: transdifferentiation, cardiomyocytes, cardiovascular diseases, transflammation, cardiovascular regeneration



Introduction
The limited ability of the human heart to regenerate explains why myocardial infarction often culminates in heart failure and sudden death.1 Existing medical therapies ameliorate adverse myocardial remodeling and support the heart, but ventricular dysfunction and dysrhythmia remain a major cause of death and disability. Accordingly, stem cell-based approaches have emerged as a promising approach for cardiovascular disease. As discussed by Wong et al. in this issue (see page 207), induced pluripotent stem cells (iPSCs) have great replicative capacity and may be differentiated into each of the major cardiovascular lineages. Preclinical studies indicate that these cells have therapeutic value.
Roadblocks to iPSC Therapies
The original approach of retroviral or lentiviral overexpression of the Yamanaka factors2-6 raised concerns that the integration of foreign DNA into the host genome could silence indispensable genes or induce dysregulation of these genes. Much work has been done since then to eliminate this concern with the use of episomal gene delivery, excisable transgenes, cell-permeable recombinant proteins, and synthetic messenger RNA,7-13 Additional concerns remain with respect to epigenetic memory, as iPSC-derived cardiomyocytes may retain some epigenetic memory from the parental cells. Similarly, given the replicative property of pluripotent stem cells, it is possible that iPSC-derived cardiomyocytes can give rise to teratomas.14 To overcome these roadblocks, methods for purifying therapeutic cells need to be improved.15 Finally, efficient methods to differentiate the iPSCs into mature cells in the cardiovascular lineage, with a high yield, have yet to be developed.
Transdifferentiation: A More Direct Route to Therapeutic Cardiovascular Cells
Accordingly, it may be more efficient to transdifferentiate cells directly and thus avoid the use of iPSCs to derive patient-specific cells. John Gurdon’s seminal studies demonstrated the ability of a somatic cell to be reprogrammed to a pluripotent stem cell. Since then, a number of investigators have shown that cell phenotype is fluid and can be altered by cytoplasmic factors such as transcription factors (TFs).16-19 Inspired by Yamanaka’s discovery that several TFs can be used to reactivate core transcriptional networks of pluripotency, Ieda et al. tried the same direct reprogramming paradigm to generate cardiac cell lineage.20 The group discovered that a combination of three TFs (MEF2C, GATA4, and TBX5) activated a cardiac program, as manifested by their expression of MYH6 in 20% of fibroblasts. However, only 1% showed functionality such as spontaneous beating, indicating that the majority of cells were only partially reprogrammed. To improve upon this approach, Efe et al.21 transfected mouse embryonic fibroblasts with the Yamanaka factors, then induced differentiation by inhibiting the JAK-STAT pathway and adding the cardiogenic factor BMP4. Their approach yielded a significant number of beating colonies, with about 40% exhibiting cardiac proteins.
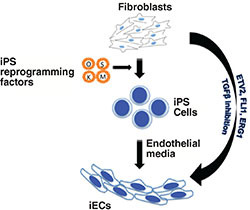
Of course, regeneration of heart tissue also requires a vascular component. We and others have used a similar approach to transdifferentiate fibroblasts into endothelial cells (Figure 1).22, 23 Human fibroblasts that are transdifferentiated into endothelial cells can express endothelial surface markers, organize into endothelial tubes in vitro, and form capillaries when injected into immunodeficient mice. Although the ability to transdifferentiate human fibroblasts into cardiac myocytes or endothelial cells in vitro is exciting, these cells need to be reimplanted into the host, which raises questions regarding methods of delivery, dosing, and whether such injected cells will survive and self-assemble into a functional architecture. Such hurdles might be obviated if transdifferentiation could be accomplished in vivo. Of great interest, several groups have shown proof of concept that transdifferentiation can be accomplished in vivo, which substantially increases the feasibility of its therapeutic application.
Figure 1.
Generation of induced endothelial cells (iECs) from fibroblasts. iECs have been shown to be generated via two different routes. An indirect route involves the conversion of fibroblasts to iPSCs using the Yamanaka factors, which could then be differentiated into endothelial cells. Alternatively, transdifferentiation does not involve a pluripotent intermediate stage and forced expression of endothelial-specific transcription factors could directly convert fibroblasts to endothelial cells.
Repairing the Broken Heart
After a myocardial infarction, cardiac fibroblasts migrate into the injured area, proliferating and generating extracellular matrix to form a scar. Although the scar restores the structural integrity of the heart, it is nonfunctional. Because the scar is avascular, residual myocytes within or at the edge of the scar are ischemic and may become foci for arrhythmia. If scar-forming fibroblasts could be transdifferentiated into cardiovascular cells, this might permit true cardiovascular regeneration with restoration of functional tissue.
