Abstract
Genetic modification of specific regions of the developing mammalian brain is a very powerful experimental approach. However, generating novel mouse mutants is often frustratingly slow. It has been shown that access to the mouse brain developing in utero with reasonable post-operatory survival is possible. Still, results with this procedure have been reported almost exclusively for the most superficial and easily accessible part of the developing brain, i.e. the cortex. The thalamus, a narrower and more medial region, has proven more difficult to target. Transfection into deeper nuclei, especially those of the hypothalamus, is perhaps the most challenging and therefore very few results have been reported. Here we demonstrate a procedure to target the entire hypothalamic neuroepithelium or part of it (hypothalamic regions) for transfection through electroporation. The keys to our approach are longer narcosis times, injection in the third ventricle, and appropriate kind and positioning of the electrodes. Additionally, we show results of targeting and subsequent histological analysis of the most recessed hypothalamic nucleus, the mammillary body.
Keywords: Neuroscience, Issue 77, Neurobiology, Genetics, Cellular Biology, Molecular Biology, Biomedical Engineering, Developmental Biology, Anatomy, Physiology, Embryo, Mammalian, Brain, Diencephalon, Hypothalamus, Genetic Techniques, Transfection, anesthesia, development, electrodes, electroporation, in utero, mammillary body, mouse, animal model
Introduction
Genetic manipulation of the embryonic mouse brain is a preferred approach to learn about developmental regulation. The generation of mutant mouse lines however is slow and expensive. One powerful method to introduce specific genetic changes in developing neurons of the mammalian brain is in utero electroporation. Essentially, the technique consists of transfecting DNA into the embryonic brain neuroepithelium by means of electric pulses, then allowing the embryo to survive for a certain period of time, collect the brain and examine them for possible novel, informative phenotypes. In this way, the experimenter can test hypotheses almost immediately without the long waiting periods necessary for the production of mouse mutants.
Transfection of DNA into developing embryos started with in ovo electroporation on chick embryos1. The essential proof-of-concept for the mouse was performed in culture2. This was soon followed by the first descriptions of the technique on the mouse in utero3,4.
The main problem is to transfect the brain of embryos developing in utero without killing them or the mother. Learning to perform the necessary surgery (laparotomy, injection, electroporation) requires a long training period. Once the surgery has been mastered to the point where the embryo survival ratio is acceptable, the next key question is: which brain structures are accessible? Not surprisingly, the first published papers showing results obtained with in utero electroporation focused on cortical development5-9. This is still true for most of the publications using this technique, since the region of the developing mouse brain most accessible to surgical procedures is the cortex (Figure 1). The procedure for in utero electroporation into the cortex has been described in print10 and in video11-14. A modification of the technique can be used to target a ventral part of the telencephalon, the basal ganglia15.
Beyond the telencephalon, the diencephalon (classically divided into thalamus and hypothalamus) is a region of the forebrain more difficult to reach. A small number of papers reports targeting of its dorsal and most accessible part, the thalamus16-19.
The hypothalamus is the most ventral part of the forebrain, therefore the one localized most deeply from the dorsal surface (cortex) (Figure 1). This region remains a difficult challenge for researchers committed to genetically manipulate the mouse brain in utero. To our knowledge, only very few articles report on results of in utero transfection into the mouse hypothalamus 20,21. However, the functional importance of the hypothalamus cannot be overstated, since it regulates behaviors like eating and drinking, mating, breeding and parenting22. Moreover, alterations in hypothalamic development contribute to originate later in life conditions like obesity, hypertension, diabetes and precocious puberty23. Being able to alter genetically the hypothalamus during development would provide a very powerful tool to understand it.
The basic surgical protocol for the laparotomy of pregnant mice that we use here is similar to that used in other protocols11,13,14,24. We will describe them here briefly for completeness. Key to our procedure, on the other hand, are the type of anesthesia, the place of injection, the type of electrodes and the insertion and position of the positive electrode with respect to the embryo's head. We prefer to induce and maintain anesthesia through gas inhalation over simple intraperitoneal anesthesia, since the former allows for the somewhat longer periods of narcosis required for a difficult surgery. Isoflurane inhalation results in faster recovery from anesthesia, since usually the mother demonstrates normal behavior already minutes after the surgery. The easiest point of injection of the DNA solution with the glass micropipette is the lateral ventricle, which however is completely unsuitable for hypothalamus electroporation. Injection directly in the third ventricle is indeed crucial to target deep diencephalic structures. It is possible to transfect the hypothalamus from E12.0 or E12.5 with standard, off-the-shelf electrodes. We have found some of the electrodes manufactured by Nepa Gene (Chiba, Japan) particularly suited to this purpose.
With our procedure we obtain transfection of the entire hypothalamic neuroepithelium or partial, regional transfection depending on electrode orientation. Here we demonstrate the technique by transfecting the mammillary body, arguably the deepest and most recessed of all hypothalamic nuclei. Additionally, we show detailed histological analysis of the transfected cells down to the cellular level of resolution.
