Abstract
We created a vaccine in which irradiated allogeneic lung adenocarcinoma cells are combined with a bystander K562 cell line transfected with hCD40L and hGM-CSF. By recruiting and activating dendritic cells, we hypothesized that the vaccine would induce tumor regression in metastatic lung adenocarcinoma. Intradermal vaccine was given q14 days x3, followed by monthly x3. Cyclophosphamide (300 mg/m2 IV) was administered before the 1st and 4th vaccines to deplete regulatory T-cells. All-trans retinoic acid was given (150/mg/m2/day) after the 1st and 4th vaccines to enhance dendritic cell differentiation. Twenty-four participants were accrued at a single institution from 10/2006 to 6/2008, with a median age 64 and median of 4 previous lines of systemic therapy. A total of 101 vaccines were administered. Common toxicities were headache (54%) and site reaction (38%). No radiologic responses were observed. Median overall survival (OS) was 7.9 months (mo) and median progression-free survival (PFS) was 1.7 mo. Of 14 patients evaluable for immunological study, 5 had peptide-induced CD8+ T-cell activation after vaccination. Overall, vaccine administration was feasible in an extensively pretreated population of metastatic lung cancer. Despite a suggestion of clinical activity in the subset with immune response, the trial did not meet the primary endpoint of inducing radiologic tumor regression.
INTRODUCTION
Due to high annual incidence and poor long-term survival, lung cancer remains an ideal target for novel agents such as immunotherapy. In particular, the treatment of patients with advanced non-small cell lung cancer (NSCLC) is frequently complicated by co-morbid conditions and older age.1 Thus, tumor vaccines may be ideal in this population due to their favorable toxicity profile.2 Unfortunately, tumor-associated antigen (TAA) vaccination alone is usually insufficient to induce innate immunity, likely due to host immune incompetence and tumor-related immune suppression.3 Therefore, strategies to induce or deregulate co-stimulatory protein interactions have been investigated. In particular, dendritic cells (DC) are the most potent antigen presenting cells (APC) that express co-stimulatory molecules.4 DCs are activated by the cytokine granulocyte-macrophage colony stimulating factor (GM-CSF).5 Furthermore, the maturation of DCs from immunosuppressive myeloid-derived suppressor cells (MDSCs) is induced by the combination of GM-CSF with IL-46 or IL-10.7
Several previous vaccine trials in NSCLC have tested methods of recruiting dendritic cells with GM-CSF. An adenoviral vector for delivery of hGM-CSF gene was safe in NSCLC8,9 and a larger trial in NSCLC suggested a correlation of cell dose to survival.10 Unfortunately, this approach was hampered by feasibility, since genetic transduction of individual tumors required a median of 50 days from harvest to treatment. A scientific advance was the creation of a "bystander" cell line derived from K562, which is universally major histocompatability complex (MHC) negative.11 This line was stably transfected with plasmid vector to secrete GM-CSF, eliminating the burden of genetic modification of autologous cells. However, when this bystander was combined with autologous TAAs in NSCLC, no tumor regression was observed.12 Subsequently, it was discovered that GM-CSF-expressing bystander vaccine at high doses may actually impair immunity by recruitment of induced Gr1+/CD11b+ myeloid suppressor cells.13,14 Similarly, anti-tumor vaccine activity is often attenuated by induction of regulatory T-cells.15,16,15 Due to the antigenic heterogeneity of NSCLC, many trials have relied upon autologous tumor for vaccine TAAs. However, autologous collection suffers from several potential drawbacks: failure to harvest, unsuccessful processing or contamination, and patient progression while awaiting vaccine preparation.17
To address these problems, we created a bystander K562 cell line which was transfected with GM-CSF and CD40L plasmids admixed with two lung adenocarcinoma cell lines as the source of shared tumor antigens.18 CD40L expression is believed to augment DC activation at the local vaccine site.19 Our bystander cell line (GM.CD40L) was more effective in inducing responses in cultures of tumor-draining lymph nodes compared to autologous vaccine alone.20 In a Phase I trial of GM.CD40L with an autologous tumor vaccine, anti-tumor immune responses as well as some durable radiologic stable disease was observed.21 Next, we designed a preparation of two lethally irradiated lung adenocarcinoma cell lines as a shared tumor antigen. This contains together HER-2/neu, CEA, GD-2, WT-1, MAGE-A1 and -A3.22 In this approach, thousands of potential lung tumor epitopes within the lysate may be taken up and cross-presented by both MHC class I and II molecules on DCs.23, 24 Thus, screening for specific TAAs or matching HLA type in individual patients is not required. All-trans-retinoic acid (ATRA) was added to induce differentiation of immature DCs at the local vaccine site.25 Cyclophosphamide pretreatment was included to reduce the number and function of CD25+ T-regulatory cells.26 We hypothesized that this vaccine preparation would induce objective responses in a clinical trial of patients with advanced lung adenocarcinoma.
METHODS
Study Design
This was an open-label, single institution, single-arm, phase II trial designed to evaluate the safety, efficacy, and immunogenicity of a GM.CD40L plus tumor cell-based vaccine. Intravenous cyclophosphamide at 300 mg/m2 was given on day 1 and 57. The vaccine was then administered intradermally in the axillary and inguinal lymph node basins; every 2 weeks for the first 4 weeks (days 4, 18, and 32), then monthly for the next 3 months (days 60, 88, and 116). All-trans retinoic acid (ATRA,) at 150 mg/m2/day, was given orally thrice daily after the first and fourth vaccine (days 5–7 and days 61–63). CT of chest, abdomen, and pelvis was done at baseline, with reassessment at week 8, week 17, and every three months thereafter using Response Evaluation Criteria in Solid Tumors (RECIST) v 1.0. This schedule was followed uniformly and consistently. Hematologic, liver, and renal function was assessed, and toxicity was graded according to National Cancer Institute Common Toxicity Criteria v 3.0. Biologic materials were registered with the Food and Drug Administration (BB-IND-13088) and Office of Biologic Activities (NIH-OBA-0608-801). The trial was nationally registered (NCT00601796). All patients signed the protocol-specific informed consent approved by local institutional review board (FWA00001669).
Study Population
Eligible patients for this study were 18 years or older, with histologically-confirmed American Joint Committee on Cancer (AJCC) clinical stage IIIB or IV lung adenocarcinoma that was refractory or relapsed after at least one previous systemic therapy. Participants had to have an Eastern Cooperative Oncology Group performance status of 0 or 1, an absolute granulocyte count > 1,500/µL, platelet count > 100,000 /µL, hematocrit >25%, total bilirubin < 2 mg/dL, and serum creatinine < 2 mg/dL. Participants were required to have progressive, measurable disease by RECIST criteria. Patients with brain metastases were eligible if the disease was asymptomatic. Patients were excluded if they had received prior systemic corticosteroids, radiation, or chemotherapy within 4 weeks of first vaccination. Patients withpregnancy or a history of HIV were not eligible to participate in the study.
