Abstract
The National Drug Abuse Treatment Clinical Trials Network (CTN) has faced many challenges over its first eleven years. This review explores some of these challenges and the paths the CTN took to meet these challenges, including: designing clinical trials that reflect the CTN’s mission and changing public health needs, finding the synergies in the varied expertise of clinical treatment providers and academic researchers, promoting evidence-based practices and expanding the Network into mainstream medical practices to reach a broader patient population. Included in this exploration are specific examples from CTN clinical trials.
Keywords: Clinical Trials Network, drug abuse, addiction
Introduction
The Institute of Medicine released the report, ‘Bridging the Gap Between Practice and Research: Forging Partnerships with Community-Based Drug and Alcohol Treatment’, in 1998, documenting the inadequate connection between research knowledge and community practice in the field of substance abuse treatment and research.1 The report urged the responsible federal agencies to establish an infrastructure to facilitate research within community-based substance abuse treatment programs and to foster true research partnerships with treatment providers. The National Institute on Drug Abuse (NIDA) of the United States (US) National Institutes of Health subsequently established the National Drug Abuse Treatment Clinical Trials Network (CTN), with the stated goal of accelerating the translation of science-based addiction treatments into community-based practice.2
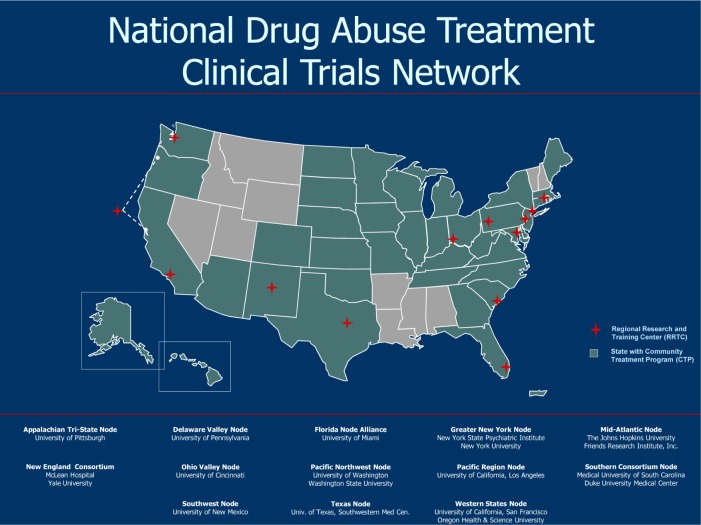
The CTN is organized into 13 nodes centered in university-based research centers that are aligned with healthcare providers from more than 240 community-based substance abuse treatment programs in 39 states across the nation, the District of Columbia, and Puerto Rico (Figure 1). The dozens of scientists in the network are affiliated with more than 50 universities. The network strives to foster collaboration between researchers and treatment providers throughout the entire research process, thereby enhancing the transferability and acceptability of research results by the practice community and their patients.2 As of December 2010, researchers in the CTN have enrolled over 12,000 trial participants, completed 24 major clinical trials (Table 1), published over 190 scientific papers in peer-reviewed journals,3 and contributed to the development of three comprehensive training and treatment tools, including Buprenorphine Treatment, Short-Term Opioid Withdrawal Using Buprenorphine, and Promoting Awareness of Motivational Incentives, for dissemination throughout the addiction treatment and research community.4,5
Figure 1.
National Drug Abuse Treatment Clinical Trials Network.
Table 1.