A comparison of in utero electroporation with other approaches to transfecting the mouse developing brain in utero can be found in the Discussion section.
Protocol
1. Preparation of DNA and Glass Micropipettes for Injection
Good quality glass micropipettes are essential to reduce initial high abortion rate due to loss of amniotic fluid. The procedure to pull glass micropipettes has been well documented13,18,25. Use 1.2 mm diameter capillaries pulled in a conventional Sutter P-97 device with the settings P=500; Heat=300; Pull=40; Velocity=50; Time=50. Fit the puller with 3 mm "trough" filaments (Sutter Instrument FT330B). The 2 mm size filaments have yielded for us less satisfactory results. On the other hand, beveling of the micropipette tips does not seem to improve results for us.
Dissolve purified, endotoxin-free plasmid DNA in PBS (Cell Culture Grade) containing 0.1% Fast Green to a final concentration of 1 to 2 μg/μl. The Fast Green will make the injected solution visible in the embryonic brain ventricle.
Load 10 μl of the DNA solution into the glass micropipette.
Connect the glass micropipette to the injection system (Pico Pump) or mouth pipette.
2. Anesthesia
Prepare the surgical table with a heating pad and the surgical instruments. Turn on the cold-light sources to facilitate the visualization of the embryos. Disinfect the surgical tools using for instance a glass bead sterilizer.
Three different anesthesia procedures are possible for this protocol (see Discussion). Here we will describe the one we consider the best, using isoflurane inhalation for anesthesia induction (flow rate 0.5 L/min) as well as maintenance (flow rate 1 L/min).
Introduce the pregnant mouse in a small transparent (so the mouse remains visible) container connected to the Komesaroff Mark 5 Anesthetic Device by a short length of tubing.
Fill the vaporizer with isoflurane, then open the oxygen bottle attached to the device. Blow one or two pulses of isoflurane/oxygen (0.5 L/min) into the container holding the mouse and watch for narcosis to set in.
Take the mouse out, cover its eyes with ointment to prevent them from drying and fit the flow anesthesia mask on its head immediately.
3. Laparotomy
Place the mouse belly up on the heating pad (otherwise its body temperature will go down very fast, decreasing chances of recovery) and secure its body into place by fixing its four legs to the sides with tape. Assess the depth of anesthesia by checking for loss of response to reflex stimulation (e.g. toe or tail pinch with firm pressure).
Shave the abdominal surface and disinfect it with the iodophorpovidone-iodine (Braunoderm).
Make a longitudinal incision (1 to 1.5 cm long) on the abdominal skin. Then cut the peritoneum. Place cotton gauze around the incision. Make one uterine horn visible and pull it out carefully with blunt forceps onto the PBS-rinsed gauze. Rinse the uterus with PBS very often to keep it always moistened (Figures 2A and 2B). During the entire procedure avoid pulling the mesometrium or the uterus tight, since high pressure inside the uterus will transmit to the embryo increasing the chances of loss of fluid upon injection resulting in abortion.
4. DNA Injection into the Brain Ventricle
Hold the uterus in such a way that the brain ventricles can be visualized. Do not extensively reposition an embryo that lies in an unfavorable position - this only increases the chances of abortion.
Looking at the embryo's head from top, localize visually the gap or fissure between the left and right cortical hemispheres. The hemispheres are easy to distinguish and the lateral ventricles inside them (not to be targeted) can usually be perceived as somewhat darker shapes. If an embryo is found to be perfectly oriented for DNA injection (Figures 2C and 2D), it is possible to pierce the uterine wall and enter the third ventricle at once. Hold the glass micropipette at 45° to the uterine wall and puncture it at the rostral end of the gap between cortical hemispheres, penetrating for about 1 mm. In this way the tip of the glass micropipette will enter the third ventricle of the brain (not the lateral ventricle) (Figures 2C and 2D). In embryos less favorably oriented it is useful to first pierce the uterine wall (always at 45°) in the vicinity of the embryonic head, place the micropipette tip in the right position between the cortical hemispheres and only then perforate the brain. Inject about 1 μl of DNA solution into the third ventricle (a good injection fills the ventricle with green fluid). Repeat the same procedure with all embryos of one uterus horn. This allows some time for the DNA solution to mix evenly with the ventricular fluid and reach the entire neuroepithelium.
5. Targeting the Hypothalamus for Electroporation
Switch the electroporator on and adjust settings according to the embryonic age (for E12.5 we use 5 square-wave pulses, 50 V, 50 msec ON/950 msec OFF). Use as positive pole the stainless steel needle electrode (CUY550-10) and as negative pole a round flat electrode (CUY700P4L). (It is important that the electrodes are smaller than the embryo, since otherwise the current just flows around the embryo but not through it.)