Two recent papers from the Olson and Srivastava laboratories provide proof of concept that such therapeutic transdifferentiation in vivo is possible. Based on their previous work,20 the Srivastava group used a retroviral approach to introduce the three TFs (GATA4, MEF2C, and TBX5) into the infarct/border zone of mice following myocardial infarction. The Olson group used a similar approach but added an extra TF (HAND2). Both groups performed lineage-tracing studies with fibroblast markers to confirm the origin of the newly generated cardiomyocytes. Importantly, both groups provided evidence that transdifferentiation was associated with improved cardiac function.
An unexpected feature of these studies included higher reprogramming efficiency in vivo when compared to their previous in vitro studies. This suggested that the native heart could provide a better microenvironment for the cardiac fibroblasts to reprogram more efficiently. Similarly, a disparity existed between functional improvement after in vivo reprogramming and the number of cardiomyocytes generated, suggesting other mechanisms playing an important role in cardiac regeneration.
Toward Clinical Application of Transdifferentiation for Cardiovascular Disease
The technology to regenerate cardiac muscle from endogenous fibroblasts avoids many of the hurdles facing the ex vivo generation and administration of therapeutic cells. That being said, numerous hurdles still remain. To begin, experimental protocols must be refined so as to increase the efficiency of the transdifferentiation process. Furthermore, we need to perform a comprehensive characterization of the transitional cells generated during the transdifferentiation process since incomplete differentiation could give rise to hamartomas or to dysfunctional myocardium and arrhythmias.24 Furthermore, the stability of the transdifferentiation needs to be determined.
One intriguing question that always remains unanswered is how so few transcriptional factors can induce transdifferentiation of somatic cells.25 This is particularly puzzling when the overexpressed gene does not encode a pioneering transcriptional factor, that is, one that does not itself initiate the transcriptional complex that includes other cofactors and epigenetic modifiers. One potential explanation is that other mechanisms might be induced during the transdifferentiation process. Recently, we discovered that the retroviral vectors used in nuclear reprogramming are more than mere vehicles for the transcription factors.17 Indeed, by activating innate immune signaling (via Toll-like receptor 3), these viral vectors cause global changes in the expression and activity of epigenetic modifiers.17 These alterations place the chromatin into an “open configuration” that increases cell epigenetic plasticity and cell transformation, a process that we have termed “transflammation.” Unpublished work from our laboratory indicates that the induction of transflammation, combined with external signals from the media and extracellular matrix, can induce therapeutic transdifferentiation in the absence of viral vectors or transcription factors.
Conclusion
Transdifferentiation, from one somatic cell type to another desired somatic cell type, is an attractive approach for regenerative medicine. Indeed, several groups have demonstrated that human fibroblasts may be converted to neurons,26 cardiomyocytes,20 or endothelial cells22, 27 by overexpressing specific transcription factors (Figure 1). Transdifferentiation offers a platform by which it is possible to reprogram in vivo. Such an approach, if accomplished with small molecules, would avoid the more complex approach of cell delivery. The clinical applications for transdifferentiation are numerous. For example, in ischemic injury such as myocardial infarction, it might be possible to convert the resident cardiac fibroblasts in the ischemic region to cardiovascular progenitor cells in order to replace scar with vascularized and functional tissue. However, to achieve the promise of this regenerative therapy, we must improve the efficiency and fidelity of the reprogramming process, and avoid viral vectors, preferably with a small-molecule approach that can be preferentially delivered to the heart. Such work is now underway at The Methodist Hospital Research Institute.
Funding Statement
Funding/Support: Dr. Cooke receives research funding from the National Institutes of Health, Dr. Sayed is supported by an NIH National Research Service Award postdoctoral fellowship and an American Heart Association Scientist Development Grant. Dr. Wong is supported by a postdoctoral fellowship from the American Heart Association.
