Abstract
The impact of donor lymphocyte infusion (DLI) initial cell dose on its outcome is known in patient with chronic myeloid leukemia, but limited in patients with other hematological malignancies. In this retrospective study, we evaluated the effect of initial DLI CD3+ cell dose on graft-versus-host disease (GVHD) and overall survival (OS) after DLI given for relapse of any hematological malignancies after allogeneic hematopoietic cell transplantation (HCT) with high or reduced intensity conditioning. The cohort included 225 patients. Initial DLI CD3+ cell dose/kg recipient body weight was ≤1×107 (n=84; Group A), >1.0 to <10 ×107 (n=58; Group B), and ≥ 10×107 (n=66; Group C). Cumulative incidence rates of GVHD at 12 months after DLI were 21%, 45% and 55% for Groups A, B, and C, respectively. Multivariate analysis showed that initial DLI CD3+ cell ≥1×107 dose/kg is associated with an increased risk of GVHD after DLI (p=0.03). Moreover, initial DLI CD3+ cell dose of 10×107 or higher did not decrease the risk of relapse and did not improve OS. Thus, these results support the use of less than 10×107 CD3+ cell/kg as the initial cell dose of DLI for treatment of persistent or recurrent hematological malignancy after HCT.
Keywords: donor lymphocyte infusion (DLI), hematopoietic cell transplantation (HCT), CD3+ T cells, graft-versus-host disease (GVHD), adoptive immunotherapy for relapse after HCT
Introduction
Allogeneic hematopoietic cell transplantation (HCT) has the potential to provide long term survival and even cure in patients with hematological malignancies (1, 2). Nonetheless, relapse of malignancy after HCT remains a major cause of transplant failure. Donor lymphocyte infusion (DLI) is one approach frequently used to treat patients with relapse hematologic malignancy after allogeneic HCT. The DLI effect is mediated through the immunologic antitumor activity of donor T cells and possibly natural killer [NK] cells (3–6). Since the first report of DLI in patients with relapsed chronic myeloid leukemia after allogeneic HCT by Kolb et al in 1990 (6), DLI has become a common approach to treat not only CML, but also acute leukemia, lymphoma, myelodysplastic syndrome and multiple myeloma that has relapsed after allogeneic HCT (7–13). The beneficial graft-versus-leukemia (GVL) effect of DLI may be offset by morbidity and mortality related to graft-versus-host disease (GVHD). While low initial cell dose followed by escalation of doses of DLI can minimize the risk of GVHD in patients treated for relapsed CML (14–16), the data regarding the impact of initial cell dose on outcomes after DLI for other relapsed hematological malignancies is limited. The primary objective of the current study was to determine the effect of the initial DLI CD3+ T cell dose on subsequent GVHD requiring systemic treatment and on overall survival (OS) after DLI.
Patients and Methods
The study cohort included 225 patients treated with DLI for relapsed hematological malignancies after allogeneic HCT from November 1993 through October 2011. Patients received high or reduced intensity conditioning regimens before HCT according to standard treatment plan or prospective clinical trials and were treated at the Fred Hutchinson Cancer Research Center (n=212) and at three participant institutions in the Seattle Nonmyeloablative HCT Consortium: the University of Torino (n=9), the Puget Sound VA Health Care System (n=2), and the Medical College of Wisconsin (n=2). Follow up was complete through July 2012. All patients provided informed consent for treatment according to transplantation protocols approved by each institutional review boards. In addition, separate institutional approval was obtained to gather data from patient records and databases retrospectively.
Donor Lymphocyte Infusion (DLI)
All 225 patients in this study received DLI for treatment of relapsed hematological malignancies after HCT. No prophylactic DLI treatment was given. 154 patients were treated with DLI in prospective clinical trials and 71 patients received DLI as a treatment plan. Patients with rapidly progressive malignancies (i.e., acute myeloid or lymphoid leukemia, CML in blast phase, high-grade myelodysplastic syndrome, intermediate-high grade Non-Hodgkin lymphoma, Hodgkin lymphoma or aggressive multiple myeloma) received chemotherapy or radiation before DLI according to specific protocols or at the discretion of the attending physician. Treatment with tyrosine kinase inhibitor or interferon was generally discontinued before DLI. Patients were eligible to receive DLI if they were not receiving systemic treatment for GVHD, had no evidence of active GVHD at the time of DLI and had evidence of donor chimerism. No immunosuppressive agents were given after DLI to prevent GVHD. Among 128 patients with available information regarding the DLI product, 32 patients received a G-CSF mobilized product for DLI. Twelve patients received IL-2 after DLI as part of a prospective clinical trial, as previously described (17).
Graft-versus-Host Disease definition
DLI-related GVHD was defined as any acute GVHD (18) or chronic GVHD (NIH criteria or historical criteria) (19, 20) after DLI that required systemic treatment. As clinically acute and chronic GVHD occurring after DLI have overlapping onset times (21, 22), for the purpose of evaluating the incidence of GVHD after DLI we defined DLI-related GVHD as any GVHD after DLI (acute or chronic) that required systemic treatment. Serious GVHD after DLI was evaluated according to previously reported criteria (23).
Statistical Methods
Overall survival after DLI was estimated by the Kaplan-Meier method. Cumulative incidence of relapse and GVHD after DLI were estimated by standard methods, treating death as a competing risk. Cox regression was used to evaluate risk factors for GVHD, OS, and relapse and disease progression after DLI. Risk factors evaluated in univariate analysis for each of the outcomes (GVHD, OS, and relapse or disease progression after DLI) included initial DLI CD3+ cell dose, patient age at DLI, donor-recipient gender, diagnosis at time of DLI, disease status at time of DLI, donor origin, donor-recipient HLA match, graft stem cell source, conditioning intensity, acute and chronic GVHD before DLI, interval between HCT to DLI, cytoreductive treatment before DLI, donor blood CD3 and whole marrow chimerism at time of DLI, lymphocyte count at time of DLI, use of G-CSF-mobilized product for DLI, use of IL-2 after DLI, and year of DLI. Multivariate models included all factors significant at the 0.05 level in univariate analysis for each outcome, as well as age and the factors most significantly disparate among the cell dose groups (donor origin, conditioning intensity, and year of DLI). In analyzing the impact of subsequent DLI on overall survival, the second DLI was treated as a time-dependent covariate in a Cox regression model. Comparisons of CD3+ cell dose between the initial and second DLI was by paired t-test.