Select for electroporation those embryos whose dorsal side is up (i.e. turned towards the experimenter) (as most of them are), and discard those whose orientation is not favorable. It is now safe to touch the uterus with ethanol-disinfected fingers or with gloves. Holding the uterus with forceps, however, would interfere with the flow of current during electroporation.
Pierce the uterine wall by the embryo's head between the amniotic sac and the placenta by thrusting downwards through it with the tip of the needle electrode. Use the index finger of the other hand as thrust block. About 5 mm of the electrode tip must be now between the amniotic sac and the uterine wall, at about the level of the midbrain (Figures 2E and 2F). Remember that this is the "targeting electrode", so its position will determine which part of the hypothalamic neuroepithelium is most likely to get transfected. The embryo has to be very gently "squeezed" between the electrodes.
With the other hand, position the round flat electrode outside of the uterus wall on the opposite side of the embryo's head (Figures 2E and 2G).
Use the pedal switch to apply voltage (50 V, 50 msec ON, 950 msec OFF, 5 square-wave pulses).
Slowly pull the needle electrode out of the uterus while holding back the uterus with the index finger of the other hand - if amniotic liquid is lost through the punctured wall, usually the embryo will undergo abortion. Repeat the procedure with all the embryos.
Return the uterus horn into the abdomen and repeat the injection and electroporation in the remnant horn.
6. Finishing the Surgery
After injection and electroporation of all embryos, place the uterus back into the abdomen very carefully, positioned exactly as they were before and moist the peritoneal cavity with saline before closing it.
Suture the peritoneum with surgical catgut (Vicryl Polyglactin 910, 5-0, Ethicon V4914). For the closure of the skin use "interrupted stitch" methodwith a more resistant suture (Supramid Nylon, 6-0, Serag Wiessner TO 07171L).
Disinfect the abdomen surface with povidone-iodine(Braunoderm) and inject a non-steroid anti-inflammatory subcutaneously (e.g. 100 μl of a 1:10 solution of Rimadyl in 0.9% NaCl) or, even better, an opioid like buprenorphine (Temgesic, 0.05 to 0.1 mg/kg body weight in 0.9% NaCl)to relieve pain.
Remove the mother from the anesthetic machine and place it in a cage which is heated by a heating pad. Monitor the mouse continually until it is completely recovered from the anesthesia. Later on, check the animals daily to insure they are recovering from the procedure without any sign of infection or pain.
7. Analyzing the Results
Harvest the embryos (or postnatals) according to the desired day of analysis. Postnatal mice (1 to 2 day old) are killed by decapitation.
Separate every embryo according to the previous electroporation annotation. Dissect the brain under a stereomicroscope and check under the microscope for green fluorescent signal (from the GFP reporter) in the appropriate region.
The selected brain can be analyzed "fresh": fix the tissue for a short time in 4% paraformaldehyde and embed in agarose 4% or in gelatin-albumin, then section on a vibratome-type device (Figure 3). For more detailed immunohistochemical analysis (Figure 4), cryo-protect the brain in 30% saccharose solution, embed in OCT mounting medium (Tissue Tek) then cut 20 μm sections in a cryostat.
Representative Results
Most hypothalamus neurons are born between E11.5 to E15.2, according to birth-dating analysis in the rat26 translated into the somewhat shorter mouse development27,28. The peak of hypothalamic neurogenesis is reached at E12.529-31. Accordingly, at the transfection age chosen for the present study (E12.5), a large proportion of hypothalamic neurons can be labeled at any given rostro-caudal level.
Analysis at E18.5 on thick vibratome-type sections (Figures 3A and 3B) shows that the entire rostro-caudal extension of the hypothalamic neuroepithelium is accessible to electroporation (Figure 3A). At any given age, neurons can be labeled at all medio-lateral levels (Figure 3B), in agreement with lineage studies32. The mammillary body (MBO) is a neuronal nucleus developing from the neuroepithelium lining a recess in the most ventral and caudal portion of the forebrain. This makes the MBO in principle very difficult to target. Functionally, the MBO is a key structure for memory consolidation and its pathological degeneration results in anterograde amnesia33. We have been able to transfect reporter plasmids very specifically into this nucleus (Figures 3C and 3D). MBO neurons extend axons forming two very characteristic and functionally important tracts which are also labeled after transfection (arrowheads in Figure 3C). Transverse sections show that the neurons born at E12.5 (age of transfection) form a curved "layer" around a non-labeled mass of neurons generated earlier (i.e. before the transfection experiment took place) (Figure 3D).
Beyond the general migration patterns shown by examination on thick sections with the help of fluorescent reporter proteins, a closer analysis of the results is possible using specific antibodies on cryostat sections (Figure 4). To analyze the example chosen here, the MBO, we prepared horizontal sections parallel to the plane of the radial glial processes emanating from the mammillary recess (Figure 4A). We then identified the MBO neurons born from neuroepithelium labeled on E12.5 by means of antibodies against GFP (Figure 4B). As expected, the antibody labeled a restricted group of MBO cells (arrowhead in Figures 4B, 4C, and 4D). This group was located between two unlabeled lateral and medial MBO areas corresponding respectively to MBO neurons born before (lateral) and after (medial) E12.5. As an example, here we co-stained with an antibody against nestin (red in Figures 4C and 4D) in order to show the migration mode of individually labeled neurons on radial glial processes of the neuroepithelium.