Study Drug
The GM.CD40L bystander cell line was prepared as previously described.21 Two human cell lines, NCI-H1944 and NCI-H2122, were used as the TAA source. These cell lines were originally isolated from two smokers with stage IIIB lung adenocarcinoma.27 H2122 and H1944 were tested for correct identification and karyology, and products were free of viral, retroviral, and bacterial contaminants (WuXi AppTec®, St Paul MN). These cell lines do not constitutively express HLA-A2.22
For the therapeutic vaccine, one vial containing 7.5 × 106 radiated H1944 adenocarcinoma cells, 1 vial containing 7.5 × 106 radiated H2122 adenocarcinoma cells, and 1 vial containing 15 × 106 radiated GM.CD40L bystander cells were thawed rapidly to 37°C, combined, diluted in 10 mL sterile saline for 15–30 min at 37°C, centrifuged, and resuspended in a final volume of 1.1 mL sterile saline. A 0.1 mL aliquot was sent for microbiological testing (including Gram stain and culturing for aerobes, anaerobes, and fungi) and immunofluorescence analysis after staining with DAPI, to eliminate the possibility of mycoplasma contamination. If the testing ruled out microbial contamination, the vaccine was transported on ice and injected intradermally. Each injection consisted of 0.25 mL of cell suspension, containing 15 × 106 irradiated tumor cells and 15 × 106 irradiated GM.CD40L bystander cells. Patients were monitored for acute toxicity for 1 hour after injection.
Immune Function Testing
At baseline and after each vaccination, 10 mL of blood was collected by phlebotomy. Peripheral blood mononuclear cells (PBMCs) were isolated using a density gradient protocol (Ficoll-PaqueTM PLUS, GE Healthcare Biosciences, PA), and frozen in liquid nitrogen. All samples from each patient were analyzed simultaneously to reduce inter-assay variability. MNC were thawed, incubated overnight in complete medium (RPMI-1640 supplemented with antibiotics and 10 % FBS), and then used in experiments. For ELISPOT assay, 2 × 105 PBMCs /well of AIM-V medium containing 10% human AB serum and 2ng/ml rhIL-2 were added to 96-well plates. HLA-A2-presented peptides derived from WT1, RMFPNAPYL28; CEA, YLSGANLNL29; and hTERT, ILAKFLHWL30 were added to the appropriate wells. If HLA-A2 negative, irradiated H1944 cells were used for peptide. After a 36 h restimulation culture, ELISPOT assays were performed with anti-γ-IFN (Serial # 552138 - BD bioscience, San Jose CA). Controls included PBMCs stimulated with an irrelevant peptide as a negative control and PBMCs stimulated with anti-CD3 as a positive control. The plates were cultured for 18 hours, washed, and the detection antibodies were added. Resultant spots were counted with Immunospot Series I Analyzer (Cellular Technology Ltd, Shaker Heights OH). A response was defined as positive if it met these criteria: 1) the mean number of spots present in the peptide wells was greater than 20 spots per 200,000 PBMC added, and 2) a greater than 3-fold increase over the standard deviation of the pre-treatment PBMC ELISPOT was observed.
Analysis of Cell Phenotype
Cell phenotype was evaluated by multicolor flow cytometry using a LSR II flow cytometer and monoclonal antibodies obtained from Becton–Dickinson. The following antibodies were used: CD4-Alexa 700; CD3-PE, CD14-PE, CD19-PE, CD56-PE (as lineage-PE); CD25-PE; CD127-Alexa 647; HLA-DR-APC; CD33-PE-Cy7. The following combinations of antibodies were used to identify cell populations: Lineage− (Lin) (CD3, 14, 19, 56) HL-DR+; Mature DC: Lin− HLA-DR+; MDSC: Lin− HLA-DR− CD33+; T-reg: CD4+CD25+CD127−. Dead cells were removed from the analysis by using DAPI staining.
For PD-L1 expression, NCI-H1944 and NCI-2122 cells were incubated in complete media with or without 50 U/mL interferon-γ (R&D Systems, Minneapolis) for 18 hours. The cells were analyzed by staining monoclonal anti-human CD274-FITC, clone MIH1 (BD Bioscience, New Jersey) in combination with corresponding isotype control, then imaged using FACScan (Becton Dickinson, Mountain View, California) and CellQuest software (Becton Dickinson).
Statistical Plan
This trial used a Simon minimax design31 based upon the primary endpoint of objective response rate, in which H0 = 15% and H1 = 30% with one-sided α = 0.05 and β = 0.20. The trial had 54% probability of stopping early at 23 patients if H0 was true, and 5% chance of stopping early if H1 was true. All patients exposed to study drug were included in the safety and survival analyses. All statistical tests were two-tailed unless otherwise specified. Mean ELISPOT responses were compared by Wilcoxon signed rank, or if two groups were present, Friedman with Dunn’s post-test to control for multiple comparisons.32 Best overall response was defined as best response recorded from first vaccine to progression or death. Progression free survival (PFS) was defined as date of treatment until date of progression or death, and overall survival (OS) defined from date of treatment until death. Survival curves were calculated according to the Kaplan-Meier method. Since no patients were censored, the effect of immune response on PFS and OS was evaluated using the Wilcoxon rank-sum test. Figures and survival plots were generated using GraphPad Prism® (version 5.04, San Diego CA).
RESULTS
Twenty-four participants were accrued at a single institution from October 2006 to June 2008, with data cut-off in January 2012. Participant demographics are presented in Table 1. Patients had a median age of 64 years (range 48 – 84) and had received a median of 4 previous lines of systemic therapy prior to entry. All were Caucasian. Twenty were former or active smokers, and 4 had brain metastases. Eleven patients were HLA-A2 positive.
Table 1. Demographics.
Participant Demographics and Baseline Measurements.
| Characteristics | Patients n (%) |
|---|---|
| Total | 24 |
| Age in years (Median) | 64.3 |
| Range | 48 – 84 |
| Race | |
| White | 24 (100) |
| Gender | |
| Female | 12 (50) |
| Male | 12 (50) |
| ECOG* performance status | |
| PS 0 | 8 (33) |
| PS 1 | 15 (63) |
| PS 2 | 1 (4) |
| Smoking status | |
| Smoker | 3 (12) |
| Former smoker | 17 (71) |
| Non smoker | 4 (17) |
| AJCC ‡ clinical stage | |
| IIIB | 1 (4) |
| IV | 23 (96) |
| Histotype of NSCLC | |
| Adenocarcinoma | 24 (100) |
| Previous treatments | |
| Surgical resection | 10 (42) |
| Radiation | 13 (54) |
| Chemotherapy | 22 (92) |
| Tyrosine kinase inhibitor | 9 (38) |
| Metastatic sites | |
| Visceral | 17 (71) |
| Bone | 7 (29) |
| Brain | 4 (17) |
Eastern Cooperative Oncology Group.