Results
Patient characteristics
225 patients underwent treatment with DLI for persistent or relapse hematological malignancies after HCT including chronic myeloid leukemia (n=56), acute myeloid leukemia (n=71), myelodysplastic syndrome (n=22), acute lymphoblastic leukemia (n=21), multiple myeloma (n=23), lymphoma (n=21),chronic lymphocytic leukemia/lymphoma (n=8), myelofibrosis (n=2), and myeloproliferative disorder (n=1). Patients were classified into (i) high risk myeloid malignancies group (AML, MDS, CML (BC, AP), myelofibrosis, and myeloproliferative disorder) (n=111), (ii) high risk lymphoid malignancies group [ALL and high grade lymphomas (Hodgkin lymphoma, DLBCL, transformed NHL)] (n=37), (iii) low risk lymphoid malignancies group (CLL, MM, other lymphomas) (n=36), and (iv) CML-CP (n=41). The median age of the 225 patient cohort was 46 years (range, 3–74) and 59% (n=132) were male. Patients received transplants from HLA-matched related (n=171) or unrelated (n=41) donors. 13 patients had HLA-mismatched donors. 58 patients (26%) received reduced intensity conditioning regimens before HCT. The median time interval from HCT to relapse was 11.3 months (range, 1–180) and from HCT to DLI was 15.5 months (range, 21.1–215). 144 patients (64%) received cytoreductive therapy before DLI, and 55 patients (24%) had achieved complete remission (CR) at time of DLI. The initial DLI CD3+ cell dose/kg was ≤1×107 in 84 patients (Group A), >1.0 to <10 ×107 in 58 patients (Group B), and ≥ 10×107 in 66 patients (Group C). Median follow-up after DLI was 78 months (range 0.1–197). Characteristics of the cohort according to the initial DLI CD3+ cell dose administered are shown in Table 1.
Table 1.
Patient characteristics
| CD3+ cell dose, per kg | |||||
|---|---|---|---|---|---|
| Characteristic |
Unknown (N=17) |
Group A ≤ 107 (N=84) |
Group B >107 to <108 (N=58) |
Group C ≥ 108 (n=66) |
p-value1 |
| Patient age at DLI | 0.14 | ||||
| 0–29 years | 4 | 12 (14) | 13 (22) | 9 (14) | |
| 30–44 years | 7 | 25 (30) | 11 (19) | 27 (41) | |
| 45–59 years | 5 | 29 (35) | 25 (43) | 22 (33) | |
| 60–74 years | 1 | 18 (21) | 9 (16) | 8 (12) | |
| Donor-recipient gender (n=220) | 0.17 | ||||
| Other | 14 | 65 (82) | 40 (69) | 52 (79) | |
| Female to male | 3 | 14 (18) | 18 (31) | 14 (21) | |
| Disease diagnosis/risk at time of DLI | 0.36 | ||||
| CML chronic phase | 6 | 16 (19) | 9 (16) | 10 (15) | |
| Low risk lymphoid malignancies2 | 0 | 20 (24) | 6 (10) | 10 (15) | |
| High risk myeloid malignancies3 | 6 | 36 (43) | 32 (55) | 37 (56) | |
| High risk lymphoid malignancies4 | 5 | 12 (14) | 11 (19) | 9 (14) | |
| Disease status at time of DLI | 0.05 | ||||
| Complete remission (CR) | 2 | 14 (17) | 19 (33) | 20 (30) | |
| Not in CR | 15 | 70 (83) | 39 (67) | 46 (70) | |
| Donor origin | <0.0001 | ||||
| Related | 13 | 48 (57) | 46 (79) | 64 (97) | |
| Unrelated | 4 | 36 (43) | 12 (21) | 2 (3) | |
| Donor-recipient HLA match | 0.07 | ||||
| Matched | 13 | 77 (92) | 57 (98) | 65 (98) | |
| Mismatched | 4 | 7 (8) | 1 (2) | 1 (2) | |
| Graft stem cell source (n=201) | 0.004 | ||||
| Bone Marrow | 12 | 31 (49) | 21 (38) | 45 (68) | |
| Mobilized blood | 5 | 32 (51) | 34 (62) | 21 (32) | |
| Conditioning intensity | <0.0001 | ||||
| Myeloablative | 16 | 47 (56) | 41 (71) | 63 (95) | |
| Nonmyeloablative | 1 | 37 (44) | 17 (29) | 3 (5) | |
| Prior acute GVHD (n=218) | 0.05 | ||||
| 0–I | 4 | 34 (44) | 25 (43) | 17 (26) | |
| II–IV | 13 | 43 (56) | 33 (57) | 49 (74) | |
| Prior chronic GVHD | 0.14 | ||||
| No | 11 | 60 (71) | 39 (67) | 37 (56) | |
| Yes | 6 | 24 (29) | 19 (33) | 29 (44) | |
| Time from HCT to DLI | 0.50 | ||||
| > 1 year | 11 | 51 (61) | 31 (53) | 42 (64) | |
| ≤ 1 year | 6 | 33 (39) | 27 (47) | 24 (36) | |
| Cytoreduction before DLI (n=220) | 0.02 | ||||
| No | 6 | 37 (45) | 18 (31) | 15 (24) | |
| Yes | 11 | 45 (55) | 40 (69) | 48 (76) | |
| Donor CD3 chimerism at time of DLI (n=91) | 0.70 | ||||
| > 95% | 1 | 32 (70) | 20 (71) | 7 (58) | |
| ≤ 95% | 4 | 14 (30) | 8 (29) | 5 (42) | |
| Donor BM chimerism at time of DLI (n=114) | 0.75 | ||||
| > 95% | 5 | 25 (54) | 18 (60) | 13 (50) | |
| ≤ 95% | 7 | 21 (46) | 12 (40) | 13 (50) | |
| Lymphocyte count at time of DLI (n=217) | 0.01 | ||||
| ≥ 103/microL | 10 | 47 (61) | 24 (42) | 25 (38) | |
| < 103/microL | 7 | 30 (39) | 33 (58) | 41 (62) | |
| G-CSF mobilized product for DLI (n=128) | 0.02 | ||||
| No | 0 | 41 (84) | 37 (79) | 18 (56) | |
| Yes | 0 | 8 (16) | 10 (21) | 14 (44) | |
| IL-2 after DLI | 0.02 | ||||
| No | 15 | 83 (99) | 55 (95) | 58 (88) | |
| Yes | 2 | 1 (1) | 3 (5) | 8 (12) | |
| Year of DLI | <0.0001 | ||||
| 1992–1996 | 6 | 5 (6) | 8 (14) | 23 (35) | |
| 1997–2001 | 6 | 26 (31) | 14 (24) | 33 (50) | |
| 2001–2006 | 2 | 30 (36) | 27 (47) | 10 (15) | |
| 2007–2011 | 3 | 23 (27) | 9 (16) | 0 | |
Among groups A, B, and C
CLL, MM, lymphomas not high grade
AML, MDS, CML (BC, AP), myelofibrosis, myeloproliferative disorders
