Abstract
Stem cell therapy is emerging as a potential new therapy for patients with advanced heart failure. In recent years, advances in molecular imaging have allowed monitoring of stem cell homing and survival. In this review article, we will discuss the clinical application and future directions of stem cell imaging in advanced heart failure.
Keywords: stem cell transplantation, heart failure, multimodality imaging, nuclear medicine

Introduction
During the last decade, cardiac stem cell therapy has evolved into a promising treatment modality for cardiovascular diseases. Several trials have investigated the effects of stem cell therapy, most commonly bone marrow-derived stem cells, in patients with ischemic and nonischemic cardiomyopathy. In the majority of the published studies, stem cell therapy was associated with an increase in left ventricular ejection fraction (LVEF) ranging from 3% to 8%, depending on the patient population and stem cell numbers injected.1 This improvement appears to be mainly mediated through paracrine effects and not tissue regeneration, because the majority of stem cells died within several weeks of stem cell injection.2 Several factors influence stem cell response, including patient selection, stem cell subtype, timing and method of stem cell delivery, functional state of myocardium (i.e., hibernating versus scar), and the initial retention and homing of stem cell therapy.3 Our group has recently shown that the response to stem cell therapy in patients with dilated cardiomyopathy was partly related to early engraftment at 1 hour post-injection.4 The following reviews the principles and clinical applications of clinical molecular imaging applied for stem cell therapy and some future direction for the field.
In Vivo Tracking of Stem Cells after Transplantation
Stem cell imaging allows the assessment of both the short- and long-term fate of delivered cells. For short-term assessment of cellular fate, stem cells are usually labeled directly by transferring a molecule into the cell, which can then be tracked, prior to delivery. Several methods are available for direct stem cell labeling, including iron particles for magnetic resonance imaging (MRI),5 microbubbles for ultrasound (US) tracking,6 and radionuclide tracers for single photon emission computed tomography (SPECT) or positron emission tomography (PET) imaging. Long-term assessment of cellular fate is usually achieved by the reporter gene imaging technique in which a specific reporter gene is transferred into stem cells, leading to expression of a reporter protein such as a receptor or an enzyme. The delivery of an exogenous reporter probe leads to an interaction with the reporter protein and generation of a signal that can be detected by various imaging modalities.7 Examples of reporter gene imaging include firefly luciferase reporter gene and D-luciferin as reporter substrate, with emitted photons detected by optical bioluminescence imaging; herpes simplex virus thymidine kinase (HSV-tk) reporter gene with F-18-9-(4-fluoro-3-hydroxymethylbutyl)guanine (18F-FHBG) reporter substrate for PET imaging;8 and sodium iodide symporter (NIS) reporter gene with 99mTc-pertechnetate (99mTcO4-) reporter substrate for SPECT imaging.9 Reporter genes and probes have also been developed for MRI in preclinical models.10 At present, however, cardiac stem cell tracking using reporter gene imaging is not in clinical use due to concerns with genetic manipulation of stem cells. However, with the recent development of site-specific integration vectors to mitigate random integrations, these concerns may be reduced in the future.11, 12
Advantages and Limitations of Radionuclide Labels
The ideal tracer molecule for clinical studies should fulfill a number of criteria (Table 1).13 To date, none of the available agents has been able to meet all of them. Radionuclide labels possess a number of characteristics that account for their preferential use in clinical practice (Table 2). First, they are relatively safe, nontoxic and inert, and have been in clinical use for labeling of mature cell types, such as leukocytes for imaging of inflammation or infection, and erythrocytes for blood pool imaging. Second, radionuclide methods are highly sensitive, allowing for detection of stem cells in concentrations as low as 10-12 mol for PET and 10-9 mol for SPECT. Third, they are quantifiable, thus allowing for assessment of stem cell retention. However, the tracking of labeled cells is limited by temporal loss of radiotracer, in part due to radionuclide decay related to its half-life, as well as by dilution of radiotracer by cell division and efflux of radiotracer from labeled cells. Moreover, physiological accumulation and excretion of free radiotracer may interfere with activity in labeled stem cells, hampering imaging and quantification. In addition, the radiation burden to the patient must be considered, though it is comparable to one received during routine diagnostic nuclear medicine imaging procedures.
