Abstract
Despite an increase in direct-to-consumer (DTC) genetic testing, little is known about how variations in website content might alter consumer behavior. We evaluated the impact of risk information provision on women’s attitudes about DTC BRCA testing. We conducted a randomized experiment; women viewed a “mock” BRCA testing website without (control group: CG) or with information on the potential risks of DTC testing (RG; framed two ways: unattributed information [UR] and information presented by experts [ER]). 767 women participated; mean age was 37 years, mean education was 15 years, and 79% of subjects were white. Women in the RG had less positive beliefs about DTC testing (mean RG=23.8, CG=25.2; p=0.001), lower intentions to get tested (RG= 2.8, CG= 3.1; p=0.03), were more likely to prefer clinic-based testing (RG=5.1, CG=4.8; p=0.03) and to report that they had seen enough risk information (RG=5.3, CG= 4.7; p<0.001). UR and ER exposure produced similar effects. Effects did not differ for women with or without a personal/family history of breast/ovarian cancer. Exposing women to the potential risks of DTC BRCA testing altered their beliefs, preferences, and intentions. Risk messages appear to be salient to women irrespective of their chance of having a BRCA mutation.
Keywords: communication, direct-to-consumer, BRCA, informed consent, cancer
Introduction
Over the past decade there has been a dramatic rise in the availability of direct-to-consumer (DTC) genetic tests. The number of DTC genetic tests has more than doubled since 2003 and approximately 30 companies sell genetic tests online.[1–2] The prominence of DTC genetic testing is highlighted by the fact that the “retail DNA test” was Time Magazine’s invention of the year in 2008 and Oprah Winfrey used DTC genetic testing to publically explore her heredity.[3–4] A recent study demonstrated substantial consumer interest with 6% of Facebook users already using DTC genome testing and 64% interested in using DTC testing in the future.[5] Companies are also trying to reach broader consumer markets as evidenced by a recent push to sell personalized genomic tests in drugstores across the United States.[6–7]
Advocates of the DTC genetic testing industry argue that DTC testing may improve access to genetic tests, lead to greater awareness of genetic conditions, enhance privacy, and lower prices.[8–14] On the other hand, some experts have expressed concerns that DTC genetic testing is not well regulated, that consumers might misunderstand test results, that DTC testing may have insufficient counseling, and that tests with unproven clinical benefit will be used prematurely.[2, 8–10, 12–18] Many different types of DTC genetic tests are available to consumers; from tests that are part of standard medical care (e.g. BRCA I) to curiosity tests that are unlikely to influence health decision-making (such as gene variants for earwax characteristics).[19] The variation in the clinical utility of tests in the United States and Canada is, in part, due to the fact that laws that regulate the medical device industry do not clearly apply to DTC genetic tests.[10, 19–20] Genetic tests are not routinely evaluated by federal agencies before they are released on the market and there is limited oversight over the types of claims that genetic testing companies can make about their products.[4, 10, 21] The lack of regulation in the United States and Canada stands in stark contrast to other countries, such as Germany, that have banned public access to testing.[22]
The American Society of Clinical Oncology (ASCO), the American College of Medical Genetics (ACMG), The American Society of Human Genetics (ASHG), The American Medical Association (AMA), and the European Society of Human Genetics (ESHG) have all issued recommendations specifically related to DTC genetic testing.[21, 23–26] In their policy statements, ASCO and the ESHG have outlined concerns related to DTC genetic testing that have previously been expressed within the medical community including concerns regarding the adequacy of counseling and informed consent.[23, 26] The ESHG policy statement also proposed a requirement that all advertising and marketing claims related to DTC genetic testing should be unbiased and fair.[26]
Many of the issues related to DTC advertising and company claims have been magnified by a recent Government Accountability Office investigation that identified egregious examples of deceptive marketing of DTC genetic tests. [27–28] Because of concerns over online testing there have been efforts in a number of states to limit access to DTC genetic testing and the ASHG has called for legislation that would require DTC genetic testing companies to provide consumers with information on all of the possible risks associated with testing.[12, 21, 29] A recent report from the Institute of Medicine and the National Research Council stressed the importance of research that might provide insight relevant to the question of how the DTC industry can be regulated in a way that provides sufficient consumer protection without unduly stifling innovation.[30] There is an urgent need to understand how the inclusion of risk information on commercial websites might alter consumer behavior because the FDA and congress are actively debating how to regulate this rapidly growing industry.[7, 31–32]
In order to determine how potential regulation of website risk information might influence consumer behavior, we conducted a randomized information exposure experiment in the context of DTC BRCA testing. Our purpose was to investigate the impact of risk information exposure on women’s beliefs about and preferences for online BRCA testing and intentions to get BRCA testing. We studied risk information provision in the setting of BRCA mutation testing because it is a setting in which adequate informed consent is essential and post-test decision making is critically important. In this manuscript we present the complete analysis for a study of women with and without a personal or family history of breast or ovarian cancer.[33] This manuscript is an extension of our prior pilot work in three key ways: 1) with a sample of over 700 women we have more precision and confidence in the estimates of our effects; 2) we have more power to detect effects that are moderate or small in size and; 3) we have the ability to evaluate whether there are any differences in the effects of risk information exposure on our outcomes for women with or without a personal or family history of breast/ovarian cancer.