ALL, High grade lymphomas (Hodgkin lymphoma, diffuse large B cell lymphoma, transformed non-Hodgkin lymphoma)
GVHD after DLI
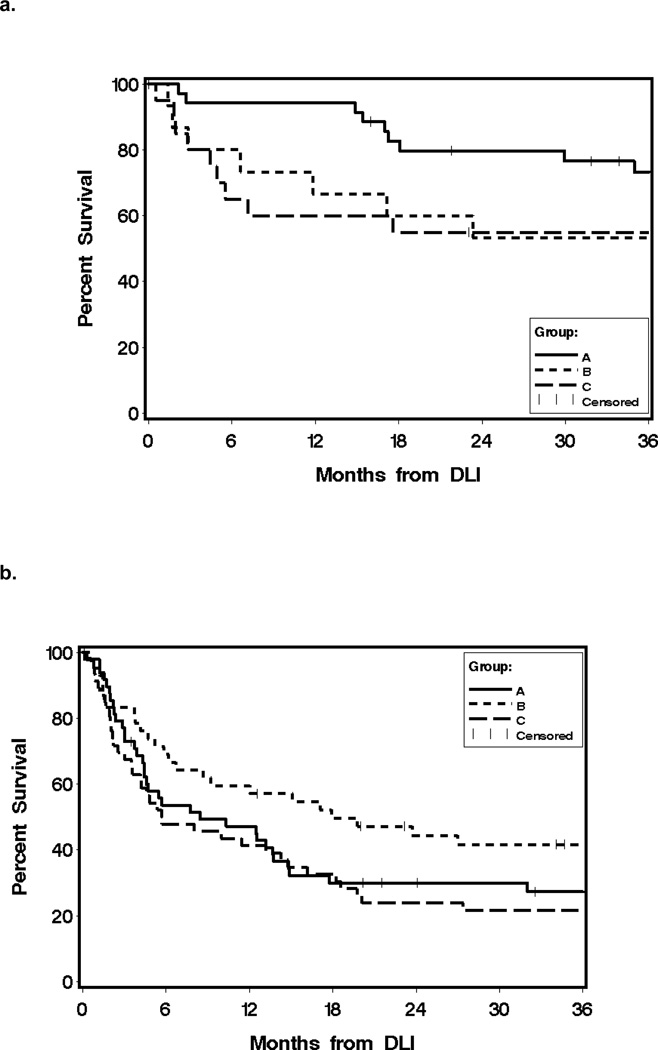
Of the 225 treated patients, 86 (39%) developed GVHD that required systemic therapy after DLI and 29 of 86 cases had serious GVHD as previously defined (23). The median interval from DLI to GVHD that required systemic treatment was 39 days (range, 6–1029). The incidence rates of GVHD at 12 months after DLI according to initial cell dose were 21%, 45% and 55% for Groups A, B, and C, respectively (Figure 1.a.).
Figure 1. Outcome after DLI according to DLI cell dose.
a. Cumulative incidence of GVHD after DLI according to initial CD3+ cell dose. 12-month cumulative incidence of GVHD for initial DLI cell dose Group A (≤ 1×107 CD3+ cell/kg) was 21%, compared to 45% (P = 0.01) for initial cell dose Group B (> 1×107 – < 10×107 CD3+ cell/kg) and 55% (P < 0.0001) for initial cell dose Group C (≥10×107 CD3+ cell/kg).
b. Overall survival after DLI according to initial CD3+ cell dose. 3-year Overall survival were 47% for cell dose A, 45% (P =0.16) for cell dose B, and 32% for cell dose C (P =0.01).
Results of univariate and multivariate analysis of risk factors for the development of GVHD after DLI are shown in Table 2. In the multivariate analysis, two factors showed a statistically significantly association with increased risk of GVHD after DLI: (i) initial DLI CD3+ cell dose ≥10×107/kg (hazard ratio (HR) 2.4; 95% CI, 1.1 – 5.4; P=0.03) and (ii) short interval between transplant to DLI of one year or less (HR, 2.95; 95% CI, 1.7–5.2; P =0.0002) (Table 2). In univariate analysis, higher initial CD3+ cell dose was associated with an increased risk for serious GVHD after DLI for Group B (HR, 4.34; 95% CI, 1.4–13.6; P=0.01) and for Group C (HR, 4.80; 95% CI, 1.6–14.7; p=0.006) compared to Group A. Due to the small number of patients experiencing serious GVHD, multivariate analysis was not performed. While DLI given in more recent years was associated with a decreased risk of GVHD in the univariate analysis, this factor did not reach statistical significance in the multivariate analysis. A history of acute or chronic GVHD before DLI or donor type was not statistically significantly associated with increased risk of GVHD after DLI (Table 2).
Table 2.
Risk factors analysis for GVHD after DLI
| Univariate | Multivariate (n=194) | |||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| CD3+ cell dose | ||||
| ≤ 107 cells/kg | 1.0 | 1.0 | ||
| >107 – <108 cells/kg | 2.74 (1.5–5.0) | 0.001 | 1.82 (0.9–3.7) | 0.10 |
| ≥108 cells/kg | 3.87 (2.2–6.9) | <0.0001 | 2.40 (1.1–5.4) | 0.03 |
| Patient age at DLI | ||||
| 0–29 years | 1.0 | 1.0 | ||
| 30–44 years | 1.06 (0.6–2.0) | 0.86 | 0.82 (0.4–1.8) | 0.62 |
| 45–59 years | 1.26 (0.7–2.3) | 0.46 | 1.35 (0.6–2.8) | 0.42 |
| 60–74 years | 0.45 (0.2–1.1) | 0.09 | 0.55 (0.2–1.8) | 0.32 |
| Donor-recipient gender | ||||
| Other | 1.0 | |||
| Female to male | 1.26 (0.8–2.1) | 0.37 | ||
| Disease diagnosis/risk at time of DLI | ||||
| CML chronic phase | 1.0 | 1.0 | ||
| Low risk lymphoid malignancies | 0.94 (0.4–2.3) | 0.89 | 1.04 (0.3–3.8) | 0.95 |
| High risk myeloid malignancies | 2.53 (1.3–4.8) | 0.005 | 1.72 (0.6–4.8) | 0.30 |
| High risk lymphoid malignancies | 1.65 (0.7–3.7) | 0.23 | 1.41 (0.4–4.7) | 0.58 |
| Disease status at time of DLI | ||||
| Complete remission (CR) | 1.0 | 1.0 | ||
| Not in CR | 0.61 (0.4–1.0) | 0.03 | 0.99 (0.5–1.8) | 0.97 |
| Donor origin | ||||
| Related | 1.0 | 1.0 | ||
| Unrelated | 0.76 (0.5–1.3) | 0.29 | 0.99 (0.5–2.0) | 0.97 |
| Donor-recipient HLA match | ||||
| Matched | 1.0 | |||
| Mismatched | 1.10 (0.4–2.7) | 0.84 | ||
| Graft stem cell source | ||||
| Bone Marrow | 1.0 | |||
| Mobilized blood | 1.04 (0.7–1.6) | 0.86 | ||
| Conditioning intensity | ||||
| Myeloablative | 1.0 | 1.0 | ||
| Nonmyeloablative | 0.57 (0.3–1.0) | 0.04 | 0.71 (0.3–1.6) | 0.42 |
| Prior acute GVHD (n=218) | ||||
| 0–I | 1.0 | |||
| II–IV | 1.32 (0.8–2.1) | 0.23 | ||
| Prior chronic GVHD | ||||
| No | 1.0 | |||
| Yes | 1.24 (0.8–1.9) | 0.34 | ||
| Time from HCT to DLI | ||||
| > 1 year | 1.0 | 1.0 | ||
| ≤ 1 year | 2.57 (1.7–3.9) | <0.0001 | 2.95 (1.7–5.2) | 0.0002 |
| Cytoreduction before DLI | ||||
| No | 1.0 | 1.0 | ||
| Yes | 1.88 (1.2–3.1) | 0.01 | 1.32 (0.6–2.9) | 0.48 |
| Donor CD3 chimerism at time of DLI | ||||
| > 95% | 1.0 | |||
| ≤ 95% | 1.87 (0.8–4.1) | 0.12 | ||
| Donor BM chimerism at time of DLI | ||||
| > 95% | 1.0 | |||
| ≤ 95% | 1.26 (0.7–2.3) | 0.46 | ||
| Lymphocyte count at time of DLI | ||||
| ≥ 103/microL | 1.0 | 1.0 | ||
| < 103/microL | 2.13 (1.4–3.3) | 0.0008 | 1.41 (0.8–2.3) | 0.19 |
| G-CSF mobilized product for DLI | ||||
| No | 1.0 | |||
| Yes | 0.95 (0.5–1.8) | 0.87 | ||
| IL-2 after DLI | ||||
| No | 1.0 | |||
| Yes | 1.22 (0.6–2.6) | 0.62 | ||
| Year of DLI | ||||
| 1992–1996 | 1.0 | 1.0 | ||