American Joint Committee on Cancer
Safety
A total of 101 vaccines were administered. One grade 3 diarrhea, one grade 3 nausea, one grade 3 abdomen pain, and three grade 3 headache were observed related to study medication. Adverse events are presented in Table 2. The most common treatment-related adverse events included injection site reaction (38%), fatigue (21%), and headache (54%). All adverse effects were manageable and without significant clinical sequela. No auto-immune adverse events such as endocrine dysfunction or vitiligo were observed. No participants stopped therapy due to adverse events or elective discontinuation. Ten patients (42%) completed the initial four month course of vaccine therapy and continued vaccination, with three remaining on treatment at one year.
Table 2. Therapy-related adverse events.
Therapy-related adverse events
| Category | Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total | % |
|---|---|---|---|---|---|---|---|
| Constitutional | Fatigue | 4 | 1 | 5 | 21% | ||
| Dizzyness | 1 | 1 | 4% | ||||
| Anorexia | 1 | 1 | 4% | ||||
| Chills | 1 | 1 | 4% | ||||
| Hematologic | Leukopenia | 1 | 1 | 4% | |||
| Low Platelet | 1 | 1 | 4% | ||||
| Dermatologic | Site Reaction | 9 | 9 | 38% | |||
| Dry skin | 2 | 2 | 8% | ||||
| Edema: limb | 1 | 1 | 4% | ||||
| GI | Diarrhea | 3 | 1 | 4 | 17% | ||
| Nausea | 4 | 2 | 1 | 7 | 29% | ||
| ALT elevation | 1 | 1 | 4% | ||||
| Pain | Headache | 7 | 3 | 3 | 13 | 54% | |
| Joint pain | 1 | 1 | 4% | ||||
| Abdomen pain | 1 | 1 | 4% | ||||
| Injection pain | 1 | 1 | 4% |
Includes all definite, probable, and possible attributions. Common toxicity criteria (CTC) version 3.0. GI: gastroenterological. ALT: alanine aminotransferase.
Survival
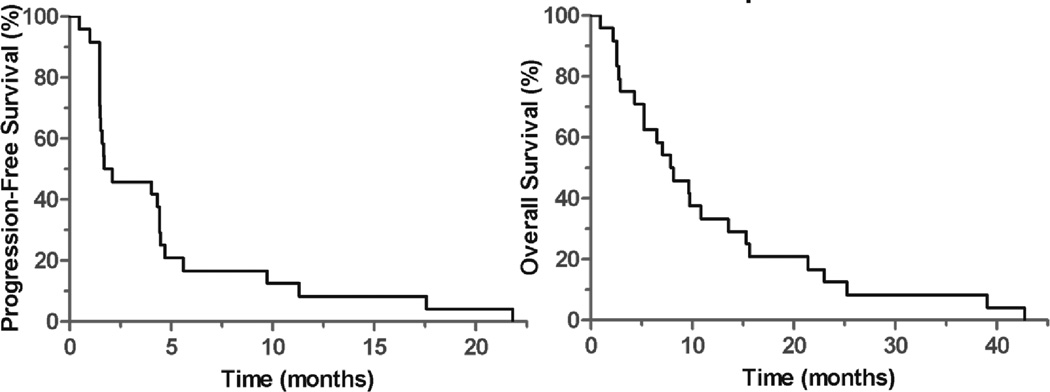
After at least three vaccines, ten (42%) of patients had stable disease by RECIST criteria. No confirmed radiologic responses were observed. Therefore, the trial met predefined stopping criteria and closed to accrual. Median progression-free survival was 1.7 months (mo) [95%CI NE – 4.6 mo], and median overall survival was 7.9 mo [95%CI 4.1 – 11.6 mo] (Figure 1). One-year survival was 33% and 3-year survival was 8%. Four patients expired from cardiac disease, four from obstructive lung disease, and the remainder died from progressive lung cancer. For unclear reasons, the eleven HLA-A2 positive participants appeared to have improved PFS compared to the thirteen HLA-A2 negative (median PFS 4.4 vs. 1.5 mo, p = 0.02) and trend towards improved OS (median OS 14.0 vs 5.0 mo, p = 0.06).
Figure 1. Progression-free and overall survival of trial participants.
Median OS = 7.9 mo [95%CI 4.1 – 11.6 mo], median PFS = 1.7 mo [95%CI 4.1 – 11.6 mo]. n = 24, no censored events.
Immune Response
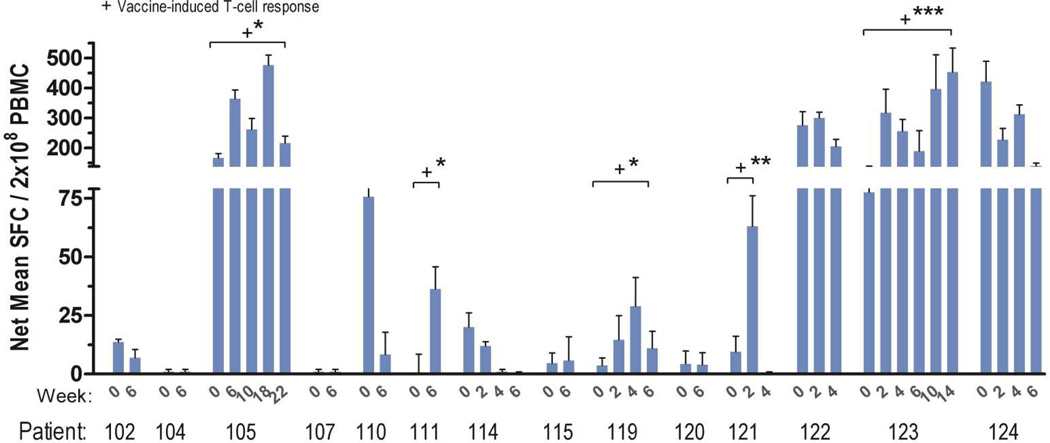
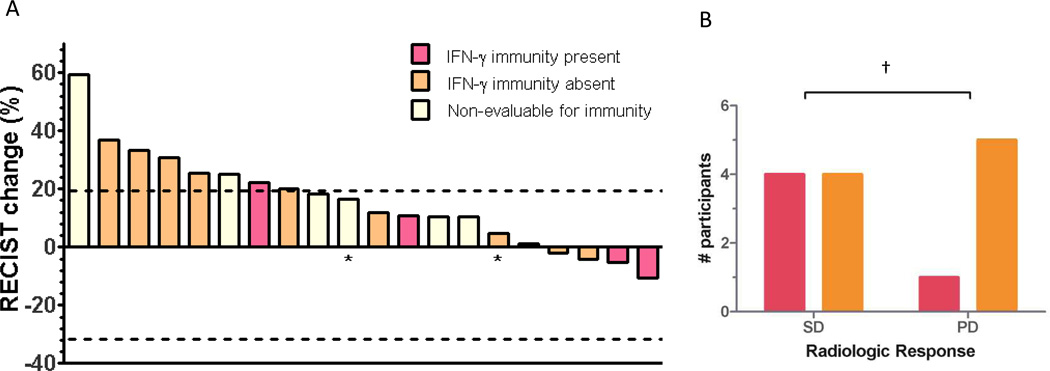
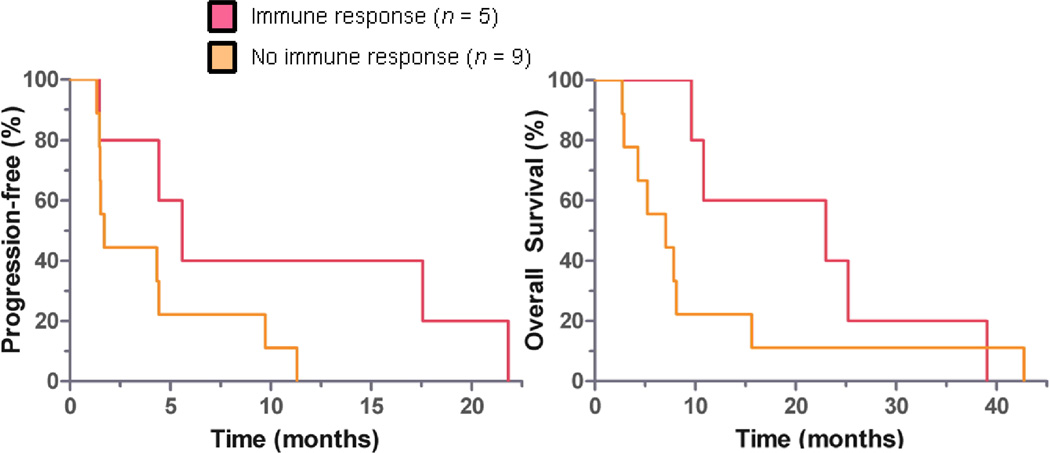
Samples for evaluation of vaccine-induced immunity were available from 14 patients. Patients who were HLA-A2 positive were tested for a response to three peptides (CEA, TERT2, and WT-1) as a sentinel measure of vaccine activity. Similarly, patients who were HLA-A2 negative were tested for response to H1944 cell lysate. Responses ranged between 40 and 700 spots/2 × 105 PBMCs, presented in Figure 2. Overall, 36% of evaluable patients met the pre-specified definition for immune response. All immune responses were statistically significant compared to baseline with p < 0.05. An association of immune response with risk of progression was not statistically significant (HR = 0.33 and p = 0.10, one-tailed χ2), as presented in Figure 3B. Compared to non-responders, immune responders appeared to have longer median OS (23.0 vs 7.1 mo, p = 0.04) and a similar but non-statistically significant median PFS (5.6 vs. 1.7 mo, p = 0.18), as presented in Figure 4. However, immune responders also appeared to have more favorable baseline prognostic features, such as less grade 3 cancer (p = 0.02) and trend toward more metastatic disease limited to the lungs alone (p=0.08), as presented in Table 3. No statistically significant association between HLA A2 and immune response was observed, although this was limited by small sample size (RR 1.8, 95% CI 0.79 – 4.2, p = 0.30).