We hypothesized that women who were exposed to information about the potential risk of getting BRCA testing online would have more negative beliefs about DTC genetic testing, lower intentions to get BRCA testing, and that risk information presented by experts would be more persuasive than unattributed risk information. We also hypothesized that women with a personal or family history of breast/ovarian cancer might find risk messages in the context of online breast/ovarian cancer genetic testing to be more personally relevant and therefore more persuasive than they would be for women without a personal/family history of breast/ovarian cancer (therefore producing more negative beliefs about DTC testing, lower preferences for online testing, and lower intentions to get tested).Our final hypothesis is based on theories of persuasion in the psychology and health communication literature (primarily the Elaboration Likelihood Model) which posit that one of the most important determinants of message processing is the perceived self-relevance of the message.[34–36] As the personal relevance of a message is increased (by a message recipient’s ability to link the message to some aspect of him/her self or his/her experience), the more able the message recipient is to process and be persuaded by the message arguments.[36]
Materials and Methods
Sample and intervention
Women aged 18–80 were invited to participate in the study. Non-English speaking women, women who had undergone BRCA testing/counseling or women who could not provide informed consent were excluded. Subjects were recruited online through Craigslist and The University of Pennsylvania’s OncoLink Cancer Resource website; through print advertisements in local Philadelphia newspapers; and with flyers that were posted around The University of Pennsylvania. There were two versions of the recruitment material; versions recruiting women with and without a personal or family history of breast or ovarian cancer. The only difference between the two versions of the recruitment material related to the criteria for study eligibility. The study was approved by The University of Pennsylvania institutional review board and conducted from October 2006 through January 2008.
The study design has been reported previously.[33] The study consisted of three parts: a baseline telephone interview, exposure to an online stimulus (a “mock” website), and an online survey. Subjects first completed a baseline telephone interview in which potential covariate information was collected (e.g. awareness of and interest in BRCA testing, personal/family history of cancer). Subjects, stratified by personal/family history of breast/ovarian cancer, were randomized to one of three groups for viewing the “mock” website. The password protected “mock” website was hosted on the Annenberg School for Communication web server. Subjects could access the “mock” website from any computer with an internet connection. The “mock” website consisted of a modified commercial website for DTC BRCA testing. The “mock” website was stripped of names, phone numbers and other identifying information. The “mock” website contained 47 pages with information on topics such as BRCA genetics, the pros and cons of BRCA testing, testing indications, and endorsements. Subjects in the control group (CG) viewed the “mock” website without added risk information. Subjects in the risk information groups viewed the same mock website with added information about the potential risks of getting a BRCA test online. The risk domains included concern over comprehension of test results, privacy, the potential lack of emotional support, and concern about post-test decision making.[33] Subjects could freely navigate the site for up to 30 minutes. Following free navigation, the mock website displayed six core web pages, plus or minus the risk page, to ensure that all subjects had exposure to key concepts related to BRCA testing and test logistics. We chose a mock website for our experimental stimulus (rather than manipulate print materials on DTC genetic testing) because DTC BRCA testing is currently sold over the internet and because the regulation of online marketing claims related to DTC genetic testing is an area of intense debate in the FDA and Congress. After subjects viewed the mock website, they were directed to an online survey where they answered questions related our outcome measures.
Risk information was presented in two ways. Subjects in the unattributed risk information (UR) group viewed unattributed statements about the potential risks of online testing where as subjects in expert risk information (ER) group viewed statements on the potential risks of online testing as described by experts. Our cited experts included individuals with degrees in law, public health, medicine, and ethics as well as statements from the Food and Drug Administration (FDA), the Centers for Disease Control and Prevention (CDC), and the Federal Trade Commission (FTC). The content of the UR and ER web pages was similar. We manipulated source credibility as numerous studies have shown that sources high in credibility are often more persuasive than those without credible sources.[37]
Measures
Socio-demographic information was self-reported by subjects. The study outcome measures have been reported previously.[33] Because there was a paucity of work examining women’s attitudes toward online BRCA testing at the time of study design, we created five outcome measures for our study (not previously validated). All outcomes were measured on a scale that ranged from 1–7 except the belief index which ranged from 1–35. The midpoint of all 7 point scales represented a neutral response option (e.g. “neither likely or unlikely” and “no difference”). We inquired about intentions to get BRCA testing with the following question. “How likely is it that you will get a BRCA test in the next 12 months?” Response options for this question were on a 1–7 point scale from very unlikely to very likely. Elicitation of where subjects would prefer to have BRCA testing was asked by the following question: “If you were to get a BRCA test, are you more likely to get a test from an internet company or by going to a cancer genetics clinic?” Response options were on a 1–7 point scale from definitely internet company to definitely cancer genetics clinic. Trust in test results was asked by the following question: “I would trust my BRCA test results more if I was tested for a BRCA mutation through…”. Response options for this question were on a 1–7 point scale from a cancer genetics clinic to an internet company. We asked subjects if they believed that internet testing was wise with the following question: “ getting a BRCA test from an internet company in the next 12 months would be”… with response options on a 1–7 point scale from foolish to wise. We asked subjects if they felt that they had seen enough risk information with the following question: “The information that you viewed on the simulated website provided enough information about the possible risks that people might face if they decided to purchase BRCA testing from an internet company” Response options for this question were on a 1–7 point scale from strongly disagree to strongly agree. Beliefs about online BRCA testing were measured by 5 questions, randomly ordered, with response options on a 1–7 point scale from very strongly disagree to strongly agree. The five belief questions were prefaced with the instructions “Please tell me how much you agree or disagree with each statement about internet testing for a breast cancer gene mutation. Using an internet company to get a BRCA test would…” “provide me with enough information on the test and its advantages and disadvantages”, “be convenient”, “provide me with the counseling resources that I need to make health and lifestyle decisions”, “allow me to keep my privacy”, “cause me to be discriminated against for health or life insurance”. After reverse coding the discrimination item, we created a belief index by summing the 5 belief items together (alpha 0.67, inter-item covariance 0.77).