| 1997–2001 | 0.77 (0.4–1.3) | 0.34 | 0.93 (0.5–1.8) | 0.83 |
| 2001–2006 | 0.57 (0.3–1.0) | 0.05 | 0.67 (0.3–1.3) | 0.25 |
| 2007–2011 | 0.21 (0.1–0.6) | 0.002 | 0.33 (0.1–1.1) | 0.07 |
Overall survival after DLI
Overall survival according to initial DLI cell dose at 3 years were 47%, 45%, and 32% for Groups A, B, and C respectively (Figure 1b). At the time of analysis, 71 patients (31%) were alive after DLI. Survivors were 28 of 41 patients (68%) treated for CML-CP, 3 of 15 patents (20%) treated for CML-AP/BC, 15 of 71 patients (21%) for AML, 4 of 21 patients (19%) for ALL, 1 of 22 patients (4%) for MDS, 7 of 23 patients (30%) for MM, 2 of 8 patients (25%) for CLL and 8 of 21 patients (38%) for lymphomas, 2 of 2 patients with myelofibrosis, and 1 patient with myeloproliferative disorder.
As demonstrated in Table 1, we found no statistically significant imbalance in the distribution of diagnoses risk groups between the three initial DLI CD3+ cell dose groups (p=0.36). Due to the small number of patients in each of initial DLI cell dose groups in the different diagnostic risk groups (i.e., CML-CP, low risk lymphoid, low risk myeloid and high risk lymphoid malignancies), these 4 diagnostic risk groups were combined into two risk categories for the analysis of OS according to initial DLI CD3+ cell dose, as follows: low risk disease including CML-CP and CLL, MM, low-grade lymphomas, and high risk disease including myeloid malignancies (AML, MDS, CML-AP/BC) myelofibrosis, and myeloproliferative disorder, and high-risk lymphoid malignancies [ALL, high grade lymphomas (Hodgkin lymphoma, diffuse large B cell lymphoma, transformed non-Hodgkin lymphoma)]. Figure 2 shows the univariate analysis of OS after DLI according to initial CD3+ cell dose and the two diagnosis risk categories. The 3-year OS for the low-risk category was 73% for cell dose A, 53% for cell dose B and 55% for cell dose C (P =0.07) (Figure 2a). The 3-year OS for the high-risk category was 42% for cell dose B, 27% for cell dose A, and 22% for cell dose C, but the difference in OS between the three cell doses was not statistically significant (P =0.35) (Figure 2b).
Figure 2. Overall survival after DLI according to initial CD3+ cell dose and disease risk category.
Low risk included CML-CP and CLL, MM, low-risk lymphomas. High risk included high risk myeloid malignancies (AML, MDS, CML –AP/BC), myelofibrosis, myeloproliferative disorders, and high risk lymphoid malignancies (ALL, High grade lymphomas (Hodgkin lymphoma, diffuse large B cell lymphoma, transformed non-Hodgkin lymphoma)).
a. Low risk category. 1- and 3-year overall survival were 94% and 73% for cell dose A, 67% and 53% for cell dose B and 60% and 55% for cell dose C (P =0.07).
b. High risk category. 1- and 3-year overall survival were 47% and 27% for cell dose A, 60% and 42% for cell dose B and 41% and 22% for cell dose C (P =0.35)
Univariate analysis of OS according to initial CD3+ cell dose for specific diagnosis such as CML and other disease categories is shown in Supplemental Figure 1. For patients treated with DLI for relapsed CML, 3-year OS according to initial DLI cell dose was 81% for cell dose A, 46% for CD3+ cell dose B, and 50% for CD3+ cell dose C, but these differences did not reach statistical significance (P =0.07) (Supplemental Figure 1a). For patients given DLI for relapsed lymphoma, CLL, and MM, the 1- and 3-year OS were 91% and 64%, respectively, for patients treated with cell dose B, 85% and 43%, respectively, for patients given cell dose A, and 46% and 31%, respectively, for cell dose C. These differences were not statistically significant (P =0.25) (Supplemental Figure 1b). No association between initial DLI CD3+ cell dose and OS was noted for patients treated for relapsed AML or MDS with 3-year OS of 32%, 40% and 28% for initial DLI CD3+ cell doses A, B and C respectively (P =0.99) (Supplemental Figure 1c).
Results of multivariate analysis for risk factors for mortality after DLI are presented in Table 3. Three factors were statistically significantly associated with an increased risk of mortality after DLI: (i) DLI within 1 year after HCT (HR, 2.66; 95% CI, 1.7 – 4.2; P <0.0001), (ii) age 60 or older (HR, 2.69; 95% CI, 1.1 – 6.3; P =0.02), and (iii) high-risk lymphoid malignancies (HR, 2.62; 95% CI, 1.0 – 6.8; P =0.05). As shown in Table 2, a trend for an association between high risk myeloid malignancies and increased mortality risk was noted (P =0.06). More recent DLI was associated with decreased risk for mortality, with a HR of 0.27 (95% CI, 0.1 – 0.6; P =0.002) for patients treated with DLI between 2007 to 2011 compared to patients treated between 1992 to 1996. Initial DLI cell dose did not affect mortality either for the entire cohort (Table 3) or for the cohort of patients with diseases other than CML-CP (cell dose B: HR-0.88; P =0.61, cell dose C: HR-1.22; P =0.51).
Table 3.