Table 1.
Characteristics of an ideal cell label for stem cell tracking in clinical studies.13
| 1. Biocompatible, safe, and nontoxic |
| 2. No genetic modification or perturbation to the stem cell |
| 3. Single-cell detection at any anatomic location |
| 4. Quantification of cell number |
| 5. Minimal or no dilution with cell division |
| 6. Minimal or no transfer of contrast agent to non-stem cells |
| 7. Noninvasive imaging in the living subject over months to years |
| 8. No requirement for injectable contrast agent |
Table 2.
Radionuclide methods for stem cell labeling and clinical in vivo tracking.
| Imaging Method | Tracer/Label | Half-life | Photon Emission Energy |
| SPECT Planar Imaging |
99mTc-HMPAO | 6 hours | 140keV |
| 111In-oxine | 2, 8 days | 173keV, 247keV | |
| PET | 18F-FDG | 110 minutes | 511keV |
Technetium (99mTc) coupled with exametazime (HMPAO) is the most widely used cell label. The low energy of this pure gamma emitter results in lower patient dose and is optimal for nuclear medicine imaging. Its half-life of 6 hours allows for imaging within 24 hours postinjection. The viability of stem cells does not appear to be significantly affected by labeling.14, 15 However, physiological excretion of 99mTc-HMPAO occurs through both the genitourinary and biliary/gastrointestinal tracts, something that should be considered on delayed imaging. Figure 1 shows 99mTc-based imaging of stem cells used to monitor CD34+ stem cell retention after intracoronary versus intramyocardial stem cell injection.
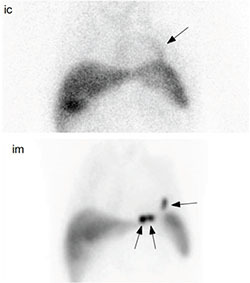
Figure 1.
Comparison of two routes of stem cell delivery 1 hour postinjection in the same patient. Using the intracoronary (ic) route, mild diffuse accumulation of labeled stem cells is noted along the LAD territory (arrow; vessel used for stem cell administration). In contrast, intramuscular (im) injection results in discrete areas (arrows) of intense stem cell accumulation in myocardium targeted by NOGA XP electrophysiological mapping.
Indium (111In) is a radionuclide with combined gamma and beta emission. It is typically coupled to oxine, which, in contrast to 99mTc-HMPAO, is minimally excreted through the biliary/gastrointestinal tract. Indium has a half-life of 2.8 days that permits tracking for up to 7 days. However, the advantage of this prolonged tracking is offset by a relatively high radiation dose and average imaging characteristics related to energy of emissions. Moreover, the beta emission in the form of Auger electrons was previously found to affect stem cell viability.16, 17
For clinical PET imaging, 18F-fluorodeoxyglucose (18F-FDG) is the most widely used radiotracer, reflecting glucose metabolism that is typically upregulated in malignant cells. As a cell label, it was initially used for leukocyte labeling similar to the use of 99mTc-HMPAO for imaging of inflammatory or infectious foci. Its short half-life limits tracking to within several hours of stem cell transplantation. The high energy of photon emission in 18F-FDG does not appear to affect stem cell viability.18, 19 The physiological uptake of 18F-FDG occurs in most organs and tissues of the body, including the myocardium, where uptake is highly variable.
Clinical Studies of Stem Cell Tracking Using Radionuclide Labels
Only a few studies have evaluated the efficiency of cardiac stem cell transplantation in terms of early stem cell retention and distribution. Most studies involved patients with ischemic heart disease in either an acute (i.e., usually defined as up to 1 month after acute coronary event) or chronic phase (i.e., usually defined as at least 6 months to 1 year after an acute coronary event or in patients with ischemic cardiomyopathy) (Table 3). In an early report using 18F-FDG as a stem cell label, Hoffmann et al. demonstrated good retention of CD34+ enriched bone marrow stem cells (BMSCs) (14% to 39%) and low retention of unselected BMSCs (1.3% to 2.6%) in the myocardium after intracoronary transfer in patients who had experienced an acute MI.18 Similar myocardial retention rates of unselected BMSCs using the same radiotracer and delivery route were reported by Kang et al. (average 1.5%, ranging from 0.2-3.3%) with no significant difference in stem cell retention as measured by elapsed time after the acute event.19 Schachinger et al. reported the early retention of intracoronarily delivered 111In-labeled CD133+ BMSCs as 6.9%, with significant decline on delayed imaging to about 2.5% at 24 hours in patients with ischemic heart disease.20 Significantly lower stem cell retention (average of 2.5%) was found in a subgroup of patients with chronic MI. Several other studies with smaller patient numbers, such as Blocklet et al. and Caveliers et al., also demonstrated comparable stem cell retention rates.21, 22
Table 3.