Statistical analysis
We used simple frequencies to describe subject characteristics. We used Pearson’s chi-square testing, t-tests, and one-way analysis of variance models (ANOVA) to examine the differences between groups. We examined the differences between the control group and the combined risk information group as well as between the UR and ER information groups. We examined the effect of subjects’ personal/family history of breast/ovarian cancer, controlling for exposure to risk information, in linear and logistic regression models. We incorporated interaction terms in our models to evaluate for effect modification. We estimated a probability of detecting a moderate (delta of 0.5) or large (delta of 0.75) effect between any two groups to be over 99% and our probability of detecting a small effect (delta of 0.25) to be approximately 58%–92% (depending on outcome). All hypothesis tests were two-tailed and used a significance of p=0.05. Analysis was conducted in STATA 10 (Stata Corp. College Station, Tex).
Results
Participant Characteristics
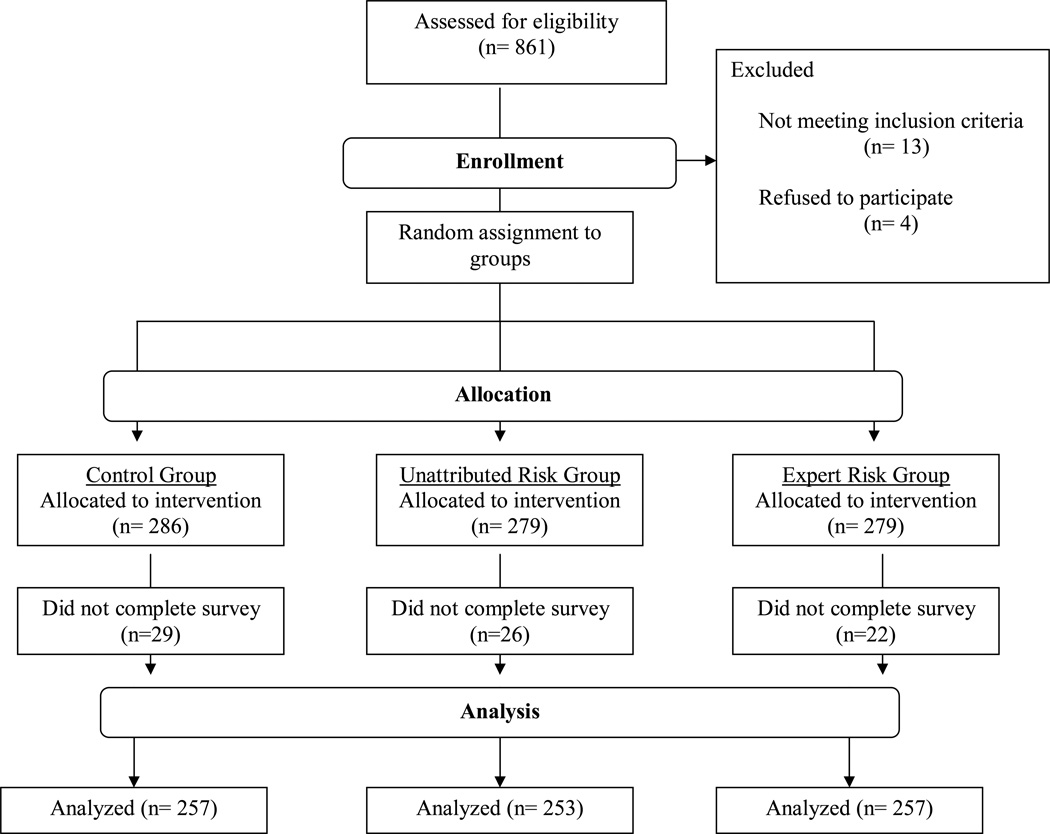
Eight hundred and sixty one women were assessed for eligibility and 844 women were randomized. Seven hundred and sixty seven subjects completed the study and are included in the analysis (Figure 1). Subject characteristics are shown in Table 1. Subjects were classified into subpopulations: those with and without a personal or family history of breast or ovarian cancer. We estimated the likelihood of carrying a BRCA gene mutation for subjects using the model on the Myriad Genetics website developed by Frank et al (“Frank score”; lowest value on the score calculator at the time of the study was 2.8%).[38–39] We used the Frank model because other models require significantly more information on personal and/or family history and would have decreased the feasibility of the study (e.g. the BRCAPRO model requires cancer/no-cancer information for all first and second degree family members and the Gail model requires pathologic data from breast biopsies). The calculated likelihood of carrying a gene mutation in our population ranged from 2.8% to 30%, with an average Frank score of 4.6%. The women without a personal/family history had an average Frank score of 3.5% and the women with a personal/family history had an average Frank score of 6.4%. Women with and without a personal/family history of breast/ovarian cancer were evenly distributed in all three of the experimental conditions (chi square value of 0.22 and a p value of 0.90). Randomization was adequate in the control, unattributed risk, and expert risk conditions with no significant differences in measured characteristics by group (Table 1).
Figure 1.
Table 1.