Risk factor analysis for overall mortality after DLI
| Univariate | Multivariate (n=181) | |||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| CD3+ cell dose | ||||
| ≤ 107 cells/kg | 1.0 | 1.0 | ||
| >107 – <108 cells/kg | 1.35 (0.9–2.0) | 0.16 | 0.98 (0.6–1.7) | 0.93 |
| ≥108 cells/kg | 1.64 (1.1–2.4) | 0.01 | 1.25 (0.7–2.3) | 0.48 |
| Patient age at DLI | ||||
| 0–29 years | 1.0 | 1.0 | ||
| 30–44 years | 0.99 (0.6–1.6) | 0.95 | 1.02 (0.6–1.9) | 0.95 |
| 45–59 years | 1.02 (0.6–1.6) | 0.94 | 1.44 (0.8–2.7) | 0.25 |
| 60–74 years | 1.42 (0.8–2.4) | 0.20 | 2.69 (1.1–6.3) | 0.02 |
| Donor-recipient gender | ||||
| Other | 1.0 | |||
| Female to male | 1.19 (0.8–1.7) | 0.38 | ||
| Disease diagnosis/risk at time of DLI | ||||
| CML chronic phase | 1.0 | 1.0 | ||
| Low risk lymphoid malignancies | 2.93 (1.5–5.8) | 0.002 | 1.47 (0.5–4.2) | 0.48 |
| High risk myeloid malignancies | 5.05 (2.8–9.1) | <0.0001 | 2.29 (1.0–5.4) | 0.06 |
| High risk lymphoid malignancies | 4.64 (2.4–9.0) | <0.0001 | 2.62 (1.0–6.8) | 0.05 |
| Disease status at time of DLI | ||||
| Complete remission (CR) | 1.0 | |||
| Not in CR | 1.18 (0.8–1.7) | 0.39 | ||
| Donor origin | ||||
| Related | 1.0 | 1.0 | ||
| Unrelated | 0.86 (0.6–1.3) | 0.44 | 1.05 (0.6–1.8) | 0.87 |
| Donor-recipient HLA match | ||||
| Matched | 1.0 | |||
| Mismatched | 1.14 (0.6–2.2) | 0.70 | ||
| Graft stem cell source | ||||
| Bone Marrow | 1.0 | 1.0 | ||
| Mobilized blood | 1.72 (1.2–2.4) | 0.002 | 1.42 (0.8–2.5) | 0.22 |
| Conditioning intensity | ||||
| Myeloablative | 1.0 | 1.0 | ||
| Nonmyeloablative | 1.24 (0.9–1.8) | 0.24 | 0.75 (0.4–1.6) | 0.46 |
| Prior acute GVHD (n=218) | ||||
| 0–I | 1.0 | |||
| II–IV | 1.07 (0.8–1.5) | 0.68 | ||
| Prior chronic GVHD | ||||
| No | 1.0 | |||
| Yes | 0.98 (0.7–1.4) | 0.89 | ||
| Time from HCT to DLI | ||||
| > 1 year | 1.0 | 1.0 | ||
| ≤ 1 year | 3.04 (2.2–4.2) | <0.0001 | 2.66 (1.7–4.2) | <0.0001 |
| Cytoreduction before DLI | ||||
| No | 1.0 | 1.0 | ||
| Yes | 2.35 (1.6–3.4) | <0.0001 | 1.37 (0.7–2.5) | 0.31 |
| Donor CD3 chimerism at time of DLI | ||||
| > 95% | 1.0 | |||
| ≤ 95% | 1.39 (0.8–2.4) | 0.25 | ||
| Donor BM chimerism at time of DLI | ||||
| > 95% | 1.0 | |||
| ≤ 95% | 2.08 (1.3–3.3) | 0.003 | ||
| Lymphocyte count at time of DLI | ||||
| ≥ 103/microL | 1.0 | 1.0 | ||
| < 103/microL | 1.72 (1.2–2.4) | 0.001 | 1.16 (0.8–1.8) | 0.50 |
| G-CSF mobilized product for DLI | ||||
| No | 1.0 | |||
| Yes | 1.06 (0.7–1.7) | 0.81 | ||
| IL-2 after DLI | ||||
| No | 1.0 | |||
| Yes | 1.41 (0.8–2.5) | 0.23 | ||
| Year of DLI | ||||
| 1992–1996 | 1.0 | 1.0 | ||
| 1997–2001 | 0.89 (0.6–1.4) | 0.59 | 0.65 (0.4–1.1) | 0.10 |
| 2001–2006 | 0.79 (0.5–1.2) | 0.30 | 0.46 (0.2–0.9) | 0.02 |
| 2007–2011 | 0.58 (0.3–1.1) | 0.09 | 0.27 (0.1–0.6) | 0.002 |
Of the 225 patients, 46 received two DLIs, 13 patients received three DLIs and one patient received four DLIs. A time-dependent Cox regression analysis showed no significant effect of subsequent DLIs on OS (HR, 0.95; 95% CI, 0.6–1.4, p=0.82).
Causes of Death
A total of 154 patients have died. Deaths occurred in 49 of 84 patients (58%) in cell Group A, in 41 of 58 patients (71%) in cell dose Group B, and in 55 of 66 patients (83%) in cell Group C. The most common cause of death after DLI was progressive disease or relapse of malignancy in all three cell doses: 90% of deaths in cell dose A, 73% of deaths in cell dose B, and 67% of deaths in cell dose C. GVHD was the primary cause of death in 4 patients (8%) in Group A, 3 patients (7%) in Group B, and 5 patients (9%) in Group C. Table 4 summarizes the cause of death according to the initial DLI cell dose groups.
Table 4.
Cause of death according to initial DLI CD3 cell/kg dose Groups
| Group A N=49 |
Group B N=41 |
Group C N=55 |
|
|---|---|---|---|
| GVHD | 4 (8%) | 3 (7%) | 5 (9%) |
| Relapse/progressive disease | 44 (90%) | 30 (73%) | 37 (67%) |
| Relapse/progressive disease and GVHD | 0 | 3 (7%) | 4 (7%) |
| Other cause death | 0 | 3 (7%) | 4 (7%) |
| Unknown cause of death | 1 (2%) | 2 (5%) | 5 (9%) |
P=0.32 (excluding unknown causes)
Relapse and disease progression after DLI
Among the 225 patients, 166 (74%) had relapse/progressive disease after DLI. Results of univariate and multivariate analysis for relapse/progressive disease after DLI are shown in Supplemental Table 1. Three factors statistically significantly affected the risk of relapse or disease progression after DLI in the multivariate analysis: (i) interval of one year or less from HCT to DLI (HR, 1.92; 95% CI, 1.3 to 2.9; P =0.002), (ii) age of 60 and older (HR 2.33; 95% CI, 1.1 to −5.1; P =0.03), and (iii) initial CD3+ cell dose of > 1×107 – <10×107/kg compared with lower cell dose (HR 0.54; 95% CI, 0.3 to −0.9; P =0.01). Initial CD3+ cell dose of ≥ 10×107/kg was not associated with decreased relapse rate. Analysis for risk of relapse according to initial DLI CD3+ cell dose among patients with diseases other than CML-CP showed similar results. The intermediate cell dose was associated with a decreased relapse rate (HR 0.57; 95% CI, 0.4 – 0.9; P =0.02) but the highest cell dose was not.