Figure 2. Patient IFN-γ immune responses. Mean CEA / hTERT / WT-1, or H1944 lysate if A2- (n = 14).
Shown are enzyme-linked immunosorbent spot assay responses from a direct assay, comparing pre-vaccination and weekly time points after vaccination. Bars = mean ± SD. + indicates responses that met pre-defined immune response criteria.
* p < 0.05, ** p <0.001, *** p < 0.0001 (Wilcoxon signed rank, or Friedman with Dunn's post-test if more than two comparators). SFC: spot-forming cells. PBMC: Peripheral blood mononuclear cells.
Figure 3.
A. Waterfall plot of best overall radiologic response to vaccine. Immunity assessed by IFN-γ ELISPOT. Of 24 total, 3 were non-evaluable for RECIST due to lung cancer death (n = 2) or missing evaluation (n =1). * PD due to unequivocal progression of malignant pleural effusion.
B. Participants who met pre-specified immune response criteria had non-statistically significant trend towards more favorable radiologic response. SD: Stable disease, PD: Progressive disease. RECIST v 1.0. † p = 0.10 (1-tailed χ2).
Figure 4. Participants who met pre-specified IFN-γ immune response criteria appeared to have improved overall survival.
Median OS 23.0 vs. 7.1 months ◦ p = 0.04. Median PFS 5.6 vs. 1.7 months, ◦ p = 0.18. Immune response determined by IFN-γ ELISPOT assay. ◦ Wilcoxon rank-sum test.
Table 3. Baseline factors related to immune response.
Baseline factors related to immune response
| Characteristic | p value | |||
|---|---|---|---|---|
| Immune category | Total | + | − | + vs all others |
| n (%) | 24 (100) | 5 (26) | 9 (38) | 5 vs. 19 |
| Weight, in kg (mean ± SD) | 69.7 ± 14.0 | 61.6 ± 12.5 | 74.6 ± 13.8 | 0.14§ |
| BMI (mean ± SD) | 25.6 ± 4.6 | 25.1 ± 4.0 | 26.8 ± 5.8 | 0.85 § |
| Albumin, g/dL (mean ± SD) | 3.91 ± 0.43 | 3.90 ± 0.47 | 4.08 ± 0.41 | 0.76 § |
| Poorly differentiated (n,%) | 7 (30) | 0 (0) | 2 (22) | 0.03† |
| Prior bevacimab (n, %) | 6 (25) | 1 (20) | 1 (11) | 0.99† |
| Lung-only mets (n,%) | 11 (46) | 4 (80) | 5 (56) | 0.08† |
‘Lung-only mets’ denotes stage IV disease with only contralateral lung metastases. “+” = IFN-γ immunity observed, “−“ = not observed. 13 patients were not evaluable for immunity.
= Wilcoxon rank-sum
= χ2test.
Peripheral Mononuclear Cells
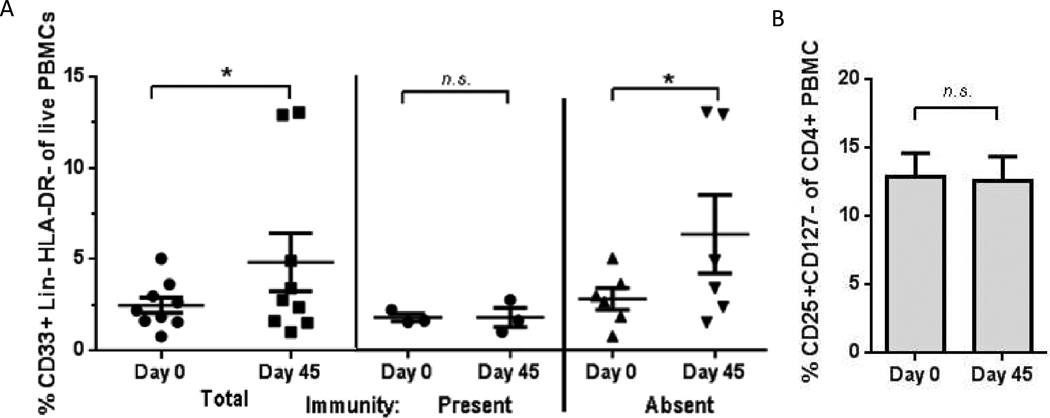
Of the patients evaluable, peripheral blood lymphocytes decreased by Day 45 (p = 0.04) and Day 88 (p=0.02) compared to baseline, as presented in Table 4. However, no significant difference in absolute blood lymphocytes between immune responders and non-responders was observed. An increase in Lin− HLA-DR− CD33+ MDSCs was observed at Day 45 compared to Day 0 (p = 0.04), which appeared to be more pronounced in non-responders (p = 0.03), as seen in Figure 5A. No change in Lin−HLA-DR+ mature dendritic cells was observed at Day 45 compared to Day 0, with mean % total live PBMCs of 6.7 vs. 6.2; p = 0.82. These findings were limited by small sample size due to only nine evaluable paired samples, with the remainder of patients non-evaluable due to off-study, deceased, or poor sample viability. Despite a bolus of cyclophosphamide at Day 0, no statistically significant change in peripheral blood CD4+CD25+CD127− T-regulatory cells was observed at Day 45, as shown in Figure 5B, although this finding was similarly limited by small sample size.