Participant Characteristics by Group
| Characteristic | Control Mean (n=257) |
Unattributed Risk Mean (n=253) |
Expert Risk Mean (n=257) |
Χ2-value (or f-value) |
p-value |
|---|---|---|---|---|---|
| Age, mean (SD) | 36.96 (11.83) | 36.92 (11.92) | 37.43 (11.84) | 1.01* | 0.7622 |
| Education, mean (SD) | 10.25 (2.4) | 10.31 (2.4) | 9.96 (2.5) | 0.82* | 0.6291 |
| Sex (% Women) | 100 | 100 | 100 | -- | -- |
| Ethnicity (%) | 2.44 | 0.97 | |||
| White | 79.53 | 77.38 | 80 | -- | -- |
| Black | 8.27 | 10.32 | 8.24 | -- | -- |
| Asian | 8.66 | 8.73 | 7.84 | -- | -- |
| Other | 3.54 | 3.57 | 3.92 | -- | -- |
| Hispanic | 4.74 | 4.76 | 2.35 | 2.57 | 0.28 |
| Employed Full-Time (%) | 53.54 | 55.95 | 50.39 | 1.58 | 0.45 |
| Married (%) | 63.39 | 64.82 | 57.54 | 3.1859 | 0.203 |
| Health Insurance (%) | 80.08 | 79.84 | 83.14 | 1.8645 | 0.761 |
| Works in Health Care (%) | 15.42 | 14 | 14.68 | 0.2009 | 0.904 |
| History of Cancer (%) | |||||
| Breast | 3.11 | 4.74 | 3.5 | 1.0141 | 0.602 |
| Ovarian | 0.39 | 0.4 | 0 | 1.0107 | 0.603 |
| Frank Score, mean (SD) | 0.047 (0.036) | 0.043 (0.03) | 0.046 (0.03) | 0.388 | 0.8236 |
| Interested in Testing at Baseline (%) | 6.4664 | 0.167 | |||
| No | 12.06 | 16.21 | 10.51 | -- | -- |
| Yes | 64.2 | 55.34 | 61.48 | -- | -- |
| Don’t Know | 23.74 | 28.46 | 28.02 | -- | -- |
f-value
Outcomes
Women who saw risk information (combined UR and ER groups) had lower intentions to get BRCA testing (17% vs. 24%, p=0.03), less positive beliefs about DTC BRCA testing (72% vs. 81%, p=0.01), and were more likely to report that they had seen enough information on the potential risks of internet based testing (73% vs. 61%, p<0.001) than women in the control group. The findings were confirmed in ANOVA models using continuous versions of our outcome variables (Table 2). In addition, the ANOVA models revealed that women who saw risk information were more likely to prefer clinic based testing (RG=5.1, CG=4.8; p=0.03) than women in the control group. We then explored the differences in outcomes for the control group, the UR group and the ER group (Table 3). There were no statistically significant differences between the UR and the BR risk information groups on any of the outcomes; however, there was a trend towards a difference in the effect of risk information exposure group on intentions to get a BRCA test. The women in the unattributed risk information group had lower intentions to get a BRCA test than the women in the expert risk information group (UR 2.63, ER 2.92; difference of 0.29, p=0.06)
Table 2.
Outcome Measures for Risk vs. No Risk for All Subjects
| Outcome | Control Mean (SD) |
Combined Risk Information Mean (SD) |
F-value | p-value |
|---|---|---|---|---|
| Intention to Get Tested | 3.07 (1.82) | 2.78 (1.73) | 4.64 | 0.032 |
| Testing Preference Site | 4.83 (1.69) | 5.11 (1.61) | 4.86 | 0.028 |
| Beliefs about Internet Testing | 25.18 (5.67) | 23.76 (5.37) | 11.26 | 0.001 |
| Trust in Internet Testing | 2.81 (1.32) | 2.65 (1.40) | 2.42 | 0.12 |
| Wisdom of Getting Internet Testing | 4.37 (1.38) | 4.22 (1.44) | 1.83 | 0.177 |
| Enough Risk Information | 4.71 (1.61) | 5.29 (1.44) | 4.99 | <0.001 |
Table 3.
Outcome Measures for All Subjects
| Outcome | Control Mean (SD) |
UR Mean (SD) |
ER Mean (SD) |
UR-ER Diff |
S.E. of Diff |
t-value of diff |
p-value |
|---|---|---|---|---|---|---|---|
| Intention to Get Tested | 3.07 (1.82) | 2.63 (1.71) | 2.92 (1.74) | −0.29 | 0.15 | −1.87 | 0.06 |
| Testing Preference Site | 4.83 (1.69) | 5.08 (1.66) | 5.14 (1.57) | −0.07 | 0.14 | −0.46 | 0.65 |
| Beliefs about Internet Testing | 25.18 (5.67) | 24.00 (5.37) | 23.53 (5.37) | 0.47 | 0.48 | 0.97 | 0.33 |
| Trust in Internet Testing | 2.81 (1.32) | 2.75 (1.38) | 2.54 (1.43) | 0.22 | 0.12 | 1.74 | 0.08 |
| Wisdom of Getting Internet Testing | 4.37 (1.38) | 4.20 (1.48) | 4.24 (1.39) | −0.04 | 0.13 | −0.31 | 0.76 |
| Enough Risk Information | 4.71 (1.61) | 5.34 (1.48) | 5.23 (1.48) | 0.11 | 0.13 | 0.84 | 0.40 |
UR= Unattributed Risk, ER=Expert Risk, S.D.= Standard Deviation, S.E.= Standard Error, Diff= Difference
Outcome by Subpopulation
Differences in the effects of exposure to risk information based on subpopulation (women with a personal/family history of breast or ovarian cancer as compared to women without a personal/family history) are displayed in Table 4. Overall, women with a personal/family history reported more negative beliefs about DTC testing and higher preferences for clinic-based testing than women without a personal/family history. There was no evidence for statistically significant effect modification by population. Linear regression analysis of the effect of population on outcomes showed similar results (analyses not shown).
Table 4.