Clinical studies using stem cell tracking with radionuclide tracers.
| Imaging Technique | Tracer/Label | Study Authors | Patient no. (overall) | Setting (Patient no., intracoronary route) |
| SPECT Conventional NM |
99mTc-HMPAO | Penicka et al.14 | 10 | Acute MI (5), Chronic MI (5) |
| Silva et al.25 | 24 | Acute MI | ||
| Musialek et al.15 | 34 | Acute MI | ||
| Goussetis et al.23 | 8 | Chronic MI | ||
| 111In-oxine | Schaechinger et al.20 | 17 | Acute MI (8), Chronic MI (5) | |
| Blocklet et al.21 | 6 | Acute MI (3) | ||
| Caveliers et al.22 | 8 | Chronic MI (2) | ||
| PET | 18F-FDG | Hoffmann et al.18 | 9 | Acute MI (3) |
| Kang et al.19 | 20 | Acute MI | ||
| Blocklet et al.21 | 6 | Acute MI | ||
| Dedobbeleer et al.24 | 7 | Chronic MI |
99mTc-HMPAO also has been used as a stem cell label in human studies. Goussetis et al.23 reported 9.2% early myocardial retention of intracoronarily injected stem cells in eight patients with chronic ischemic cardiomyopathy (ICMP), with a decrease to 6.8% at 24 hours. Penicka et al. compared two patient groups with acute anterior MI (single vessel disease, LAD) and chronic ICMP (EF <35%) using intracoronary delivery of unselected BMSCs to the LAD territory.14 The average myocardial retention was higher in the acute MI group (1.3-5.1%) than in the chronic ICMP group (0-3.0%). A significant efflux of stem cells was found from the site of delivery within 24 hours in both patient groups, with relevant differences in stem cell kinetics. None of the five patients within the chronic ICMP group had myocardial retention after 24 hours, whereas only two patients out of five in the acute MI group showed no myocardial retention. Dedobbeleer et al.24 found comparably low retention of 99mTc-HMPAO labeled stem cells (average 3.0%) in patients in the chronic phase of MI in contrast to Silva et al.,25 who reported significantly higher average retention rates of 14.1% and 10.3% on early and delayed imaging, respectively. Musialek et al.15 investigated two methods of stem cell delivery in 34 patients with acute anterior MI. Only early imaging (1 hour postinjection) of 99mTc-HMPAO labeled stem cells was performed, with an average stem cell engraftment of 4.98%; there was no significant difference between the balloon-over-the-wire versus the perfusion-catheter approach.
Results of stem cell tracking studies are difficult to compare directly due to differences in methods of delivery, patient selection, stem cell number and subtype, and timing of imaging among the studies. All of the studies discussed above have used an intracoronary administration route, and several studies used an intravenous route in parallel that was proven to be inferior in terms of cardiac engraftment.18, 19 Overall, myocardial retention expressed as a percentage of stem cells administered appears to be rather low, with lower retention in chronic MI and ICMP compared to acute MI.15, 20, 21 Furthermore, there is significant efflux of stem cells from the site of delivery on delayed imaging.