Footnotes
Conflict of Interest Disclosure: All authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
Contributor Information
Nazish Sayed, Houston Methodist DeBakey Heart & Vascular Center, Houston Methodist Hospital, Houston, Texas
Wing Tak Wong, Houston Methodist DeBakey Heart & Vascular Center, Houston Methodist Hospital, Houston, Texas
John P. Cooke, Houston Methodist DeBakey Heart & Vascular Center, Houston Methodist Hospital, Houston, Texas
References
- 1.van den Borne SW, Diez J, Blankesteijn WM, Verjans J, Hofstra L, Narula J. Myocardial remodeling after infarction: the role of myofibroblasts. Nat Rev Cardiol. 2010 Jan;7(1):30–7.. doi: 10.1038/nrcardio.2009.199. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006 Aug 25;126(4):663–76.. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007 Nov 30;131(5):861–72.. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Maherali N, Sridharan R, Xie W, Utikal J, Eminli S, Arnold K, et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007 Jun 7;1(1):55–70.. doi: 10.1016/j.stem.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007 Jul 19; 448(7151):318–24.. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 6.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007 Jul 19;448(7151):313–7.. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 7.Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P, Woltjen K. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009 Apr 9;458(7239):771–5.. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soldner F, Hockemeyer D, Beard C, Gao Q, Bell GW, Cook EG, et al. Parkinson’s disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009 Mar 6;136(5):964–77.. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang CW, Lai YS, Pawlik KM, Liu K, Sun CW, Li C, et al. Polycistronic lentiviral vector for “hit and run” reprogramming of adult skin fibroblasts to induced pluripotent stem cells. Stem Cells. 2009 May;27(5):1042–9.. doi: 10.1002/stem.39. [DOI] [PubMed] [Google Scholar]
- 10.Woltjen K, Michael IP, Mohseni P, Desai R, Mileikovsky M, Hämäläinen R, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009 Apr 9;458(7239):766–70.. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yusa K, Rad R, Takeda J, Bradley A. Generation of transgene-free induced pluripotent mouse stem cells by the piggyBac transposon. Nat Methods. 2009 May;6(5):363–9.. doi: 10.1038/nmeth.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010 Nov 5;7(5):618–30.. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009 Jun 5;4(6):472–6.. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nussbaum J, Minami E, Laflamme MA, Virag JA, Ware CB, Masino A, et al. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. FASEB J. 2007 May;21(7):1345–57.. doi: 10.1096/fj.06-6769com. [DOI] [PubMed] [Google Scholar]
- 15.Tang C, Lee AS, Volkmer JP, Sahoo D, Nag D, Mosley AR, et al. An antibody against SSEA-5 glycan on human pluripotent stem cells enables removal of teratoma-forming cells. Nat Biotechnol. 2011 Aug 14;29(9):829–34.. doi: 10.1038/nbt.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blau HM, Chiu CP, Webster C. Cytoplasmic activation of human nuclear genes in stable heterocaryons. Cell. 1983 Apr;32(4):1171–80.. doi: 10.1016/0092-8674(83)90300-8. [DOI] [PubMed] [Google Scholar]
- 17.Lee J, Sayed N, Hunter A, Au KF, Wong WH, Mocarski ES, et al. Activation of innate immunity is required for efficient nuclear reprogramming. Cell. 2012 Oct 26;151(3):547–58.. doi: 10.1016/j.cell.2012.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blau HM, Pavlath GK, Hardeman EC, Chiu CP, Silberstein L, Webster SG, et al. Plasticity of the differentiated state. Science. 1985 Nov 15;230(4727):758–66.. doi: 10.1126/science.2414846. [DOI] [PubMed] [Google Scholar]
- 19.James D, Nam HS, Seandel M, Nolan D, Janovitz T, Tomishima M, et al. Expansion and maintenance of human embryonic stem cell-derived endothelial cells by TGFbeta inhibition is Id1 dependent. Nat Biotechnol. 2010 Feb;28(2):161–6.. doi: 10.1038/nbt.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010 Aug 6;142(3):375–86.. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Efe JA, Hilcove S, Kim J, Zhou H, Ouyang K, Wang G, et al. Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nat Cell Biol. 2011 Mar;13(3):215–22.. doi: 10.1038/ncb2164. [DOI] [PubMed] [Google Scholar]
- 22.Margariti A, Winkler B, Karamariti E, Zampetaki A, Tsai TN, Baban D, et al. Direct reprogramming of fibroblasts into endothelial cells capable of angiogenesis and reendothelialization in tissue-engineered vessels. Proc Natl Acad Sci U S A. 2012 Aug 21;109(34):13793–8.. doi: 10.1073/pnas.1205526109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Huang NF, Zou J, Laurent TJ, Lee JC, Okogbaa J, et al. Conversion of human fibroblasts to functional endothelial cells by defined factors. Arterioscler Thromb Vasc Biol. 2013 Jun;33(6):1366–75.. doi: 10.1161/ATVBAHA.112.301167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao SY, Liu Y, Siu CW, Zhang Y, Lai WH, Au KW, et al. Proarrhythmic risk of embryonic stem cell-derived cardiomyocyte transplantation in infarcted myocardium. Heart Rhythm. 2010 Dec;7(12):1852–9.. doi: 10.1016/j.hrthm.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Lujan E, Wernig M. The many roads to Rome: induction of neural precursor cells from fibroblasts. Curr Opin Genet Dev. 2012 Oct;22(5):517–22.. doi: 10.1016/j.gde.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010 Feb 25;463(7284):1035–41.. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ginsberg M, James D, Ding BS, Nolan D, Geng F, Butler JM, et al. Efficient direct reprogramming of mature amniotic cells into endothelial cells by ETS factors and TGFβ suppression. Cell. 2012 Oct 26;151(3):559–75.. doi: 10.1016/j.cell.2012.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]