Logistic regression; effects of personal/family history sub-population on outcomes
| Outcome Measure | Odds Ratio | 95% CI | p-value | |
|---|---|---|---|---|
| Intend to get BRCA Testing | ||||
| Population: women with a personal/family history of breast/ovarian cancers* | 1.27 | 0.87– 1.83 | 0.21 | |
| Exposure to Risk Information** | 0.65 | 0.45–0.95 | 0.02 | |
| Interaction*** | 0.21 | |||
| Prefer Clinic Testing | ||||
| Population: women with a personal/family history of breast/ovarian cancers* | 1.46 | 1.07– 1.99 | 0.02 | |
| Exposure to Risk Information** | 1.22 | 0.89–1.67 | 0.21 | |
| Interaction*** | 0.25 | |||
| Positive Attitudes about Internet Testing | ||||
| Population: women with a personal/family history of breast/ovarian cancers* | 0.71 | 0.50– 0.99 | 0.05 | |
| Exposure to Risk Information** | 0.63 | 0.44–0.92 | 0.02 | |
| Interaction*** | 0.31 | |||
| Trust Internet Testing | ||||
| Population: women with a personal/family history of breast/ovarian cancers* | 1.28 | 0.94– 1.73 | 0.12 | |
| Exposure to Risk Information** | 1.13 | 0.83–1.54 | 0.44 | |
| Interaction*** | 0.72 | |||
| Believe Internet Testing is Wise | ||||
| Population: women with a personal/family history of breast/ovarian cancers* | 1.08 | 0.80– 1.46 | 0.63 | |
| Exposure to Risk Information** | 0.84 | 0.62–1.15 | 0.28 | |
| Interaction*** | 0.46 | |||
| Site Provides Enough Risk Information | ||||
| Population: women with a personal/family history of breast/ovarian cancers* | 0.81 | 0.59– 1.12 | 0.20 | |
| Exposure to Risk Information** | 1.78 | 1.29–2.46 | <0.001 | |
| Interaction*** | 0.118 | |||
CI= 95% confidence interval, Info=information
control group is women without a personal/family history of breast/ovarian cancer.
control group is women who were not exposed to risk information.
interaction between sub-population (women with and without a personal/family history of breast/ovarian cancer) and exposure to risk information.
Discussion
Our study aimed to examine the impact of risk information provision on women’s beliefs about DTC BRCA testing and intentions to obtain BRCA testing. Similar to our previous report in a sample of women with a personal/family history of breast or ovarian cancer, our findings in this larger population that includes women without a personal/family history of breast/ovarian cancer show that women who are exposed to information about the potential risk of online BRCA testing have lower intentions to get BRCA testing and have more negative beliefs about DTC genetic testing.[33] Our findings are consistent with those of Sweeny and colleagues who demonstrated that people have lower intentions to get DTC genetic testing when they are exposed to information on the risks of DTC testing than when they are only exposed to information on the benefits of DTC genetic testing.[40]
In this larger population of women we also found a more robust effect on participants’ preferences for clinic based testing; women who were exposed to messages about the possible risks of online testing had higher preferences for clinic based testing. We previously identified this finding only in the group of women who saw information that was attributed to expert sources whereas in this larger study, we found that preferences for clinic based testing were significant for women who viewed both versions of the risk messages (expert risk and unattributed risk information). Because of our larger sample size, we are more confident that the effect of risk information exposure on participant’s preferences for clinic based testing is a true effect. While clinic-based testing is currently the standard of care in the U.S., there is a critical shortage of qualified genetic providers.[41–44] If risk information redirects consumers from online genetic testing to clinic-based testing, educators, providers and policy makers will have to consider how additional consumer demand will impact an already strained system.[33] In addition, this study revealed a new finding; that participants who were exposed to risk information were more likely to report that they had seen enough information on the possible risks of online testing. The fact that women have less positive beliefs about DTC genetic testing and that they are more likely to report having seen enough risk information suggests that regulations dictating the inclusion of risk information on DTC websites may increase awareness about the possible risks of online genetic testing; therefore potentially improving informed decision-making.
Contrary to our expectation, we found that the effects of risk information exposure on intentions, beliefs, and preferences did not differ based on personal or family history of breast/ovarian cancer. This finding is interesting because it suggests that general risk messages that are not tailored to women’s personal experiences may still be highly persuasive. Lowering intentions to get BRCA testing in a population with a low likelihood of having a mutation is appropriate because BRCA testing is not recommended for individuals without a personal or family history suggestive of a genetic cancer susceptibility condition and testing in unselected populations may subject individuals to unnecessary costs and complications with little benefit.[45–49] On the other hand, these sites are also likely to be viewed by people who have a high likelihood of carrying a BRCA mutation and lowering intentions to get BRCA testing in this group may result in decreased testing and negative health outcomes.[33] Ideally, one would want to ensure that website content contains information that is complete and balanced and that facilitates informed decision making but does not decrease utilization of tests with clinical utility in populations of women who are likely to be BRCA mutation carriers.
Finally, this study has given us greater insight into how different variations in risk message framing may potentially influence consumer behavior. Counter to our hypothesis, “expert” risk information was not more persuasive than unattributed risk information. We found that exposure to expert risk information and unattributed risk information produced similar effects on all outcomes. In fact, the only difference that we observed was that exposure to the unattributed risk information may produce lower intentions to have a BRCA test than exposure to the expert risk information (trend to significance with a p=0.06). If this finding is replicated in future studies it would be interesting because it contradicts much of the known communication literature which demonstrates that credible expert sources tend to be highly persuasive.[37] A possible explanation for this trend is that some people may have high levels of trust in internet information and that the attribution to expert sources was distracting. One study found that 60% of patients in a primary care practice felt that information on the internet was the same as or better than information that they received from their doctor.[50] A second explanation is that women may have found the website itself to be a credible source which may have then diluted the effect of the expert attributions. Source credibility and other types of information frames should be explored further in future studies.