Aplasia after DLI
Aplasia after DLI was evaluated in 154 patients who participated in prospective DLI studies for relapsed hematological malignancies after HCT. Fifteen of 154 (9.7%) patients developed aplasia after DLI. Five of 15 patients received an initial DLI dose of ≥10×107 CD3/kg, two patients received a dose of 9×107 CD3/kg, one patient received a dose of 2.5×107 CD3/kg, and the rest of the patients received a dose of 1×107 CD3/kg.
Discussion
DLI is an attractive salvage treatment option for patients with persistent or relapsed hematological malignancies after high or reduced intensity HCT (7–11). Previous studies have suggested optimal initial total nucleated cells (TNC) and lymphocyte doses of DLI associated with low risk of GVHD and mortality and yet maintaining the desirable graft-versus-malignancy effect for treatment of relapsed CML after allogeneic HCT (14–16). However, limited data is available on the impact of DLI CD3+ cell dose on GVHD and mortality after DLI in patients treated for other hematological malignancies, and the appropriate initial cell dose of DLI for treatment of recurrent non-CML hematological malignancies after HCT remained unsettled. Thus, the primary objective of our study was to determine the effect of the initial DLI cell dose on GVHD and OS after DLI in patients treated for any hematological malignancies that relapsed after allogeneic HCT.
DLI contains a variety of different cell types, and the response to DLI could be mediated by several mechanisms. T lymphocytes have significant effects on both GVL and GVHD, due to their longevity after transfusion in vivo and their ability to target minor histocompatibility antigens shared between leukemic and normal host tissue as well as antigens unique to leukemia cells (24–26). Therefore, we focused our analysis on the effect of the initial CD3+ T cell dose on GVHD and survival after DLI. This retrospective analysis of 225 patients confirms that adoptive immunotherapy with donor lymphocytes may be an effective treatment of patients with hematological malignancies who have relapse after allogeneic HCT, and the results suggest that the initial CD3+ cell dose may influence the outcome independently of other relevant factors.
Our multivariate analysis suggest that the risk for developing GVHD after DLI significantly increases with CD3+ cell dose ≥10×107/kg, irrespective of diagnosis, pre-DLI acute or chronic GVHD or interval between HCT and DLI. GVHD, a pathological process initiated by the activation of donor T cell after adoptive transfer into the allogeneic recipient (27), has been a major direct complication after DLI (7, 17, 24, 28–34). Prior reports demonstrated that the dose of allogeneic TNC and lymphocytes infused for DLI is a risk factor of GVHD after DLI in patients with relapsed CML (14, 21, 30). Chalendon et al. showed that >1×107 CD3+ cells/kg was correlated with higher frequency of GVHD after DLI in patients with relapsed CML after HCT (21). In our study, initial DLI CD3+ cell dose of ≥10×107/kg was associated, with a 2.4 fold increase in the risk of GVHD after DLI compared to cell doses ≤ 1.0×107 in patients treated for any hematological malignancy that relapsed or progressed after allogeneic HCT following either high intensity or reduced intensity conditioning. Initial DLI CD3+ cell dose of > 1.0×107 – < 10×107/kg was not associated with increased risk for GVHD compared to lower cell dose.
The next question we asked was the effect of the initial CD3+ cell dose on OS after DLI. Prior studies evaluated the effect of DLI mononuclear cells (MNC) or T cell dose for the treatment of CML (14, 15). Guglielmi et al. demonstrated that for treatment of relapsed CML, an initial DLI cell dose of ≤0.20×108 MNC/kg was associated with less GVHD and better survival than higher MNC doses (14). Similar to the findings by Guglielmi et al, we demonstrated better OS for patient with relapsed CML who were treated with lower initial DLI CD3+ cell dose. Although our association did not reach statistical significance, likely due to the small cohort, our and prior results demonstrate that for patients with CML, initial CD3+ cell dose of 1×107 or lower has survival advantage as compared to higher CD3+ cell dose. In contrast to the association between initial DLI CD3+ cell dose and OS in CML, we did not demonstrate such association for patients with AML or MDS. Prior analyses to evaluate the correlation between cell dose and response rate in AML showed that increasing the cell dose beyond 1.5×108 T cell/kg did not add to the response rate (35). A study by Choi et al., however, appeared to show a better response rate with higher dose of T cells (36). In our study, we demonstrated 3-year overall survival of 32%, 40% and 28% for patient with relapsed AML or MDS treated with ≤1×107 CD3+ cells/kg, 1.1–9.9×107 CD3+ cells/kg and ≥10×107 CD3+ cells/kg respectively (p=0.99). Although these results do not demonstrate correlation between initial DLI CD3+ cell dose and OS, they do demonstrate that initial DLI CD3+ cell dose ≥ 10×107/kg does not provide survival benefit. Therefore, considering that an initial DLI cell dose of ≥10×107 CD3+ cells/kg is associated with increased risk of GVHD after DLI, our results suggest that initial CD3+ cell doses ≥10×107/kg should be avoided. While we found no statically significant difference between initial cell dose groups and OS, patients in the low risk disease category might achieve better survival with lower DLI cell dose (Figure 2a). Our evaluation of the relationship between CD3+ cell dose and OS for lymphoma, CLL, and MM showed association between initial DLI CD3+ cell dose and OS, however this association did not reach statistical significance (Supplemental Figure 1b). The number of ALL patients in our cohort was too small to derive significant conclusions.
Relapse or progressive disease was the main cause of death after DLI at all three cell doses, with no statistically significance decrease in the proportion of patients who died due to relapse among patients who were treated with higher CD3+ cell doses.
We then evaluated the association between initial DLI cell dose and relapse or disease progression after DLI. Our data demonstrate a decreased risk of relapse/disease progression with initial DLI CD3+ dose of >1×107 – <10×107, but not with higher cell dose. The results were not different when CML-CP patients were excluded from the analysis. Similar observations have been made previously in ALL patients treated with DLI (35). While no conclusive statement can be made due to small numbers, the lower response rates with higher CD3+ cell dose could reflect higher numbers of infused T-regulatory cells, which might dampen the graft-versus tumor effect.
This study has several limitations. The data were mostly collected retrospectively, the patient population is heterogeneous, patients were treated according to a variety of protocols with different treatment strategies, and methods and timing of follow-up were not standardized. Additionally, better supportive care has improved survival of patients who were treated in more recent years compared to patients who were treated earlier. Despite those limitations, we believe that this study gives a reliable estimate of the effect of initial CD3+ cell dose on GVHD and survival after DLI for treatment of relapsed hematological malignancies after HCT.
Our results demonstrate that an initial DLI CD3+ cells dose/kg ≥ 10×107 is associated with increased risks of GVHD after DLI, without improving survival. These findings are clinically relevant, since they support a recommendation to infuse less than 10×107 CD3+ cell/kg as the initial cell dose of DLI for treatment of recurrent hematological malignancy, including non-CML after allogeneic HCT.