Table 4. Effect upon peripheral blood count.
Effect upon peripheral blood count.
| Time | Day 0 | Day 45 | Day 88 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Immune category | Total | + | − | Total | + | − | Total | + | − |
| n (%) | 24 (100) | 5 (26) | 9 (38) | 22 (100) | 5 (23) | 9 (41) | 15 (100) | 5 (33) | 6 (40) |
| WBC (×106/ml) | 7.1 ± 1.8 | 5.6 ± 0.9 | 7.1 ± 1.5 | 7.4 ± 2.0 | 6.3 ± 1.8 | 6.5 ± 1.2 | 9.4 ± 4.5 | 5.8 ± 1.0 | 10.6 ± 5.0 |
| Neutrophils (×106/ml) | 4.7 ± 1.4 | 3.6 ± 1.0 | 4.8 ± 0.9 | 5.3 ± 1.6* | 4.2 ± 1.4 | 4.9 ± 1.3 | 7.1 ± 4.3* | 4.1 ± 0.6 | 8.1 ± 4.6 |
| Lymphocytes (×106/ml) | 1.5 ± 0.8 | 1.4 ± 0.7 | 1.5 ± 0.6 | 1.4 ± 0.7* | 1.4 ± 0.6 | 1.2 ± 0.7 | 1.3 ± 0.6* | 1.1 ± 0.4 | 1.4 ± 0.4 |
| Monocytes (×106/ml) | 0.6 ± 0.2 | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.6 ± 0.3 | 0.5 ± 0.1 | 0.5 ± 0.2 | 0.6 ± 0.3 | 0.5 ± 0.1 | 0.7 ± 0.3 |
Mean absolute cell count ± standard deviation are shown. “ +” = IFN-γ immune response observed by Day 88, “−“ = immune response not observed. 13 patients were not evaluable for immunity. ‘Day 0’ is day of cytoxan pretreatment. Day 45 and 88 are ±3 days.
Asterisks indicate p < 0.05, with each timepoint compared to pretreatment level by Wilcoxon signed rank.
No significant differences were observed between “+’ and “−“ groups by Wilcoxon rank-sum
Figure 5.
A. MDSCs increased from baseline (day 0) to next consecutive evaluation (day 45). MDSCs may have contributed to failure to achieve an IFN-γ response to vaccine antigen. Fraction CD33+ of Lin-DR- were analyzed by flow cytometry. Remaining patients were not evaluable due to poor cell viability, sample not collected, or death.
Bars: mean ± standard error. * p < 0.05, Wilcoxon signed rank test. n.s. = not significant. For pairing, Spearman r2 = 0.94.
B. Peripheral T-regulatory cells did not appreciably decrease from baseline (day 0) to next consecutive evaluation (day 45), despite single bolus of intravenous cyclophosphamide. n = 8. Fraction CD25+CD127- of CD4+ were analyzed by flow cytometry. Remaining patients were not evaluable due to poor cell viability, sample not collected, or death.
Bars: mean ± standard error. n.s. = not significant, Wilcoxon signed rank test. For pairing, Spearman r2 = 1.0.
Tumor-associated Vaccine Antigen
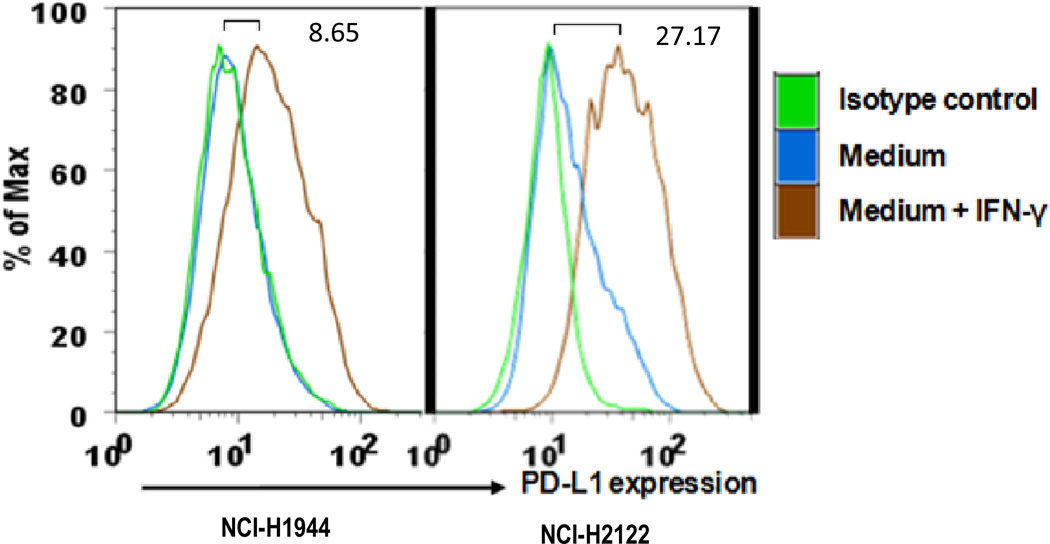
To help identify why the allogeneic vaccine may have been unsuccessful, we tested the cell lines used as TAA for cell surface PD-L1 expression. As shown in Figure 6, no constitutive PD-L1 expression was observed in either H1944 or H2122. However, expression was inducible after incubation with IFN-γ, in accordance with reports in other lung cancer cell lines.33,34
Figure 6. FACS analysis of cell-surface PD-L1 expression in the two human adenocarcinoma cell lines used as tumor antigen.
Cell lines were incubated for 18 hours with or without 50 U/mL IFN-γ and stained with monoclonal antibody against human B7-H1. Numbers on the bar indicate the mean fluorescent intensity of IFN-γ incubated cells as compared with media-incubated cells alone. Positive staining is indicated by the blue or brown lines and control antibody (mouse IgG1) staining is shown as green lines.