In the absence of an outright ban on DTC genetic testing, policy makers are faced with the challenge of determining how to ensure adequate consumer protection. While a one-size-fits-all approach to regulation may be difficult because of the diversity of genetic testing,[21] regulations related to website information content and the provision of risk information may be needed. Without regulation, DTC genetic testing companies are likely to maintain websites that try to maximize test sales; potentially excluding information that consumers need to make informed decisions. Recent studies have concluded that many DTC genetic testing websites mislead consumers, have incomplete information, have low agreement with professional recommendations, often appear to broaden the population for whom testing is indicated, and fail to disclose the risks and limitation of online testing.[27, 51–53] A study by Leighton and colleagues found that people in the general public were more likely to misunderstand DTC genetic testing results and rate them as more helpful as compared to genetic counselors.[54] Prompted by claims made on DTC genetic testing websites, the FDA has recently decided to reevaluate the lack of regulation of DTC genetic testing.[31] Additionally, a bill introduced into the House last year, H.R. 5440, deals with many facets of genetic testing, including the regulation of the claims and advertising of personalized medicine products.[32]
There are few published studies to date that reveal how DTC genetic marketing and testing impacts actual DTC genetic testing consumers and consumer behavior. A recent study by Bloss and colleagues (which evaluated the impact of DTC single nucleotide polymorphism (SNP) testing) reported that over 97% of customers had no clinically significant test-related distress after testing.[55] The study also found that consumers who had elevated estimates for developing some cancers reported higher intentions to engage in cancer screening behaviors; notably consumers with elevated estimates for breast cancer reported higher intentions to perform breast self exam and higher intentions to get mammograms [55]. Our knowledge about the impact of DTC genetic testing marketing and sales on consumer behavior will greatly expand in the next five years as there are a number of ongoing, longitudinal studies in this area.[55–58]
Our study has a number of limitations. First, we recruited a convenience sample of female internet users; however, because we conducted a randomized experiment, our findings related to the effects of exposure to risk information on outcomes are robust. Additionally, it is likely that female internet users are the target audience for DTC BRCA testing and the population that would most likely use these websites. Second, our experiment used a “mock” website and there were constraints on website navigation. While we tried to closely replicate an actual DTC website by using a modified commercial website, similar risk information interventions on differently formatted sites might have different effects. Third, since BRAC testing through a cancer genetics clinic is standard-of-care, we did not include information on the risks associated with clinic-based testing in our experiment. Also, we have studied the impact of exposure to a small number of potential risks of online genetic testing in the setting of BRCA mutations. Our findings can not be generalized to other types of risk information or other online genetic tests. Finally, our study examines beliefs about and intentions related to DTC BRCA testing but does not investigate women’s actual behavior.
We are rapidly approaching the “$1000 dollar genome”, a tipping point that many argue will lead to widespread consumer genetic testing.[44, 59] If DTC genetic testing is inevitable, we need to better understand how this testing can be done in a way that optimizes patient outcomes. A recent policy statement noted that providers and consumers will need to think in new ways about education, counseling and informed consent in the setting of DTC genetic testing.[23] Our study provides some evidence that risk information provision in the setting of DTC BRCA testing alters women’s beliefs and preferences related to online genetic testing and that risk information exposure may lead to lower intentions to get BRCA testing. Future work should be directed at understanding the impact of regulation on the uptake of DTC testing and the overall impact of DTC genetic testing on health behaviors.
Acknowledgments
Funding/Support: NCI 5P50CA095856-05, Robert Wood Johnson Foundation, The American Society for Clinical Oncology, NCI R25 CA092203
Footnotes
Conflicts of interest statement: All authors have contributed sufficiently to the project to be included as authors. All authors have read and approved the manuscript. To the best of our knowledge, none of the authors had a conflict of interest related to this manuscript.
References
- 1. [cited 2011 June 20];Genetics and Public Policy Center; Direct-to-consumer genetic testing companies. 2010 May 28; Available from: http://www.dnapolicy.org/resources/AlphabetizedDTCGeneticTestingCompanies.pdf.
- 2.Gollust SE, Wilfond BS, Hull SC. Direct-to-consumer sales of genetic services on the Internet. Genet Med. 2003;5(4):332–337. doi: 10.1097/01.GIM.0000076972.83711.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamilton A. 50 Best Inventions 2008: The Retail DNA Test. Time. 2008 [Google Scholar]
- 4.Schickedanz AD, Herdman RC. Direct-to-consumer genetic testing: the need to get retail genomics right. Clin Pharmacol Ther. 2009;86(1):17–20. doi: 10.1038/clpt.2009.56. [DOI] [PubMed] [Google Scholar]
- 5.McGuire AL, et al. Social networkers' attitudes toward direct-to-consumer personal genome testing. Am J Bioeth. 2009;9(6–7):3–10. doi: 10.1080/15265160902928209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stein R. The Washington Post. Washington, DC: 2010. Company plans to sell genetic testing kit at drugstores. [Google Scholar]
- 7.Corbett Dooren J. The Wall Street Journal. New York: 2010. House panel probes gene tests. [Google Scholar]
- 8.Williams-Jones B. Where there's a web, there's a way: commercial genetic testing and the Internet. Community Genet. 2003;6(1):46–57. doi: 10.1159/000069538. [DOI] [PubMed] [Google Scholar]
- 9.Wolfberg AJ. Genes on the Web--direct-to-consumer marketing of genetic testing. N Engl J Med. 2006;355(6):543–545. doi: 10.1056/NEJMp068079. [DOI] [PubMed] [Google Scholar]
- 10.Javitt GH, Stanley E, Hudson K. Direct-to-consumer genetic tests, government oversight, and the First Amendment: what the government can (and can't) do to protect the public's health. Oklahoma Law Rev. 2004;57(2):251–302. [PubMed] [Google Scholar]
- 11.Siegel R, Palca J. DNA: The Machinery Behind Human Beings - Part 1. National Public Radio, All things Considered. 2005 Oct 10; Available from: http://www.npr.org/templates/story/story.php?storyId=4953053.