Supplementary Material
a. Chronic myeloid leukemia (CML). 1- and 3-year overall survival for patients who received DLI for relapsed CML after allogeneic HCT were 81% and 81% for cell dose A, 64% and 46% for cell dose B and 50% and 50% cell dose C (P =0.07).
b. Multiple myeloma(MM)/Chronic lymphocytic leukemia (CLL)/Lymphoma. 1- and 3-year overall survival for patients who received DLI for relapsed MM/CLL/Lymphoma were 85% and 43% for cell dose A, 91% and 64% for cell dose B and 46% and 31% cell dose C (P =0.25).
c. Acute myeloid leukemia (AML)/Myelodysplastic syndrome (MDS). 1- and 3-year overall survival for patients who received DLI for relapsed AML or MDS were 45% and 32% for cell dose A, 47% and 40% for cell dose B and 52% and 28% cell dose C (P =0.99).
Acknowledgments
We thank the patients who participated. We thank members of the research and clinical staff at the FHCRC and referring physicians for their contribution to the care of our patients after hematopoietic cell transplantation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare no competing financial interests
REFERENCES
- 1.Appelbaum FR. The current status of hematopoietic cell transplantation. Annu Rev Med. 2003;54:491–512. doi: 10.1146/annurev.med.54.101601.152456. [DOI] [PubMed] [Google Scholar]
- 2.Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, Martin PJ, Sandmaier BM, Marr KA, Appelbaum FR, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiden PL, Flournoy N, Thomas ED, Prentice R, Fefer A, Buckner CD, Storb R. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med. 1979;300:1068–1073. doi: 10.1056/NEJM197905103001902. [DOI] [PubMed] [Google Scholar]
- 4.Weiden PL, Sullivan KM, Flournoy N, Storb R, Thomas ED. Antileukemic effect of chronic graft-versus-host disease: contribution to improved survival after allogeneic marrow transplantation. N Engl J Med. 1981;304:1529–1533. doi: 10.1056/NEJM198106183042507. [DOI] [PubMed] [Google Scholar]
- 5.Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, Rimm AA, Ringden O, Rozman C, Speck B, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–562. [PubMed] [Google Scholar]
- 6.Kolb HJ, Mittermuller J, Clemm C, Holler E, Ledderose G, Brehm G, Heim M, Wilmanns W. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;76:2462–2465. [PubMed] [Google Scholar]
- 7.Collins RH, Jr, Shpilberg O, Drobyski WR, Porter DL, Giralt S, Champlin R, Goodman SA, Wolff SN, Hu W, Verfaillie C, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol. 1997;15:433–444. doi: 10.1200/JCO.1997.15.2.433. [DOI] [PubMed] [Google Scholar]
- 8.Bethge WA, Hegenbart U, Stuart MJ, Storer BE, Maris MB, Flowers ME, Maloney DG, Chauncey T, Bruno B, Agura E, et al. Adoptive immunotherapy with donor lymphocyte infusions after allogeneic hematopoietic cell transplantation following nonmyeloablative conditioning. Blood. 2004;103:790–795. doi: 10.1182/blood-2003-07-2344. [DOI] [PubMed] [Google Scholar]
- 9.Huff CA, Fuchs EJ, Smith BD, Blackford A, Garrett-Mayer E, Brodsky RA, Flinn IW, Ambinder RF, Borrello IM, Matsui WH, et al. Graft-versus-host reactions and the effectiveness of donor lymphocyte infusions. Biol Blood Marrow Transplant. 2006;12:414–421. doi: 10.1016/j.bbmt.2005.11.520. [DOI] [PubMed] [Google Scholar]
- 10.Michallet AS, Nicolini F, Furst S, Le QH, Dubois V, Hayette S, Bourgeot JP, Tremisi JP, Thomas X, Gebuhrer L, et al. Outcome and long-term follow-up of alloreactive donor lymphocyte infusions given for relapse after myeloablative allogeneic hematopoietic stem cell transplantations (HSCT) Bone Marrow Transplant. 2005;35:601–608. doi: 10.1038/sj.bmt.1704807. [DOI] [PubMed] [Google Scholar]
- 11.Schmid C, Labopin M, Nagler A, Bornhauser M, Finke J, Fassas A, Volin L, Gurman G, Maertens J, Bordigoni P, et al. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party. J Clin Oncol. 2007;25:4938–4945. doi: 10.1200/JCO.2007.11.6053. [DOI] [PubMed] [Google Scholar]
- 12.Bloor AJ, Thomson K, Chowdhry N, Verfuerth S, Ings SJ, Chakraverty R, Linch DC, Goldstone AH, Peggs KS, Mackinnon S. High response rate to donor lymphocyte infusion after allogeneic stem cell transplantation for indolent non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2008;14:50–58. doi: 10.1016/j.bbmt.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Porter D, Levine JE. Graft-versus-host disease and graft-versus-leukemia after donor leukocyte infusion. Semin Hematol. 2006;43:53–61. doi: 10.1053/j.seminhematol.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Guglielmi C, Arcese W, Dazzi F, Brand R, Bunjes D, Verdonck LF, Schattenberg A, Kolb HJ, Ljungman P, Devergie A, et al. Donor lymphocyte infusion for relapsed chronic myelogenous leukemia: prognostic relevance of the initial cell dose. Blood. 2002;100:397–405. doi: 10.1182/blood.v100.2.397. [DOI] [PubMed] [Google Scholar]
- 15.Mackinnon S, Papadopoulos EB, Carabasi MH, Reich L, Collins NH, Boulad F, Castro-Malaspina H, Childs BH, Gillio AP, Kernan NA, et al. Adoptive immunotherapy evaluating escalating doses of donor leukocytes for relapse of chronic myeloid leukemia after bone marrow transplantation: separation of graft-versus-leukemia responses from graft-versus-host disease. Blood. 1995;86:1261–1268. [PubMed] [Google Scholar]
- 16.Dazzi F, Szydlo RM, Craddock C, Cross NC, Kaeda J, Chase A, Olavarria E, van Rhee F, Kanfer E, Apperley JF, et al. Comparison of single-dose and escalating-dose regimens of donor lymphocyte infusion for relapse after allografting for chronic myeloid leukemia. Blood. 2000;95:67–71. [PubMed] [Google Scholar]
- 17.Inamoto Y, Fefer A, Sandmaier BM, Gooley TA, Warren EH, Petersdorf SH, Sanders JE, Storb RF, Appelbaum FR, Martin PJ, et al. A phase I/II study of chemotherapy followed by donor lymphocyte infusion plus interleukin-2 for relapsed acute leukemia after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17:1308–1315. doi: 10.1016/j.bbmt.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 19.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, Martin P, Chien J, Przepiorka D, Couriel D, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Atkinson K, Horowitz MM, Gale RP, Lee MB, Rimm AA, Bortin MM. Consensus among bone marrow transplanters for diagnosis, grading and treatment of chronic graft-versus-host disease. Committee of the International Bone Marrow Transplant Registry. Bone Marrow Transplant. 1989;4:247–254. [PubMed] [Google Scholar]