Status of Clinical Trials Network clinical trials (December 2010)
| Protocol # (Referencea) | Principal Investigator | Study | Sites | N | Status |
|---|---|---|---|---|---|
| CTN 0001 | Ling | Buprenorphine/naloxone for inpatient detoxification | 6 | 113 | Completed (Ling 2005)37 |
| CTN 0002 | Ling | Buprenorphine/naloxone for outpatient detoxification | 6 | 230 | Completed (Ling 2005)37 |
| CTN 0003 | Ling | Comparison of two buprenorphine/naloxone taper schedules | 11 | 516 | Completed (Ling 2009)38 |
| CTN 0004 | Carroll | Motivational enhancement therapy for patients in treatment for substance use disorders | 6 | 496 | Completed (Ball 2007)39 |
| CTN 0005 | Carroll | Motivational interviewing for patients in treatment for substance use disorders | 5 | 423 | Completed (Carroll 2006)40 |
| CTN 0006 | Stitzer | Low cost motivational incentives for stimulant-abusing patients in outpatient psychosocial treatment programs | 8 | 454 | Completed (Petry 2005)28 |
| CTN 0007 | Stitzer | Low cost motivational incentives for stimulant-abusing patients in methadone maintenance treatment | 6 | 403 | Completed (Peirce 2006)27 |
| CTN 0009 | Reid | Incorporating smoking cessation treatment into substance abuse treatment programs | 12 | 225 | Completed (Reid 2008)41 |
| CTN 0010 | Woody | Buprenorphine/naloxone-facilitated rehabilitation for opioid-dependent adolescents and young adults | 6 | 154 | Completed (Woody 2008)42 |
| CTN 0011 | Hubbard | Telephone enhancement procedure for long-term engagement in continuing care | 4 | 339 | Completed (Hubbard 2007)43 |
| CTN 0013 | Winhusen | Motivational enhancement therapy for pregnant women in treatment for substance use disorders | 4 | 200 | Completed (Winhusen 2008)44 |
| CTN 0014 | Szapocznik, Robbins | Brief Strategic Family Therapy for adolescents in treatment for substance use disorders | 8 | 457 | Manuscript in preparation |
| CTN 0015 | Hien | Seeking Safety therapy for women with PTSD in treatment for substance use disorders | 7 | 353 | Completed (Hien 2009)45 |
| CTN 0017 | Booth | HIV/HCV risk reduction interventions for injection substance users | 8 | 632 | Completed (Booth 2010)46 |
| CTN 0018 | Calsyn | HIV/STD risk reduction interventions for men in treatment for substance use disorders | 14 | 594 | Completed (Calsyn 2009)47 |
| CTN 0019 | Tross | HIV/STD risk reduction interventions for women in treatment for substance use disorders | 12 | 517 | Completed (Tross 2008)48 |
| CTN 0020 | Svikis | Job seekers training for patients in treatment for substance use disorders | 12 | 628 | Manuscript in preparation |
| CTN 0021 | Carroll, Szapocznik | Motivational enhancement therapy for Spanish-speaking patients in treatment for substance use disorders | 6 | 463 | Completed (Carroll 2009)49 |
| CTN 0027 | Ling | Liver function in patients maintained on buprenorphine/naloxone or methadone | 9 | 1269 | Manuscript in preparation |
| CTN 0028 | Riggs, Winhusen | Osmotic-release methylphenidate for ADHD in adolescents in treatment for substance use disorders | 11 | 303 | Manuscript in preparation |
| CTN 0029 | Somoza, Winhusen | Osmotic-release methylphenidate for ADHD in patients receiving smoking cessation treatment | 6 | 255 | Completed (Winhusen 2010)50 |
| CTN 0030 | Ling, Weiss | Treatment of prescription opioid addiction | 11 | 653 | Manuscript in preparation |
| CTN 0031 | Donovan | Twelve-step engagement for patients in treatment for stimulant use disorders | 10 | 471 | Manuscript in preparation |
| CTN 0032 | Metsch | HIV rapid testing and counseling in substance abuse treatment programs | 12 | 1281 | Manuscript in preparation |
| CTN 0037 | Trivedi | Exercise as an adjunctive treatment for substance use disorders | 9 | 330b | Enrolling |
| CTN 0044 | Nunes | Web-delivered treatment for substance use disorders | 10 | 500b | Enrolling |
| CTN 0046 | Winhusen | Smoking cessation intervention for patients in treatment for stimulant use disorders | 12 | 528b | Enrolling |
| CTN 0047 | Bogenschutz, Donovan | Screening, motivational assessment, referral, and treatment in emergency departments | 6 | 1285b | Enrolling |
| CTN 0048 | Ling | Buprenorphine and naltrexone for treatment of cocaine dependence | 12b | 300b | Development |
| CTN 0049 | Metsch | Linkage-to-care interventions for HIV-infected substance-using hospital inpatients | 10b | 800b | Development |
Notes:
See References for full citation;
Planned study parameters.