- 12.Marietta C, McGuire AL. Currents in contemporary ethics. Direct-to-consumer genetic testing: is it the practice of medicine? J Law Med Ethics. 2009;37(2):369–374. doi: 10.1111/j.1748-720X.2009.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altman RB. Direct-to-consumer genetic testing: failure is not an option. Clin Pharmacol Ther. 2009;86(1):15–17. doi: 10.1038/clpt.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kontos EZ, Viswanath K. Cancer-related direct-to-consumer advertising: a critical review. Nature reviews. Cancer. 2011;11:142–150. doi: 10.1038/nrc2999. [DOI] [PubMed] [Google Scholar]
- 15.Hunter DJ, Khoury MJ, Drazen JM. Letting the genome out of the bottle--will we get our wish? N Engl J Med. 2008;358(2):105–107. doi: 10.1056/NEJMp0708162. [DOI] [PubMed] [Google Scholar]
- 16.McCabe LL, McCabe ER. Direct-to-consumer genetic testing: access and marketing. Genet Med. 2004;6(1):58–59. doi: 10.1097/01.gim.0000105753.01536.be. [DOI] [PubMed] [Google Scholar]
- 17.Mykitiuk R. Caveat emptor: direct-to-consumer supply and advertising of genetic testing. Clin Invest Med. 2004;27(1):23–32. [PubMed] [Google Scholar]
- 18.Gray S, Olopade OI. Direct-to-consumer marketing of genetic tests for cancer: buyer beware. J Clin Oncol. 2003;21(17):3191–3193. doi: 10.1200/JCO.2003.12.069. [DOI] [PubMed] [Google Scholar]
- 19.Caulfield T, et al. Direct-to-consumer genetic testing: good, bad or benign? Clinical genetics. 2010;77:101–105. doi: 10.1111/j.1399-0004.2009.01291.x. [DOI] [PubMed] [Google Scholar]
- 20.Javitt GH. Policy implications of genetic testing: not just for geneticists anymore. Adv Chronic Kidney Dis. 2006;13(2):178–182. doi: 10.1053/j.ackd.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Hudson K, et al. ASHG Statement on direct-to-consumer genetic testing in the United States. Obstet Gynecol. 2007;110(6):1392–1395. doi: 10.1097/01.AOG.0000292086.98514.8b. [DOI] [PubMed] [Google Scholar]
- 22.McGuire A, et al. Regulating Direct-to-Consumer Personal Genome Testing. Science. 2010;330:181–182. doi: 10.1126/science.1194006. [DOI] [PubMed] [Google Scholar]
- 23.Robson ME, et al. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2010;28(5):893–901. doi: 10.1200/JCO.2009.27.0660. [DOI] [PubMed] [Google Scholar]
- 24.ACMG statement on direct-to-consumer genetic testing. Genet Med. 2004;6(1):60. doi: 10.1097/01.GIM.0000106164.59722.CE. [DOI] [PubMed] [Google Scholar]
- 25.Keating NL, et al. Factors affecting influential discussions among physicians: a social network analysis of a primary care practice. Journal of general internal medicine. 2007;22:794–798. doi: 10.1007/s11606-007-0190-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Statement of the ESHG on direct-to-consumer genetic testing for health-related purposes. European journal of human genetics : EJHG. 2010;18:1271–1273. doi: 10.1038/ejhg.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Government Accountability Office, Direct-to-Consumer Genetic Tests: Misleading Test Results Are Further Complicated by Deceptive Marketing and Other Questionable Practices. Washington, D.C.: 2010. [Google Scholar]
- 28.Kuehn BM. Inconsistent results, inaccurate claims plague direct-to-consumer gene tests. JAMA : the journal of the American Medical Association. 2010;304:1313–1315. doi: 10.1001/jama.2010.1328. [DOI] [PubMed] [Google Scholar]
- 29.Pollack A. The New York Times. New York: 2008. Jun 26, Gene Testing Questioned by Regulators. [Google Scholar]
- 30.Fraker M, Mazza A-M. National Research Council and Institute of Medicine; Direct-to-Consumer Genetic Testing: Summary of a Workshop. Washington, D.C.: National Academies Press; 2010. [PubMed] [Google Scholar]
- 31.Rubin R. FDA groups genetic tests with medical devices, requiring approval. [cited 2010, June 22];USA Today.com. 2010 Jun 15; Available from: http://www.usatoday.com/news/health/2010-06-15-genetictesting15_st_n.htm.