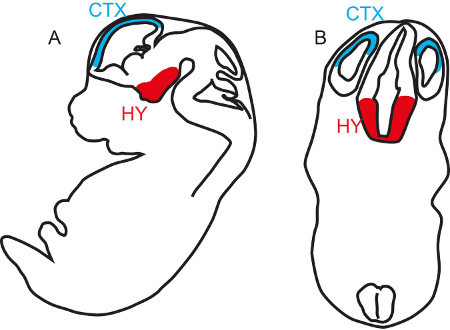 Figure 1. Relative positions of Hypothalamus and Cortex at midgestation. Sagittal (A) and transverse (B) diagrams of the brain of a midgestation mouse embryo showing the most dorsal and most superficial forebrain structure, the cortex (CTX, blue) as well as the most deeply placed forebrain structure, the hypothalamus (HY, red).
Figure 1. Relative positions of Hypothalamus and Cortex at midgestation. Sagittal (A) and transverse (B) diagrams of the brain of a midgestation mouse embryo showing the most dorsal and most superficial forebrain structure, the cortex (CTX, blue) as well as the most deeply placed forebrain structure, the hypothalamus (HY, red).
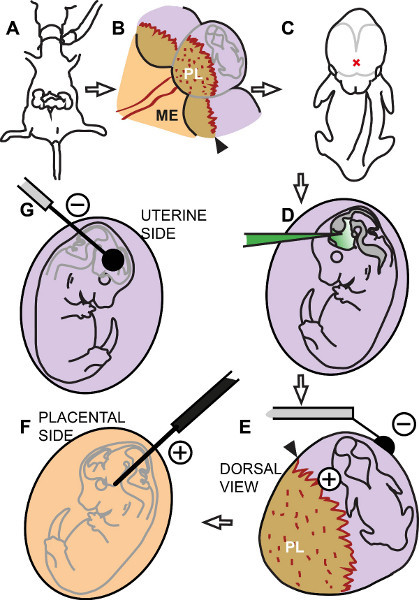 Figure 2. Diagram of the procedure. (A) The pregnant dam is anesthetized and the uterus exposed. (B) In each uterine swelling identify the placental insertion (black arrowhead), placenta (PL) and embryo. ME, mesometrium. (C) Identify the injection point in the middle of the left and right cortical hemispheres (red X). (D) Inject DNA solution into the third ventricle. (E) The needle electrode (positive) pierces the uterine wall between embryo and placental insertion. The flat electrode (negative) rests on the free side of the uterus. (F) Hypothetical view from the placental side showing position of the positive electrode along the hypothalamus. (G) View from the free side of the uterus showing the position of the negative electrode.
Figure 2. Diagram of the procedure. (A) The pregnant dam is anesthetized and the uterus exposed. (B) In each uterine swelling identify the placental insertion (black arrowhead), placenta (PL) and embryo. ME, mesometrium. (C) Identify the injection point in the middle of the left and right cortical hemispheres (red X). (D) Inject DNA solution into the third ventricle. (E) The needle electrode (positive) pierces the uterine wall between embryo and placental insertion. The flat electrode (negative) rests on the free side of the uterus. (F) Hypothetical view from the placental side showing position of the positive electrode along the hypothalamus. (G) View from the free side of the uterus showing the position of the negative electrode.