Abbreviations: HIV, human immunodeficiency virus; HCV, hepatitis C virus; STD, sexually transmitted disease; PTSD, posttraumatic stress disorder; ADHD, attention deficit hyperactivity disorder.
However, implementing randomized clinical trials across multiple and diverse substance abuse treatment programs presents several unique challenges that need to be addressed in order to achieve the stated goals. In this review, we discuss the nature of these challenges and the network’s strategies to address them in the following four areas:
Designing clinical trial protocols: new trial paradigms were developed with the aim of efficiently answering the practical clinical questions that often fall outside the scope of more traditional randomized trial models.
Implementing quality clinical trials in practice settings unfamiliar with research logistics: balance must be kept between the practical needs and research knowledge of the clinician’s practice and the need to fulfill ethical, data integrity, and regulatory requirements for human subjects’ protection in clinical research.
Promoting the adoption of evidence-based treatment practices: research products must meet the community’s needs and stay within community resource constraints.
Expanding the network to include research sites that are part of US mainstream medical care: new populations of patients who do not typically seek treatment in specialty-care clinics devoted to substance abuse treatment must be reached.
Designing clinical trial protocols
The CTN’s first group of studies evaluated the effectiveness of contingency management, motivational interviewing, and buprenorphine detoxification.4 Those studies met one of the CTN’s original criteria for developing new research: that the efficacy of the particular therapy under consideration for CTN testing must have been demonstrated in prior research. This criterion set the expectation that therapies selected for CTN trials would be ready to implement in “real world” settings soon after the evidence supporting their use was validated in an effectiveness trial.6
Because there were a limited number of interventions that had reached this late stage of development, it became necessary to apply the original criteria more judiciously for choosing promising treatment interventions. It was recognized that the network’s “blended” infrastructure of researchers and community-based treatment providers lends itself to studies aimed at developing and testing interventions on which relatively little prior efficacy research has been conducted, with an eye on ensuring that the treatments are sustainable in actual practice. Thus, the CTN broadened its research agenda to include evaluations of an expanded range of promising treatment interventions.
In some cases, the CTN’s treatment providers bring to the table experience with, or interest in, an intervention or clinical practice that is widely employed despite uncertain scientific grounding and hence warrants rigorous evaluation. One such example is Seeking Safety, an integrated cognitive-behavioral treatment intervention designed for patients with comorbid posttraumatic stress disorder (PTSD) symptoms and substance use disorders.7 The CTN undertook a randomized multisite trial of Seeking Safety, for which there was limited empirical support at the time, because it was among the most promising approaches for targeting this type of comorbidity and had been adopted by many treatment programs.8–11 The CTN-affiliated treatment providers’ experience with this intervention contributed greatly to the success of this trial’s design and execution. Two significant adaptations were made to the Seeking Safety treatment program to improve the feasibility of studying it in the CTN: 1) the number of sessions was reduced from 25 to 12; and 2) new enrollees immediately entered rolling therapy groups instead of waiting for a new group to start the program with its introductory first session. Thus, therapy groups did not consist of the same cohort of individuals from start to finish. Both adaptations were made to adjust to standard practices carried out in community treatment programs (CTPs) participating in the CTN. The study’s findings have provided important support for addressing PTSD early in substance abuse treatment and have spurred further research in this area.12
At other times, new disorders, or newly affected populations, may rapidly emerge as urgent public health concerns. The CTN’s standing translational research platform can facilitate a telescoped trial program to quickly test and refine treatment approaches that address such needs. One example is the recent increase in the prevalence of prescription opioid abuse problems in the US.13,14 Most treatment studies of opioid-dependent populations have focused on heroin users,15 and the utility of applying those studies’ findings to a potentially quite different patient population is uncertain. Many of the CTN-affiliated treatment providers have dealt with this gap in knowledge firsthand as they see a growing number of patients with problems related to prescription opioid abuse and dependence. As a result, the CTN launched the Prescription Opioid Addiction Treatment Study in 2006 to address this pressing public health concern. Building from existing opioid dependence treatment modalities, this trial applied an innovative two-phase adaptive, sequential treatment design to test the effectiveness of buprenorphine/naloxone treatment plus individual drug counseling for opioid analgesic dependence.16 This trial’s primary outcome findings are expected to be published in 2011.