- 32.To secure the promise of personalized medicine. H.R. 5440. 2010 [Google Scholar]
- 33.Gray SW, et al. Risk information exposure and direct-to-consumer genetic testing for BRCA mutations among women with a personal or family history of breast or ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1303–1311. doi: 10.1158/1055-9965.EPI-08-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petty RE, Cacioppo JT. Advances in experimental social psychology. New York: Academic Press; 1986. The Elaboration LIkelihood Model Of Persuasion; pp. 123–205. [Google Scholar]
- 35.Petty RE, Wegener DT. The elaboration likelihood model: current status and controversies. In: Chaiken S, Trope Y, editors. Dual-process theories in social psychology. New York: The Guilford Press; 1999. pp. 41–72. [Google Scholar]
- 36.Brinol P, Richard EP. Fundamental Processes Leading to Attitude Change: Implications for Cancer Prevention Communications. Journal of Communication. 2006;56:S81–S104. [Google Scholar]
- 37.Pornpitakpan C. The Persuasiveness of Source Credibility: A Critical Review of Five Decades' Evidence. Journal of Applied Social Psychology. 2004;34(2):243–281. [Google Scholar]
- 38.Epstein R, Teagarden JR. Comparative effectiveness and personalized medicine: evolving together or apart? Health affairs (Project Hope) 2010;29:1783–1787. doi: 10.1377/hlthaff.2010.0642. [DOI] [PubMed] [Google Scholar]
- 39.Frank TS, et al. Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: analysis of 10,000 individuals. J Clin Oncol. 2002;20(6):1480–1490. doi: 10.1200/JCO.2002.20.6.1480. [DOI] [PubMed] [Google Scholar]
- 40.Sweeny K, Legg AM. Predictors of interest in direct-to-consumer genetic testing. Psychology & health. 2011:1–14. doi: 10.1080/08870446.2010.514607. [DOI] [PubMed] [Google Scholar]
- 41.Kinmonth AL, et al. The new genetics. Implications for clinical services in Britain and the United States. Bmj. 1998;316(7133):767–770. doi: 10.1136/bmj.316.7133.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watson EK, et al. The 'new genetics' and primary care: GPs' views on their role and their educational needs. Fam Pract. 1999;16(4):420–425. doi: 10.1093/fampra/16.4.420. [DOI] [PubMed] [Google Scholar]
- 43.Hayflick SJ, Eiff MP. Role of primary care providers in the delivery of genetics services. Community Genet. 1998;1(1):18–22. doi: 10.1159/000016131. [DOI] [PubMed] [Google Scholar]
- 44.SACGHS. Genetics education and training of health care professionals, public health providers, and consumers. O.o.S. Policy. 2010 Editor. [Google Scholar]
- 45.Ford D, Easton DF, Peto J. Estimates of the gene frequency of BRCA1 and its contribution to breast and ovarian cancer incidence. Am J Hum Genet. 1995;57(6):1457–1462. [PMC free article] [PubMed] [Google Scholar]
- 46.Khoury MJ, Wagener DK. Epidemiological evaluation of the use of genetics to improve the predictive value of disease risk factors. Am J Hum Genet. 1995;56(4):835–844. [PMC free article] [PubMed] [Google Scholar]
- 47.Kohane IS, Masys DR, Altman RB. The incidentalome: a threat to genomic medicine. JAMA. 2006;296(2):212–215. doi: 10.1001/jama.296.2.212. [DOI] [PubMed] [Google Scholar]
- 48.Risch HA, et al. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin-cohort study in Ontario, Canada. J Natl Cancer Inst. 2006;98(23):1694–1706. doi: 10.1093/jnci/djj465. [DOI] [PubMed] [Google Scholar]
- 49.American Society of Clinical Oncology policy statement update: genetic testing for cancer susceptibility. J Clin Oncol. 2003;21(12):2397–2406. doi: 10.1200/JCO.2003.03.189. [DOI] [PubMed] [Google Scholar]
- 50.Diaz JA, et al. Patients' use of the Internet for medical information. J Gen Intern Med. 2002;17(3):180–185. doi: 10.1046/j.1525-1497.2002.10603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Delbaldo C, et al. Benefits of adding a drug to a single-agent or a 2-agent chemotherapy regimen in advanced non-small-cell lung cancer: a meta-analysis. JAMA : the journal of the American Medical Association. 2004;292:470–484. doi: 10.1001/jama.292.4.470. [DOI] [PubMed] [Google Scholar]
- 52.Goddard KA, et al. Health-related direct-to-consumer genetic tests: a public health assessment and analysis of practices related to Internet-based tests for risk of thrombosis. Public Health Genomics. 2009;12(2):92–104. doi: 10.1159/000176794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lachance CR, et al. Informational content, literacy demands, and usability of websites offering health-related genetic tests directly to consumers. Genet Med. 2010 doi: 10.1097/GIM.0b013e3181dbd8b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leighton JW, Valvered K, Bernhardt B. The General Public’s Understanding and Perception of Direct-to-Consumer Genetic Test Results. The American Society of Human Genetics Meeting; The American Society of Human Genetics; Washington, D.C. 2010. [DOI] [PubMed] [Google Scholar]
- 55.Bloss CS, Schork NJ, Topol EJ. Effect of Direct-to-Consumer Genomewide Profiling to Assess Disease Risk. The New England journal of medicine. 2011 doi: 10.1056/NEJMoa1011893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McBride CM, Wade CH, Kaphingst KA. Consumers' views of direct-to-consumer genetic information. Annual review of genomics and human genetics. 2010;11:427–446. doi: 10.1146/annurev-genom-082509-141604. [DOI] [PubMed] [Google Scholar]
- 57.Gordon ES, et al. Perceived Risk, Anxiety and Sharing Behavior in Response to Personalized Risk Information in a Cohort Study; American Society of Human Genetics Meeting; Washington, D.C. 2010. [Google Scholar]
- 58.Kaufman D, et al. Direct from consumers: A survey of 1,048 customers of three direct-to-consumer personal genomic testing companies about motivations, attitudes, and responses to testing; American Society of Human Genetics Meeting; Washington, D.C. 2010. [Google Scholar]
- 59.Feero WG, Guttmacher AE, Collins FS. Genomic medicine--an updated primer. N Engl J Med. 2010;362(21):2001–2011. doi: 10.1056/NEJMra0907175. [DOI] [PubMed] [Google Scholar]