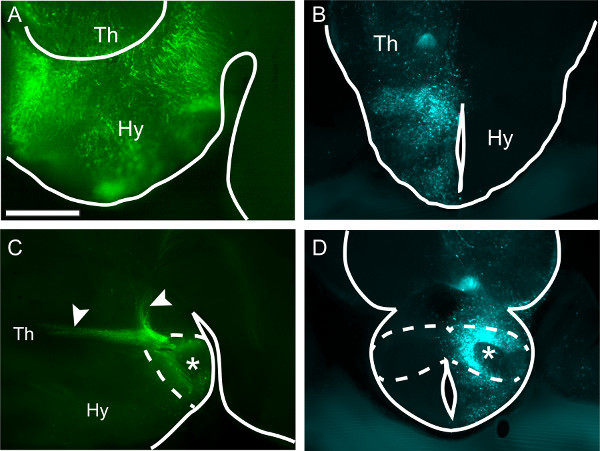 Figure 3. Transfecting the entire hypothalamus or part of it. Thick vibrating-microtome sections of four different wild type E18.5 mouse brains showing the hypothalamus after in utero transfection of plasmid DNA carrying fluorescent reporter genes GFP (A, C) or CFP (B, D) at E12.5. (A) Sagittal section (rostral to the left) showing a hypothalamus transfected from rostral to caudal. Scale bar 500 μm. (B) Transverse section. On the transfected side, labeled cells can be found at all medio-lateral levels. (C) Sagittal section showing a specifically transfected mammillary body (dashed line, asterisk) and labeling of its characteristic axonal tree (arrowheads). (D) Transverse section through the mammillary body (dashed line). On the electroporated side (asterisk), a labeled band corresponding to neurons born on E12.5 can be seen. Hy, hypothalamus; Th, thalamus
Figure 3. Transfecting the entire hypothalamus or part of it. Thick vibrating-microtome sections of four different wild type E18.5 mouse brains showing the hypothalamus after in utero transfection of plasmid DNA carrying fluorescent reporter genes GFP (A, C) or CFP (B, D) at E12.5. (A) Sagittal section (rostral to the left) showing a hypothalamus transfected from rostral to caudal. Scale bar 500 μm. (B) Transverse section. On the transfected side, labeled cells can be found at all medio-lateral levels. (C) Sagittal section showing a specifically transfected mammillary body (dashed line, asterisk) and labeling of its characteristic axonal tree (arrowheads). (D) Transverse section through the mammillary body (dashed line). On the electroporated side (asterisk), a labeled band corresponding to neurons born on E12.5 can be seen. Hy, hypothalamus; Th, thalamus
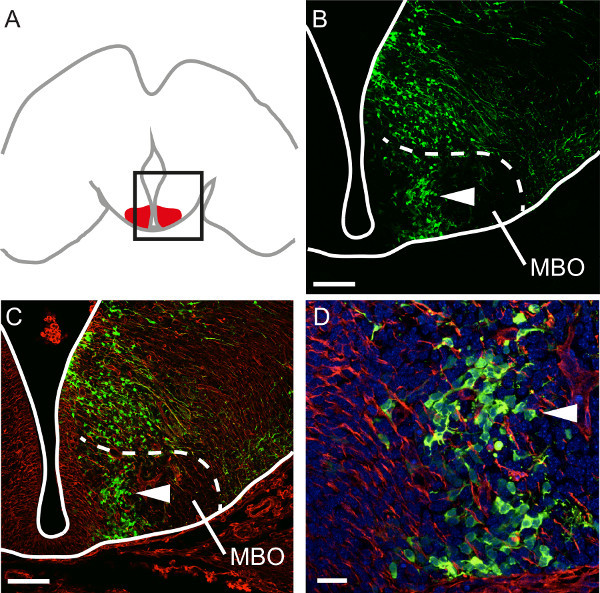 Figure 4. Analyzing specific steps in hypothalamic development. (A) Diagram of a horizontal section through the developing brain showing the mammillary body (red). The region depicted in (B) and (C) is framed. (B) Antibody labeling of GFP on a horizontal section through the E18.5 mammillary body after GFP transfection at E12. Cells born at E12.5 form a distinct band in the nucleus (arrowhead in B, C and D). Scale bar 100 μm. (C) Antibody labeling of neuroepithelial marker nestin (red) on the section shown in (B). Scale bar 100 μm. (D) Individual cells can be identified in the specific E12.5 band in the mammillary body under high magnification. Scale bar 20 μm.
Figure 4. Analyzing specific steps in hypothalamic development. (A) Diagram of a horizontal section through the developing brain showing the mammillary body (red). The region depicted in (B) and (C) is framed. (B) Antibody labeling of GFP on a horizontal section through the E18.5 mammillary body after GFP transfection at E12. Cells born at E12.5 form a distinct band in the nucleus (arrowhead in B, C and D). Scale bar 100 μm. (C) Antibody labeling of neuroepithelial marker nestin (red) on the section shown in (B). Scale bar 100 μm. (D) Individual cells can be identified in the specific E12.5 band in the mammillary body under high magnification. Scale bar 20 μm.
Discussion
About the anesthesia: Since in utero electroporation into the hypothalamus can be technically arduous and require longer narcosis times, we prefer to induce and maintain anesthesia through administration of a mixture of oxygen and isoflurane. In our experience, animals can remain suitably anaesthetized in this way for periods of up to one hour at least, the recovery of the mother is very fast, and embryo survival improved. Other approaches to anesthesia are also available. The most simple procedure consists of inducing and maintaining narcosis by intraperitoneal injection of 2.5 ml/kg of body weight of a mixture of Ketamine (25 mg/ml), Xylazin (1.2 mg/ml) and Acepromazin (0.35 mg/ml) in 0.9% sterile NaCl ("triple combo"). With this mixture alone, at the doses recommended here, a narcosis of about 30 min (up to 45 min in some cases) is induced. This is usually enough for in utero electroporation into the cortex at E12.5 (through injection in the lateral ventricle). The advantage of this method is that it completely avoids the need for a narcosis-device and other equipment. However, this type of intraperitoneal anesthesia is not recommended if longer times of surgery are necessary, since further injections in the anesthetized animal in order to prolong the anesthesia period are often followed by death. It is also possible to combine intraperitoneal and inhalation anesthesia by first using an injection of the "triple combo" (as above) in order to induce narcosis, then maintain it by inhalation of isoflurane/oxygen. Although with this particular method the narcosis can be maintained for as long as it is needed, the recovery of the pregnant mouse after the operation is not as good as with inhalation alone. Besides, whenever we use the "triple combo" intraperitoneal injection, embryo survival decreases.