DISCUSSION
This GM-CSF-producing and CD40L-expressing bystander cell line combined with an allogeneic (adenocarcinoma) tumor cell-based vaccine appeared to be safe, despite an extensively pretreated study population. However, the trial failed to meet its primary endpoint. Even in participants with an immune response to vaccination, no radiologic responses were observed. These findings are not dissimilar to previous peptide or whole-cell lysate vaccine trials, in which successful immunogenicity only rarely translated into tumor regression.35,36 Several mechanisms may have contributed to the historical failure of this vaccine strategy: inappropriate clinical response endpoints37, presence of tumor-induced immune suppression38, and application to populations suffering from extensive disease or impaired host immunity.39 In this regard, the current success of checkpoint inhibitors in overcoming immune suppression is of particular interest because it presents an attractive companion for future immunotherapy strategies in general, and for tumor cell-based vaccines specifically.40 In particular, combination of GM-CSF-secreting vaccines with CTLA-4 inhibitors has shown some promise.41,42 Furthermore, optimal timing and context of vaccination may also be particularly relevant in contributing to the failure of this trial. For example, a GM-CSF bystander vaccine, GVAX ®, appeared effective only if given after chemotherapy in an autologous mice model of lung cancer.43
Our findings are characterized by important limitations. First, this study had a small sample size, and only a proportion were evaluable for immune correlates. Next, we used IFN-γ release as an indirect surrogate for host immune response to sentinel epitopes within the whole cell lysate. This method has established limitations in reproducibility and specificity,44and also does not confirm that effective cross-priming occurred.45 Next, the likelihood of confounding due to nutritional status, organ function, or subsequent medications upon immune response was not addressed.46 This is because the sample size was too small to permit the use of a multivariable regression model.47 These co-morbid factors may confound any association between host immunity and survival, and would be expected to result in bias away from the null. As shown in Table 3, the immune responders did have more favorable tumor grade and extent of metastatic disease, so it is plausible that this explains the difference in survival observed. Therefore, conclusions about the relationship between immune response and survival should be regarded as exploratory. Moreover, the relationship between IFN-γ immune response and other important correlates of T-cell immunity, such as delayed hypersensitivity response (DTH)48 and presence of TGF-β-producing FOXP3+ regulatory T-cells49 was not examined. In addition, measurement of T-cells in the peripheral blood may not directly correlate with the activity of T-cells at the site of the tumor.50 Thus, testing of pre- and post-treatment tumor biopsies for the density of either CD 8 + or memory T-cell infiltration would have provided corroboration for the serologic findings in this trial.
The significance of improved PFS in HLA-A2 patients is unclear. Neither the K562 bystander nor allogeneic tumor cell lines express this haplotype.22 Since the allogeneic cells were lysed and irradiated, it is unlikely their MHC Class I components would have impacted the presentation of their derived proteins., and the bystander K562 cell line does not express any MHC Class I components.51 It is conceivable that HLA-A2 performed better due to random chance, or unknown confounders unrelated to immunity.
Based upon a limited subset of patients, no durable change in the number of T-regs was observed, which suggests that single-bolus cyclophosphamide may be suboptimal. Despite a report that cyclophosphamide pretreatment improves G-CSF vaccine-induced immunity52, bolus cyclophosphamide has since been shown to be inefficient in reducing T-regs.26 In contrast, metronomic dosing appears to be more effective53, perhaps mediated through ATP.54 Since the peripheral blood MDSCs did not decrease during treatment, the utility of the ATRA treatment in this trial also remains uncertain. However, we have previously demonstrated that ATRA reduces immature myeloid derived cells in patients receiving IL-2 for metastatic renal cell55, as well as small cell lung cancer56 and the latter trial is ongoing (NCT00617409).57
Some lung cancer cell lines can be induced to express cell surface PD-L1, while others have constitutive PD-L1 expression.33 Since the cell lines used as TAA in this trial were lysed and irradiated prior to injection, it is unclear if PD-L1 was present in sufficient quantity at the local vaccination site to interfere with effector T-cell activity. Despite the negative clinical result, the immune responses observed in this trial suggest that this vaccine strategy may still hold potential with further improvement. Along these lines, the expression of the chemokine C-C motif ligand (CCL21) has been shown to augment anti-tumor immune responses and has been explored as an adjuvant in several vaccines.58,59 This evidence has led us to design another clinical trial, incorporating GM.CD40L, in combination with a cell line engineered to express exogenous CCL21, in metastatic NSCLC (NCT01433172).60 This randomized phase II trial is currently underway and is expected to be completed in late 2013.61
ACKNOWLEDGEMENTS
This work was supported in part by P30CA076292 and P50-CA119997 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURE STATEMENT:
The University of South Florida holds a patent for GM.CD40L and CCL21 with one author (S.A.), application: 12/933,568, publication: US 2011/0059137, Filing date: Mar 19, 2009.
The authors have no other conflicts of interest to disclose.
HUMAN SUBJECTS STATEMENT:
The procedures within were approved by the Institution Review Board (IRB) approval from the University of South Florida (FWA00001669), in accordance with the Helsinki Declaration of 1975.
REFERENCES
- 1.Janssen-Heijnen MLG, Schipper RM, Razenberg P, et al. Prevalence of co-morbidity in lung cancer patients and its relationship with treatment: a population-based study. Lung Cancer. 1998;21:105–113. doi: 10.1016/s0169-5002(98)00039-7. [DOI] [PubMed] [Google Scholar]
- 2.Brunsvig PF, Kyte JA, Kersten C, et al. Telomerase peptide vaccination in NSCLC: a phase II trial in stage III patients vaccinated after chemoradiotherapy and an 8-year update on a phase I/II trial. Clin. Cancer Res. 2011;17:6847–6857. doi: 10.1158/1078-0432.CCR-11-1385. [DOI] [PubMed] [Google Scholar]
- 3.Dalyot-Herman N, Bathe OF, Malek TR. Reversal of CD8+ T cell ignorance and induction of anti-tumor immunity by peptide-pulsed APC. J. Immunol. 2000;165:6731–6737. doi: 10.4049/jimmunol.165.12.6731. [DOI] [PubMed] [Google Scholar]