In our experience, homing of intracoronarily injected 99mTc-HMPAO-labeled CD34+ stem cells in patients with nonischemic dilated cardiomyopathy is comparable to patients with ICMP, with stem cell retention rates around 1%. Again, a significant efflux of stem cells from delivery site occurs within the first 18 hours postinjection. When direct intramuscular/subendocardial injection of stem cells is used under the guidance of electrophysiological LV mapping, using NOGA® XP Cardiac Navigation System (Biologics Delivery Systems, Irwindale, CA), significantly higher stem cell retention rates are achieved (up to approximately 10%).26 Our findings confirm those from preclinical models that directly compared different methods of stem cell delivery, showing the direct intramuscular route to be superior.27
Clinical Relevance of Stem Cell Tracking and Future Directions
Stem cell tracking by radionuclide labels will continue to play a role in clinical studies, as it provides a feasible, robust, and safe method for the evaluation of stem cell transplantation procedures (Table 4). In recent years, important questions regarding cardiac stem cell transplantation, such as early stem cell retention, engraftment, and migration as well as relative efficiency of available stem cell delivery methods have been answered by radionuclide labeling techniques. More recently, experimental and clinical studies have demonstrated that higher early engraftment is associated with better functional response to stem cell therapy.4,28 Ongoing studies are investigating different methods to improve stem cell engraftment.29 In summary, imaging will continue to play a greater role in stem cell therapy by helping to identify the areas of hibernation (Figure 2), optimize stem cell delivery to target areas, and enable follow-up of the stem cell transplantation, with monitoring of both functional and perfusion parameters of the myocardium.3
Table 4.
Use of multimodality imaging in stem cell therapy trials.
| Use | Comment |
| Selection of target area | MRI, PET scan, or NOGA mapping can allow selection of hibernating myocardium (viable but noncontractile) for injection of stem cell therapy. |
| Preprocedure phenotyping | Imaging and functional assessment can assess the percentage of scar, ischemia, and hibernation as well as indentify contractile reserve of the myocardium. |
| Evaluation of early retention | Evaluation is used to monitor early effectiveness of stem cell delivery; it also provides potential predictors of response to stem cell therapy. |
| Monitor long-term retention of stem cells | Novel methods are being investigated in clinical practice. Reporter gene methodology is used in clinical cancer application but not yet in clinical cardiovascular application. |
| Monitor effectiveness and mechanisms of response | This allows quantification of LVEF, perfusion scores, and hibernation following the procedure. |
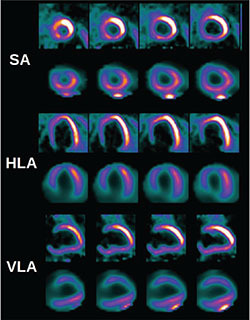
Figure 2.
Comparison of myocardial glucose metabolism (18F-FDG PET) and myocardial perfusion (99mTc-tetrofosmin SPECT). Myocardial metabolism is shown in the top row and myocardial perfusion in the bottom row in each axis. Presence of myocardial metabolism in areas of reduced myocardial perfusion in the anterior wall signifies areas of hibernating myocardium. SA: short axis; HLA: horizontal long axis; VLA: vertical long axis.
Funding Statement
Funding/Support: The authors have no funding disclosures.
Footnotes
Conflict of Interest Disclosure: The authors have completed and submitted the Cardiovascular Journal Conflict of Interest Statement and none were reported.
References