- 21.Chalandon Y, Passweg JR, Schmid C, Olavarria E, Dazzi F, Simula MP, Ljungman P, Schattenberg A, de Witte T, Lenhoff S, et al. Outcome of patients developing GVHD after DLI given to treat CML relapse: a study by the Chronic Leukemia Working Party of the EBMT. Bone Marrow Transplant. 2010;45:558–564. doi: 10.1038/bmt.2009.177. [DOI] [PubMed] [Google Scholar]
- 22.Alyea EP, Soiffer RJ, Canning C, Neuberg D, Schlossman R, Pickett C, Collins H, Wang Y, Anderson KC, Ritz J. Toxicity and efficacy of defined doses of CD4(+) donor lymphocytes for treatment of relapse after allogeneic bone marrow transplant. Blood. 1998;91:3671–3680. [PubMed] [Google Scholar]
- 23.Flowers ME, Traina F, Storer B, Maris M, Bethge WA, Carpenter P, Appelbaum F, Storb R, Sandmaier BM, Martin PJ. Serious graft-versus-host disease after hematopoietic cell transplantation following nonmyeloablative conditioning. Bone Marrow Transplant. 2005;35:277–282. doi: 10.1038/sj.bmt.1704767. [DOI] [PubMed] [Google Scholar]
- 24.Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W, Ljungman P, Ferrant A, Verdonck L, Niederwieser D, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86:2041–2050. [PubMed] [Google Scholar]
- 25.Bleakley M, Riddell SR. Molecules and mechanisms of the graft-versus-leukaemia effect. Nat Rev Cancer. 2004;4:371–380. doi: 10.1038/nrc1365. [DOI] [PubMed] [Google Scholar]
- 26.Liu Z, Savoldo B, Huls H, Lopez T, Gee A, Wilson J, Brenner MK, Heslop HE, Rooney CM. Epstein-Barr virus (EBV)-specific cytotoxic T lymphocytes for the prevention and treatment of EBV-associated post-transplant lymphomas. Recent Results Cancer Res. 2002;159:123–133. doi: 10.1007/978-3-642-56352-2_15. [DOI] [PubMed] [Google Scholar]
- 27.Vogelsang GB, Lee L, Bensen-Kennedy DM. Pathogenesis and treatment of graft-versus-host disease after bone marrow transplant. Annu Rev Med. 2003;54:29–52. doi: 10.1146/annurev.med.54.101601.152339. [DOI] [PubMed] [Google Scholar]
- 28.Collins RH, Jr, Goldstein S, Giralt S, Levine J, Porter D, Drobyski W, Barrett J, Johnson M, Kirk A, Horowitz M, et al. Donor leukocyte infusions in acute lymphocytic leukemia. Bone Marrow Transplant. 2000;26:511–516. doi: 10.1038/sj.bmt.1702555. [DOI] [PubMed] [Google Scholar]
- 29.Levine JE, Braun T, Penza SL, Beatty P, Cornetta K, Martino R, Drobyski WR, Barrett AJ, Porter DL, Giralt S, et al. Prospective trial of chemotherapy and donor leukocyte infusions for relapse of advanced myeloid malignancies after allogeneic stem-cell transplantation. J Clin Oncol. 2002;20:405–412. doi: 10.1200/JCO.2002.20.2.405. [DOI] [PubMed] [Google Scholar]
- 30.Dazzi F, Szydlo RM, Cross NC, Craddock C, Kaeda J, Kanfer E, Cwynarski K, Olavarria E, Yong A, Apperley JF, et al. Durability of responses following donor lymphocyte infusions for patients who relapse after allogeneic stem cell transplantation for chronic myeloid leukemia. Blood. 2000;96:2712–2716. [PubMed] [Google Scholar]
- 31.Salama M, Nevill T, Marcellus D, Parker P, Johnson M, Kirk A, Porter D, Giralt S, Levine JE, Drobyski W, et al. Donor leukocyte infusions for multiple myeloma. Bone Marrow Transplant. 2000;26:1179–1184. doi: 10.1038/sj.bmt.1702685. [DOI] [PubMed] [Google Scholar]
- 32.Porter DL, Collins RH, Jr, Hardy C, Kernan NA, Drobyski WR, Giralt S, Flowers ME, Casper J, Leahey A, Parker P, et al. Treatment of relapsed leukemia after unrelated donor marrow transplantation with unrelated donor leukocyte infusions. Blood. 2000;95:1214–1221. [PubMed] [Google Scholar]
- 33.van Rhee F, Savage D, Blackwell J, Orchard K, Dazzi F, Lin F, Chase A, Bungey J, Cross NC, Apperley J, et al. Adoptive immunotherapy for relapse of chronic myeloid leukemia after allogeneic bone marrow transplant: equal efficacy of lymphocytes from sibling and matched unrelated donors. Bone Marrow Transplant. 1998;21:1055–1061. doi: 10.1038/sj.bmt.1701224. [DOI] [PubMed] [Google Scholar]
- 34.Lokhorst HM, Wu K, Verdonck LF, Laterveer LL, van de Donk NW, van Oers MH, Cornelissen JJ, Schattenberg AV. The occurrence of graft-versus-host disease is the major predictive factor for response to donor lymphocyte infusions in multiple myeloma. Blood. 2004;103:4362–4364. doi: 10.1182/blood-2003-11-3862. [DOI] [PubMed] [Google Scholar]
- 35.Deol A, Lum LG. Role of donor lymphocyte infusions in relapsed hematological malignancies after stem cell transplantation revisited. Cancer Treat Rev. 2010;36:528–538. doi: 10.1016/j.ctrv.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi SJ, Lee JH, Kim S, Seol M, Lee YS, Lee JS, Kim WK, Chi HS, Lee KH. Treatment of relapsed acute myeloid leukemia after allogeneic bone marrow transplantation with chemotherapy followed by G-CSF-primed donor leukocyte infusion: a high incidence of isolated extramedullary relapse. Leukemia. 2004;18:1789–1797. doi: 10.1038/sj.leu.2403523. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
a. Chronic myeloid leukemia (CML). 1- and 3-year overall survival for patients who received DLI for relapsed CML after allogeneic HCT were 81% and 81% for cell dose A, 64% and 46% for cell dose B and 50% and 50% cell dose C (P =0.07).
b. Multiple myeloma(MM)/Chronic lymphocytic leukemia (CLL)/Lymphoma. 1- and 3-year overall survival for patients who received DLI for relapsed MM/CLL/Lymphoma were 85% and 43% for cell dose A, 91% and 64% for cell dose B and 46% and 31% cell dose C (P =0.25).
c. Acute myeloid leukemia (AML)/Myelodysplastic syndrome (MDS). 1- and 3-year overall survival for patients who received DLI for relapsed AML or MDS were 45% and 32% for cell dose A, 47% and 40% for cell dose B and 52% and 28% cell dose C (P =0.99).