- 4.Boczkowski D, Nair SK, Snyder D, et al. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J. Exp. Med. 1996;184:465–472. doi: 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiodoni C, Paglia P, Stoppacciaro A, et al. Dendritic cells infiltrating tumors cotransduced with granulocyte/macrophage colony-stimulating factor (GM-CSF) and CD40 ligand genes take up and present endogenous tumor-associated antigens, and prime naive mice for a cytotoxic T lymphocyte response. J. Exp. Med. 1999;190:125–134. doi: 10.1084/jem.190.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. The Journal of experimental medicine. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talmadge JE. Pathways mediating the expansion and immunosuppressive activity of myeloid-derived suppressor cells and their relevance to cancer therapy. Clinical Cancer Research. 2007;13:5243–5248. doi: 10.1158/1078-0432.CCR-07-0182. [DOI] [PubMed] [Google Scholar]
- 8.Salgia R, Lynch T, Skarin A, et al. Vaccination with irradiated autologous tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor augments antitumor immunity in some patients with metastatic non–small-cell lung carcinoma. J. Clin. Oncol. 2003;21:624–630. doi: 10.1200/JCO.2003.03.091. [DOI] [PubMed] [Google Scholar]
- 9.Nemunaitis J, Vorhies J, Pappen B, et al. 10-year follow-up of gene-modified adenoviral-based therapy in 146 non-small-cell lung cancer patients. Cancer Gene Ther. 2007;14:762–763. doi: 10.1038/sj.cgt.7701048. [DOI] [PubMed] [Google Scholar]
- 10.Nemunaitis J, Sterman D, Jablons D, et al. Granulocyte–macrophage colony-stimulating factor gene-modified autologous tumor vaccines in non–small-cell lung cancer. J. Natl. Cancer Inst. 2004;96:326–331. doi: 10.1093/jnci/djh028. [DOI] [PubMed] [Google Scholar]
- 11.Borrello I, Sotomayor EM, Cooke S, et al. A universal granulocyte-macrophage colony-stimulating factor-producing bystander cell line for use in the formulation of autologous tumor cell-based vaccines. Hum. Gene Ther. 1999;10:1983–1991. doi: 10.1089/10430349950017347. [DOI] [PubMed] [Google Scholar]
- 12.Nemunaitis J, Jahan T, Ross H, et al. Phase 1/2 trial of autologous tumor mixed with an allogeneic GVAX® vaccine in advanced-stage non-small-cell lung cancer. Cancer Gene Ther. 2006;13:555–562. doi: 10.1038/sj.cgt.7700922. [DOI] [PubMed] [Google Scholar]
- 13.Morales JK, Kmieciak M, Knutson KL, et al. GM-CSF is one of the main breast tumor-derived soluble factors involved in the differentiation of CD11b-Gr1-bone marrow progenitor cells into myeloid-derived suppressor cells. Breast cancer research and treatment. 2010;123:39–49. doi: 10.1007/s10549-009-0622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serafini P, Carbley R, Noonan KA, et al. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 2004;64:6337. doi: 10.1158/0008-5472.CAN-04-0757. [DOI] [PubMed] [Google Scholar]
- 15.Morse MA, Hobeika AC, Osada T, et al. Depletion of human regulatory T cells specifically enhances antigen-specific immune responses to cancer vaccines. Blood. 2008;112:610–618. doi: 10.1182/blood-2008-01-135319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicholaou T, Ebert LM, Davis ID, et al. Regulatory T-Cell–Mediated Attenuation of T-Cell Responses to the NY-ESO-1 ISCOMATRIX Vaccine in Patients with Advanced Malignant Melanoma. Clinical Cancer Research. 2009;15:2166–2173. doi: 10.1158/1078-0432.CCR-08-2484. [DOI] [PubMed] [Google Scholar]
- 17.Chapman PB. Vaccinating patients with autologous tumor. J. Clin. Oncol. 2002;20:4139–4140. doi: 10.1200/JCO.2002.20.20.4139. [DOI] [PubMed] [Google Scholar]
- 18.Antonia SJ, Mule JJ. Chemokine Gene-modified Cells for Cancer Immunotherapy: Google Patents. 2009 [Google Scholar]
- 19.Cella M, Scheidegger D, Palmer-Lehmann K, et al. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: TT help via APC activation. The Journal of experimental medicine. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dessureault S, Alsarraj M, McCarthy S, et al. A GM-CSF/CD40L producing cell augments anti-tumor T cell responses. Journal of Surgical Research. 2005;125:173–181. doi: 10.1016/j.jss.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 21.Dessureault S, Noyes D, Lee D, et al. A phase-I trial using a universal GM-CSF-producing and CD40L-expressing bystander cell line (GM. CD40L) in the formulation of autologous tumor cell-based vaccines for cancer patients with stage IV disease. Ann. Surg. Oncol. 2007;14:869–884. doi: 10.1245/s10434-006-9196-4. [DOI] [PubMed] [Google Scholar]
- 22.Wroblewski JM, Bixby DL, Borowski C, et al. Characterization of human non-small cell lung cancer (NSCLC) cell lines for expression of MHC, co-stimulatory molecules and tumor-associated antigens. Lung Cancer. 2001;33:181–194. doi: 10.1016/s0169-5002(01)00210-0. [DOI] [PubMed] [Google Scholar]
- 23.Michael A, Ball G, Quatan N, et al. Delayed disease progression after allogeneic cell vaccination in hormone-resistant prostate cancer and correlation with immunologic variables. Clin. Cancer Res. 2005;11:4469–4478. doi: 10.1158/1078-0432.CCR-04-2337. [DOI] [PubMed] [Google Scholar]
- 24.Bercovici N, Haicheur N, Massicard S, et al. Analysis and characterization of antitumor T-cell response after administration of dendritic cells loaded with allogeneic tumor lysate to metastatic melanoma patients. J. Immunother. 2008;31:101. doi: 10.1097/CJI.0b013e318159f5ba. [DOI] [PubMed] [Google Scholar]
- 25.Kusmartsev S, Cheng F, Yu B, et al. All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res. 2003;63:4441–4449. [PubMed] [Google Scholar]
- 26.Audia S, Nicolas A, Cathelin D, et al. Increase of CD4+ CD25+ regulatory T cells in the peripheral blood of patients with metastatic carcinoma: a Phase I clinical trial using cyclophosphamide and immunotherapy to eliminate CD4+ CD25+ T lymphocytes. Clin. Exp. Immunol. 2007;150:523–530. doi: 10.1111/j.1365-2249.2007.03521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phelps RM, Johnson BE, Ihde DC, et al. NCI-navy medical oncology branch cell line data base. J. Cell. Biochem. 1996;63:32–91. doi: 10.1002/jcb.240630505. [DOI] [PubMed] [Google Scholar]
- 28.Savage P, Gao L, Vento K, et al. Use of B cell-bound HLA-A2 class I monomers to generate high-avidity, allo-restricted CTLs against the leukemia-associated protein Wilms tumor antigen. Blood. 2004;103:4613–4615. doi: 10.1182/blood-2003-11-3903. [DOI] [PubMed] [Google Scholar]
- 29.Nagorsen D, Scheibenbogen C, Schaller G, et al. Differences in T-cell immunity toward tumor-associated antigens in colorectal cancer and breast cancer patients. Int J Cancer. 2003;105:221–225. doi: 10.1002/ijc.11052. [DOI] [PubMed] [Google Scholar]
- 30.Minev B, Hipp J, Firat H, et al. Cytotoxic T cell immunity against telomerase reverse transcriptase in humans. Proc Natl Acad Sci U S A. 2000;97:4796–4801. doi: 10.1073/pnas.070560797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simon R. Optimal two-stage designs for phase II clinical trials. Control. Clin. Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 32.Dunn OJ. Multiple comparisons among means. Journal of the American Statistical Association. 1961;56:52–64. [Google Scholar]