The positioning of the electrodes on the embryo's head as depicted in Figure 2 is crucial. Notice that it is not necessary to place an electrode "under" the diencephalon (which would be very difficult), but on the side of it. The needle electrode has a sharp tip, but it is useful to have it sharpened additionally (this can increase however the chances to injure the amniotic sac). We have also tried polishing the entire exposed 10 mm portion of the CUY550-10 into a very thin wire. This however dramatically decreased the chances of obtaining transfection.
The laparotomy is straightforward enough. However, the experimenter reaches the level of skill that allows for high survival ratio of embryos (after intraventricular injection followed by the administration of electric pulses) after a flat learning curve. Individual skill is an important factor so that in utero electroporation in general and its application to the hypothalamus in particular can prove not entirely practical for some students. To shorten the learning period as well as to increase reproducibility and statistical analysis we have found it useful to fill a detailed protocol including information about the number and position of the embryos, and the appearance, quality of the injection and approximate position of the electrodes for the individual embryos. Comparing these data about the experiment with the final results, on an embryo-by-embryo basis has proven useful to understand and improve the procedure.
Other reports20,21 have targeted the hypothalamus with Nepagene tweezer-type electrodes. We consistently obtain better results with the application of one needle-electrode and one flat cover-electrode of the same manufacturer. In our experience, the use of this method makes rostro-caudal targeting easier. Tweezer electrodes seem to require either more experience/skill or a much larger number of experiments in order to yield useful results.
At least two other approaches to transfect DNA constructs into the developing mouse hypothalamus can be compared to in utero electroporation. The first one is also based on laparotomy and injection into the embryonic brain ventricle, the difference being that the DNA of interest is carried by a viral particle. The virus infects the neuroepithelial cells and in this way transfects our experimental construct - no electroporation is needed. Advantages: the procedure avoids the electric pulses necessary for electroporation, therefore embryo survival probably improves. The main disadvantages of this method are: 1) work with viruses requires special precautions and S2 facilities; 2) targeting is out of the question, since viral particles will spread over the entire ventricle making it in principle equally likely to transfect any neuroepithelial region (of course some degree of transfection into the region of interest is likely). Of course, in utero delivery of viruses poses essentially the same problem as in utero electroporation, i.e. the relatively difficult surgery procedure, with a long learning curve depending strongly on the individual skills of the experimenter.
The other group of approaches avoids surgery completely, since it is based on injection of the constructs to transfect into the blood stream of the pregnant mouse. Tail vein (caudal vein) injection of shRNA34,35 or plasmids36 has the potential to manipulate genetically very early embryos, although region-specific targeting is not possible with this method. This promising approach does not seem yet ready for general application. Viral particles (adeno-associated virus) injected in the facial vein of neonatal mice (but not older mice) is able to transfect brain neurons37. Finally, nanoparticles, which could eventually be used as DNA carriers, can reach the brain after tail vein injection in adult mice38. These two methods, which open the possibility of transfecting very early embryos, have to our knowledge not yet been used on pregnant mice.
Age of transfection and collection: For learning the procedure, it is probably best to try to transfect E14.5 embryos, and then graduate to earlier, more difficult embryonic ages. In our experience, GFP expression is visible under UV light already 24 hr after transfection. After E12.5 transfection, GFP expression on E18.5 is very strong. We have electroporated at E12.5, E13.5, E14.5, and E15.5 and analyzed at E17.5, E18.5, P0 and P1 being able to find strong reporter expression in all cases. When embryos whose brains have been transfected in utero are allowed to reach term (for postnatal data collection), sometimes they are selectively eaten by the mother. This suggests subtle changes in pup appearance or behavior induced by brain manipulation (their temperature could be lower, or maybe they do not emit the right sound signals). In those postnatal animals that survive maternal culling, we have observed reporter expression up until P1 at least. Reports in the literature show expression of transfected constructs even four months after birth10.
Vectors for electroporation: we use bicistronic reporter plasmids driven by the CAG promoter39, followed by the cDNA of the gene of interest, separated by an internal ribosome entry site (IRES)40, from the cDNA of a fluorescent reporter, for instance the enhanced green fluorescent protein41. The CAG promoter is so strong in neurons that it can produce levels of DNA too high for the transfected cells, killing them. Although we have never observed massive apoptosis after transfection, a possible solution, should the problem appear, would be the use of a less efficient promoter, like the EF1 alpha42.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
This work was funded by the German Research Foundation (Deutsche Forschungsgemeinschaft).