- 1.Strauer BE, Steinhoff G. 10 years of intracoronary and intramyocardial bone marrow stem cell therapy of the heart: from the methodological origin to clinical practice. J Am Coll Cardiol. 2011 Sep 6;58(11):1095–104.. doi: 10.1016/j.jacc.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 2.Wang J, Zhang S, Rabinovich B, Bidaut L, Soghomonyan S, Alauddin MM, et al. Human CD34+ cells in experimental myocardial infarction: long-term survival, sustained functional improvement, and mechanism of action. Circ Res. 2010 Jun 25;106(12):1904–11.. doi: 10.1161/CIRCRESAHA.110.221762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Psaltis PJ, Simari RD, Rodriguez-Porcel M. Emerging roles for integrated imaging modalities in cardiovascular cell-based therapeutics: a clinical perspective. Eur J Nucl Med Mol Imaging. 2012 Jan;39(1):165–81.. doi: 10.1007/s00259-011-1925-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vrtovec B, Poglajen G, Lezaic L, Sever M, Domanovic D, Cernelc P, et al. Effects of intracoronary CD34+ stem cell transplantation in nonischemic dilated cardiomyopathy patients: 5-year follow-up. Circ Res. 2013 Jan 4;112(1):165–73.. doi: 10.1161/CIRCRESAHA.112.276519. [DOI] [PubMed] [Google Scholar]
- 5.Bulte JW, Kraitchman DL. Monitoring cell therapy using iron oxide MR contrast agents. Curr Pharm Biotechnol. 2004 Dec;5(6):567–84.. doi: 10.2174/1389201043376526. [DOI] [PubMed] [Google Scholar]
- 6.Kuliszewski MA, Fujii H, Liao C, Smith AH, Xie A, Lindner JR, et al. Molecular imaging of endothelial progenitor cell engraftment using contrast-enhanced ultrasound and targeted microbubbles. Cardiovasc Res. 2009 Sep 1;83(4):653–62.. doi: 10.1093/cvr/cvp218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen PK, Lan F, Wang Y, Wu JC. Imaging: guiding the clinical translation of cardiac stem cell therapy. Circ Res. 2011 Sep 30;109(8):962–79.. doi: 10.1161/CIRCRESAHA.111.242909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu JC, Chen IY, Sundaresan G, Min JJ, De A, Qiao JH, et al. Molecular imaging of cardiac cell transplantation in living animals using optical bioluminescence and positron emission tomography. Circulation. 2003 Sep 16;108(11):1302–5.. doi: 10.1161/01.CIR.0000091252.20010.6E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terrovitis J, Kwok KF, Lautamäki R, Engles JM, Barth AS, Kizana E, et al. Ectopic expression of the sodium-iodide symporter enables imaging of transplanted cardiac stem cells in vivo by single-photon emission computed tomography or positron emission tomography. J Am Coll Cardiol. 2008 Nov 11;52(20):1652–60.. doi: 10.1016/j.jacc.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Budde MD, Frank JA. Magnetic tagging of therapeutic cells for MRI. J Nucl Med. 2009 Feb;50(2):171–4.. doi: 10.2967/jnumed.108.053546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Zhang WY, Hu S, Lan F, Lee AS, Huber B, et al. Genome editing of human embryonic stem cells and induced pluripotent stem cells with zinc finger nucleases for cellular imaging. Circ Res. 2012 Dec 7;111(12):1494–503.. doi: 10.1161/CIRCRESAHA.112.274969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lan F, Liu J, Narsinh KH, Hu S, Han L, Lee AS, et al. Safe genetic modification of cardiac stem cells using a site- specific integration technique. Circulation. 2012 Sep 11;126 (11 Suppl 1): S20–8.. doi: 10.1161/CIRCULATIONAHA.111.084913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frangioni JV, Hajjar RJ. In vivo tracking of stem cells for clinical trials in cardiovascular disease. Circulation. 2004 Nov 23;110(21):3378–83.. doi: 10.1161/01.CIR.0000149840.46523.FC. [DOI] [PubMed] [Google Scholar]
- 14.Penicka M, Lang O, Widimsky P, Kobylka P, Kozak T, Vanek T, et al. One-day kinetics of myocardial engraftment after intracoronary injection of bone marrow mononuclear cells in patients with acute and chronic myocardial infarction. Heart. 2007 Jul;93(7):837–41.. doi: 10.1136/hrt.2006.091934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Musialek P, Tekieli L, Kostkiewicz M, Majka M, Szot W, Walter Z, et al. Randomized transcoronary delivery of CD34(+) cells with perfusion versus stop-flow method in patients with recent myocardial infarction: Early cardiac retention of 99(m)Tc-labeled cells activity. J Nucl Cardiol. 2011 Feb;18(1): 104–16.. doi: 10.1007/s12350-010-9326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brenner W, Aicher A, Eckey T, Massoudi S, Zuhayra M, Koehl U, et al. 111In-labeled CD34+ hematopoietic progenitor cells in a rat myocardial infarction model. J Nucl Med. 2004 Mar;45(3):512–8.. [PubMed] [Google Scholar]