- 33.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 34.Lee S-J, Jang B-C, Lee S-W, et al. Interferon regulatory factor-1 is prerequisite to the constitutive expression and IFN-γ-induced upregulation of B7-H1 (CD274) FEBS Lett. 2006;580:755–762. doi: 10.1016/j.febslet.2005.12.093. [DOI] [PubMed] [Google Scholar]
- 35.Barve M, Bender J, Senzer N, et al. Induction of Immune Responses and Clinical Efficacy in a Phase II Trial of IDM-2101, a 10-Epitope Cytotoxic T-Lymphocyte Vaccine, in Metastatic Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2008;26:4418–4425. doi: 10.1200/JCO.2008.16.6462. [DOI] [PubMed] [Google Scholar]
- 36.Kotsakis A, Vetsika EK, Christou S, et al. Clinical outcome of patients with various advanced cancer types vaccinated with an optimized cryptic human telomerase reverse transcriptase (TERT) peptide: results of an expanded phase II study. Ann. Oncol. 2012;23:442–449. doi: 10.1093/annonc/mdr396. [DOI] [PubMed] [Google Scholar]
- 37.Tuma RS. New response criteria proposed for immunotherapies. J. Natl. Cancer Inst. 2008;100:1280–1281. doi: 10.1093/jnci/djn334. [DOI] [PubMed] [Google Scholar]
- 38.Hsieh CL, Chen DS, Hwang LH. Tumor-induced immunosuppression: a barrier to immunotherapy of large tumors by cytokine-secreting tumor vaccine. Hum. Gene Ther. 2000;11:681–692. doi: 10.1089/10430340050015581. [DOI] [PubMed] [Google Scholar]
- 39.Krug LM, Dao T, Brown AB, et al. WT1 peptide vaccinations induce CD4 and CD8 T cell immune responses in patients with mesothelioma and non-small cell lung cancer. Cancer Immunol. Immunother. 2010;59:1467–1479. doi: 10.1007/s00262-010-0871-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N. J. Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van den Eertwegh AJM, Versluis J, van den Berg HP, et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012 doi: 10.1016/S1470-2045(12)70007-4. [DOI] [PubMed] [Google Scholar]
- 42.Hodi FS, Butler M, Oble DA, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proceedings of the National Academy of Sciences. 2008;105:3005–3010. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chu Y, Wang LX, Yang G, et al. Efficacy of GM-CSF-producing tumor vaccine after docetaxel chemotherapy in mice bearing established Lewis lung carcinoma. J. Immunother. 2006;29:367. doi: 10.1097/01.cji.0000199198.43587.ba. [DOI] [PubMed] [Google Scholar]
- 44.Janetzki S, Panageas KS, Ben-Porat L, et al. Results and harmonization guidelines from two large-scale international Elispot proficiency panels conducted by the Cancer Vaccine Consortium (CVC/SVI) Cancer Immunol. Immunother. 2008;57:303–315. doi: 10.1007/s00262-007-0380-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Disis ML. Immunologic biomarkers as correlates of clinical response to cancer immunotherapy. Cancer Immunol. Immunother. 2011;60:433–442. doi: 10.1007/s00262-010-0960-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamza SA, Mousa SM, Taha SE, et al. Immune response of 23-valent pneumococcal polysaccharide vaccinated elderly and its relation to frailty indices, nutritional status, and serum zinc levels. Geriatrics & Gerontology International. 2012 doi: 10.1111/j.1447-0594.2011.00749.x. [DOI] [PubMed] [Google Scholar]
- 47.Peduzzi P, Concato J, Kemper E, et al. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996;49:1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 48.Disis ML, Schiffman K, Gooley TA, et al. Delayed-type hypersensitivity response is a predictor of peripheral blood T-cell immunity after HER-2/neu peptide immunization. Clin. Cancer Res. 2000;6:1347–1350. [PubMed] [Google Scholar]
- 49.López MN, Pereda C, Segal G, et al. Prolonged survival of dendritic cell–vaccinated melanoma patients correlates with tumor-specific delayed type IV hypersensitivity response and reduction of tumor growth factor β-expressing T cells. J. Clin. Oncol. 2009;27:945–952. doi: 10.1200/JCO.2008.18.0794. [DOI] [PubMed] [Google Scholar]
- 50.Di Rosa F, Pabst R. The bone marrow: a nest for migratory memory T cells. Trends Immunol. 2005;26:360–366. doi: 10.1016/j.it.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 51.Maziarz RT, Mentzer SJ, Burakoff SJ, et al. Distinct effects of interferon-γ and MHC class I surface antigen levels on resistance of the K562 tumor cell line to natural killer-mediated lysis. Cell. Immunol. 1990;130:329–338. doi: 10.1016/0008-8749(90)90276-w. [DOI] [PubMed] [Google Scholar]
- 52.Laheru D, Lutz E, Burke J, et al. Allogeneic Granulocyte Macrophage Colony-Stimulating Factor–Secreting Tumor Immunotherapy Alone or in Sequence with Cyclophosphamide for Metastatic Pancreatic Cancer: A Pilot Study of Safety, Feasibility, and Immune Activation. Clinical Cancer Research. 2008;14:1455–1463. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghiringhelli F, Menard C, Puig PE, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+ CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol. Immunother. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao J, Cao Y, Lei Z, et al. Selective depletion of CD4+ CD25+ Foxp3+ regulatory T cells by low-dose cyclophosphamide is explained by reduced intracellular ATP levels. Cancer Res. 2010;70:4850. doi: 10.1158/0008-5472.CAN-10-0283. [DOI] [PubMed] [Google Scholar]
- 55.Mirza N, Fishman M, Fricke I, et al. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 2006;66:9299–9307. doi: 10.1158/0008-5472.CAN-06-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iclozan C, Antonia S, Chiappori A, et al. Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer Immunol. Immunother. 2013:1–10. doi: 10.1007/s00262-013-1396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Galluzzi L, Senovilla L, Vacchelli E, et al. Trial Watch: Dendritic cell-based interventions for cancer therapy. OncoImmunology. 2012;1:0–1. doi: 10.4161/onci.21494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamano T, Kaneda Y, Hiramatsu S, et al. Immunity against breast cancer by TERT DNA vaccine primed with chemokine CCL21. Cancer Gene Ther. 2007;14:451–459. doi: 10.1038/sj.cgt.7701035. [DOI] [PubMed] [Google Scholar]
- 59.Nguyen-Hoai T, Baldenhofer G, Ahmed MSS, et al. CCL21 (SLC) improves tumor protection by a DNA vaccine in a Her2/neu mouse tumor model. Cancer Gene Ther. 2011;19:69–76. doi: 10.1038/cgt.2011.69. [DOI] [PubMed] [Google Scholar]
- 60.Vacchelli E, Galluzzi L, Eggermont A, et al. Trial Watch: Immunostimulatory cytokines. OncoImmunology. 2012;1:493–506. doi: 10.4161/onci.20459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hall RD, Gray JE, Chiappori AA. Beyond the Standard of Care: A Review of Novel Immunotherapy Trials for the Treatment of Lung Cancer. Cancer Control. 2013:20. doi: 10.1177/107327481302000105. [DOI] [PubMed] [Google Scholar]