References
- Itasaki N, Bel-Vialar S, Krumlauf R. Shocking' developments in chick embryology: electroporation and in ovo gene expression. Nat. Cell Biol. 1999;1:E203–E207. doi: 10.1038/70231. [DOI] [PubMed] [Google Scholar]
- Miyasaka N, Arimatsu Y, Takiguchihayashi K. Foreign gene expression in an organotypic culture of cortical anlage after in vivo electroporation. Neuroreport. 1999;10:2319–2323. doi: 10.1097/00001756-199908020-00018. [DOI] [PubMed] [Google Scholar]
- Saito T, Nakatsuji N. Efficient gene transfer into the embryonic mouse brain using in vivo electroporation. Dev. Biol. 2001;240:237–246. doi: 10.1006/dbio.2001.0439. [DOI] [PubMed] [Google Scholar]
- Tabata H, Nakajima K. Efficient in utero gene transfer system to the developing mouse brain using electroporation: visualization of neuronal migration in the developing cortex. Neuroscience. 2001;103:865–872. doi: 10.1016/s0306-4522(01)00016-1. [DOI] [PubMed] [Google Scholar]
- Fukuchi-Shimogori T, Grove EA. Neocortex patterning by the secreted signaling molecule FGF8. Science. 2001;294:1071–1074. doi: 10.1126/science.1064252. [DOI] [PubMed] [Google Scholar]
- Tabata H, Nakajima K. Neurons tend to stop migration and differentiate along the cortical internal plexiform zones in the Reelin signal-deficient mice. J. Neurosci. Res. 2002;69:723–730. doi: 10.1002/jnr.10345. [DOI] [PubMed] [Google Scholar]
- Fukuchi-Shimogori T, Grove EA. Emx2 patterns the neocortex by regulating FGF positional signaling. Nat. Neurosci. 2003;6:825–831. doi: 10.1038/nn1093. [DOI] [PubMed] [Google Scholar]
- Shimogori T, Banuchi V, Ng HY, Strauss JB, Grove EA. Embryonic signaling centers expressing BMP, WNT and FGF proteins interact to pattern the cerebral cortex. Development. 2004;131:5639–5647. doi: 10.1242/dev.01428. [DOI] [PubMed] [Google Scholar]
- Shimogori T, Grove EA. Fibroblast growth factor 8 regulates neocortical guidance of area-specific thalamic innervation. J. Neurosci. 2005;25:6550–6560. doi: 10.1523/JNEUROSCI.0453-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T. In vivo electroporation in the embryonic mouse central nervous system. Nat. Protoc. 2006;1:1552–1558. doi: 10.1038/nprot.2006.276. [DOI] [PubMed] [Google Scholar]
- Dixit R, et al. Efficient Gene Delivery into Multiple CNS Territories Using In Utero Electroporation. J. Vis. Exp. 2011. p. e2957. [DOI] [PMC free article] [PubMed]
- Rice H, Suth S, Cavanaugh W, Bai J, Young-Pearse TL. In utero Electroporation followed by Primary Neuronal Culture for Studying Gene Function in Subset of Cortical Neurons. J. Vis. Exp. 2010. p. e2103. [DOI] [PMC free article] [PubMed]
- Walantus W, Castaneda D, Elias L, Kriegstein A. In Utero Intraventricular Injection and Electroporation of E15 Mouse Embryos. J. Vis. Exp. 2007. p. e239. [DOI] [PMC free article] [PubMed]
- Walantus W, Elias L, Kriegstein A. In Utero Intraventricular Injection and Electroporation of E16 Rat Embryos. J. Vis. Exp. 2007. p. e236. [DOI] [PMC free article] [PubMed]
- Borrell V, Yoshimura Y, Callaway EM. Targeted gene delivery to telencephalic inhibitory neurons by directional in utero electroporation. J. Neurosci. Methods. 2005;143:151–158. doi: 10.1016/j.jneumeth.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Haddad-Tovolli R, Heide M, Zhou X, Blaess S, Alvarez-Bolado G. Mouse thalamic differentiation: gli-dependent pattern and gli-independent prepattern. Front Neurosci. 2012;6:27. doi: 10.3389/fnins.2012.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka A, Shimogori T. Fgf8 controls regional identity in the developing thalamus. Development. 2008;135:2873–2881. doi: 10.1242/dev.021618. [DOI] [PubMed] [Google Scholar]
- Matsui A, Yoshida AC, Kubota M, Ogawa M, Shimogori T. Mouse in Utero Electroporation: Controlled Spatiotemporal Gene Transfection. J. Vis. Exp. 2011. p. e3024. [DOI] [PMC free article] [PubMed]