- 17.Nowak B, Weber C, Schober A, Zeiffer U, Liehn EA, von Hundelshausen P, et al. Indium-111 oxine labelling affects the cellular integrity of haematopoietic progenitor cells. Eur J Nucl Med Mol Imaging. 2007 May;34(5):715–21.. doi: 10.1007/s00259-006-0275-3. [DOI] [PubMed] [Google Scholar]
- 18.Hofmann M, Wollert KC, Meyer GP, Menke A, Arseniev L, Hertenstein B, et al. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005 May 3; 111(17):2198–202.. doi: 10.1161/01.CIR.0000163546.27639.AA. [DOI] [PubMed] [Google Scholar]
- 19.Kang WJ, Kang HJ, Kim HS, Chung JK, Lee MC, Lee DS. Tissue distribution of 18F-FDG-labeled peripheral hematopoietic stem cells after intracoronary administration in patients with myocardial infarction. J Nucl Med. 2006 Aug;47(8):1295–301.. [PubMed] [Google Scholar]
- 20.Schächinger V, Aicher A, Döbert N, Röver R, Diener J, Fichtlscherer S, et al. Pilot trial on determinants of progenitor cell recruitment to the infarcted human myocardium. Circulation. 2008 Sep 30;118(14):1425–32.. doi: 10.1161/CIRCULATIONAHA.108.777102. [DOI] [PubMed] [Google Scholar]
- 21.Blocklet D, Toungouz M, Berkenboom G, Lambermont M, Unger P, Preumont N, et al. Myocardial homing of nonmobilized peripheral-blood CD34+ cells after intracoronary injection. Stem Cells. 2006 Feb;24(2):333–6.. doi: 10.1634/stemcells.2005-0201. [DOI] [PubMed] [Google Scholar]
- 22.Caveliers V, De Keulenaer G, Everaert H, Van Riet I, Van Camp G, Verheye S, et al. In vivo visualization of 111In labeled CD133+ peripheral blood stem cells after intracoronary administration in patients with chronic ischemic heart disease. Q J Nucl Med Mol Imaging. 2007 Mar;51(1):61–6.. [PubMed] [Google Scholar]
- 23.Goussetis E, Manginas A, Koutelou M, Peristeri I, Theodosaki M, Kollaros N, et al. Intracoronary infusion of CD133+ and CD133-CD34+ selected autologous bone marrow progenitor cells in patients with chronic ischemic cardiomyopathy: cell isolation, adherence to the infarcted area, and body distribution. Stem Cells. 2006 Oct;24(10):2279–83.. doi: 10.1634/stemcells.2005-0589. [DOI] [PubMed] [Google Scholar]
- 24.Dedobbeleer C, Blocklet D, Toungouz M, Lambermont M, Unger P, Degaute JP, et al. Myocardial homing and coronary endothelial function after autologous blood CD34+ progenitor cells intracoronary injection in the chronic phase of myocardial infarction. J Cardiovasc Pharmacol. 2009 Jun;53(6):480–5.. doi: 10.1097/FJC.0b013e3181a7b572. [DOI] [PubMed] [Google Scholar]
- 25.Silva SA, Sousa AL, Haddad AF, Azevedo JC, Soares VE, Peixoto CM, et al. Autologous bone-marrow mononuclear cell transplantation after acute myocardial infarction: comparison of two delivery techniques. Cell Transplant. 2009;18(3):343–52.. doi: 10.3727/096368909788534951. [DOI] [PubMed] [Google Scholar]
- 26.Vrtovec B, Poglajen G, Lezaic L, Sever M, Socan A, Domanovic D, et al. Comparison of transendocardial and intracoronary CD34+ cell transplantation in non-ischemic dilated cardiomyopathy patients. Circulation 2013; in press. doi: 10.1161/CIRCULATIONAHA.112.000230. [DOI] [PubMed] [Google Scholar]
- 27.Hou D, Youssef EA, Brinton TJ, Zhang P, Rogers P, Price ET, et al. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation. 2005 Aug 30;112(9 Suppl):I150–6.. doi: 10.1161/CIRCULATIONAHA.104.526749. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Narsinh KH, Lan F, Wang L, Nguyen PK, Hu S, et al. Early stem cell engraftment predicts late cardiac functional recovery: preclinical insights from molecular imaging. Circ Cardiovasc Imaging. 2012 Jul;5(4):481–90.. doi: 10.1161/CIRCIMAGING.111.969329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chavakis E, Koyanagi M, Dimmeler S. Enhancing the outcome of cell therapy for cardiac repair: progress from bench to bedside and back. Circulation. 2010 Jan 19;121(2):325–35.. doi: 10.1161/CIRCULATIONAHA.109.901405. [DOI] [PubMed] [Google Scholar]