- Vue TY, et al. Sonic hedgehog signaling controls thalamic progenitor identity and nuclei specification in mice. J. Neurosci. 2009;29:4484–4497. doi: 10.1523/JNEUROSCI.0656-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, et al. Transcriptional regulation of the hypocretin/orexin gene by NR6A1. Biochem. Biophys. Res. Commun. 2010;403:178–183. doi: 10.1016/j.bbrc.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya R, Takahashi K, Liu FC, Takahashi H. Aberrant axonal projections from mammillary bodies in Pax6 mutant mice: possible roles of Netrin-1 and Slit 2 in mammillary projections. J. Neurosci. Res. 2009;87:1620–1633. doi: 10.1002/jnr.21966. [DOI] [PubMed] [Google Scholar]
- Canteras NS. In: The Mouse Nervous System. Watson C, Paxinos G, Puelles L, editors. Academic Press; 2011. pp. 539–562. [Google Scholar]
- Caqueret A, Yang C, Duplan S, Boucher F, Michaud JL. Looking for trouble: a search for developmental defects of the hypothalamus. Horm. Res. 2005;64:222–230. doi: 10.1159/000088977. [DOI] [PubMed] [Google Scholar]
- Petros TJ, Rebsam A, Mason CA. In utero and ex vivo Electroporation for Gene Expression in Mouse Retinal Ganglion Cells. J. Vis. Exp. 2009. p. e1333. [DOI] [PMC free article] [PubMed]
- Nijagal A, Le T, Wegorzewska M, Mackenzie TC. A Mouse Model of in Utero Transplantation. J. Vis. Exp. 2011. p. e2303. [DOI] [PMC free article] [PubMed]
- Altman J, Bayer SA. The Development of the Rat Hypothalamus. Adv. Anat. Embryol. Cell Biol. 1986;100:1–178. [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Clancy B, et al. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics. 2007;5:79–94. doi: 10.1385/ni:5:1:79. [DOI] [PubMed] [Google Scholar]
- Ishii Y, Bouret SG. Embryonic birthdate of hypothalamic leptin-activated neurons in mice. Endocrinology. 2012;153:3657–3667. doi: 10.1210/en.2012-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim MA, Sloper JC. Histogenesis of the supraoptic and paraventricular neurosecretory cells of the mouse hypothalamus. J. Anat. 1980;130:341–347. [PMC free article] [PubMed] [Google Scholar]
- Shimada M, Nakamura T. Time of neuron origin in mouse hypothalamic nuclei. Exp. Neurol. 1973;41:163–173. doi: 10.1016/0014-4886(73)90187-8. [DOI] [PubMed] [Google Scholar]
- Alvarez-Bolado G, Paul FA, Blaess S. Sonic hedgehog lineage in the mouse hypothalamus: from progenitor domains to hypothalamic regions. Neural. Dev. 2012;7:4. doi: 10.1186/1749-8104-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP. The mammillary bodies: two memory systems in one. Nat. Rev. Neurosci. 2004;5:35–44. doi: 10.1038/nrn1299. [DOI] [PubMed] [Google Scholar]
- Gratsch TE, De Boer LS, O'Shea KS. RNA inhibition of BMP-4 gene expression in postimplantation mouse embryos. Genesis. 2003;37:12–17. doi: 10.1002/gene.10221. [DOI] [PubMed] [Google Scholar]
- Wu N, Yu AB, Zhu HB, Lin XK. Effective silencing of Sry gene with RNA interference in developing mouse embryos resulted in feminization of XY gonad. J. Biomed. Biotechnol. 2012;2012:343891. doi: 10.1155/2012/343891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi N, Nakamura S, Ohtsuka M, Kimura M, Sato M. Possible mechanism of gene transfer into early to mid-gestational mouse fetuses by tail vein injection. Gene Ther. 2002;9:1529–1541. doi: 10.1038/sj.gt.3301818. [DOI] [PubMed] [Google Scholar]
- Foust KD, et al. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat. Biotechnol. 2009;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, et al. The brain targeting mechanism of Angiopep-conjugated poly(ethylene glycol)-co-poly(epsilon-caprolactone) nanoparticles. Biomaterials. 2012;33:1673–1681. doi: 10.1016/j.biomaterials.2011.11.018. [DOI] [PubMed] [Google Scholar]
- Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Kaufman RJ, Davies MV, Wasley LC, Michnick D. Improved vectors for stable expression of foreign genes in mammalian cells by use of the untranslated leader sequence from EMC virus. Nucleic Acids Res. 1991;19:4485–4490. doi: 10.1093/nar/19.16.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien RY. The green fluorescent protein. Annu. Rev. Biochem. 1998;67:509–544. doi: 10.1146/annurev.biochem.67.1.509. [DOI] [PubMed] [Google Scholar]
- Londrigan SL, et al. Evaluation of promoters for driving efficient transgene expression in neonatal porcine islets. Xenotransplantation. 2007;14:119–125. doi: 10.1111/j.1399-3089.2007.00376.x. [DOI] [PubMed] [Google Scholar]