Implementing quality multisite trials in community-based treatment settings
Moving research from academic, well controlled environments to the context of community-based real world healthcare settings may strengthen the external validity of the interventions and enhance the generalizability and adopt-ability of study results. During the CTN’s first decade, addiction treatment centers affiliated with the CTN were typically specialty substance abuse treatment programs in community settings. These have included hospital-based programs, inpatient and outpatient rehabilitation facilities, and mental health centers. CTN trials conducted in these programs have drawn upon a diverse population of treatment-seeking patients. However, the various CTPs are operated under very different treatment philosophies, funding streams, regulatory rules/policies, and organizational management and staffing.17 These differences, in turn, impact the choice of patient populations and considerations of specific types of CTPs to be included in each CTN trial. They also influence choices of realistic primary outcome measures appropriate to the trial design.18 For instance, the study protocol of the Motivational Incentives (also known as contingency management) trials (CTN 0006, 0007) was divided into two separate trials to address patients enrolled in two different treatment settings: methadone treatment programs and psychosocial outpatient treatment centers.
Another challenge of designing multisite, controlled effectiveness studies in the CTN involves the choice of the most appropriate comparison treatment. Experimental treatments are often added to existing or commonly used treatments in the experimental group, and the existing treatment alone is offered to participants in the control group. Many CTN trials have used Treatment as Usual (TAU) as their control condition, but the variety of treatment settings described above requires an equally wide variety of TAU practices. The actual treatment delivered at one CTP as TAU may be very different from the TAU practices delivered at another CTP.19 To ensure the interpretability of trial findings, the CTN generally seeks to select sites with similar TAU practices for a given trial. McCarty et al baseline study results17 and Roman et al independent surveys20,21 of CTPs within the CTN provide some of the information required to evaluate potential sites on this criterion. From there, study investigators survey and interview prospective CTP sites to gather more specific information about their treatment settings and TAU practices. The ultimate aims are to conduct trials with reasonably homogeneous background or control treatments, and to be able to precisely characterize these elements of the trial design.
Promoting the adoption of evidence-based treatment practices
Facilitating the dissemination of evidence-based treatments into community practice has always been an integral part of the CTN mission. The CTN established a Dissemination Subcommittee (later renamed the Research Utilization Committee) to plan and implement dissemination strategies for the network. The CTN’s current dissemination strategy emphasizes a) dissemination within the CTN and b) close collaboration with the Substance Abuse and Mental Health Services Administration and other stakeholders (eg, the National Association of State Alcohol/Drug Abuse Directors) that devote considerable resources and expertise to dissemination-related research and active dissemination efforts.
Operationally, the CTN is guided by the following approaches: a) bringing researchers and providers together to generate research questions that can impact practice, b) conducting trials in real world treatment settings with diverse treatment-seeking populations to improve external validity, and c) exposing clinicians to evidence-based treatments via participation in CTN trials.21 It is recognized that the process of disseminating research results to impact practice is slow and difficult and that it should be considered from the beginning of trial planning.22,23
These approaches have made a notable impact on the dissemination of motivational incentives as studied in the CTN.4,24 CTN providers raised several major dissemination issues during the research development process. The providers objected to the high costs of, and the appropriateness of, positive reinforcement for addiction treatment; some believed that it could be counterproductive to provide incentives. Their concern regarding the cost of the incentives stemmed in part from the fixed level of reimbursement provided by third-party payers for the care of substance use disorders. Incentive payments were unlikely to be added to the reimbursement if clinically implemented, and were too costly to be supported by the provider alone.25,26 The CTN therefore modified the research protocols to employ low-cost prize-drawing incentives that would be affordable in community programs.24,27,28 Once these CTN studies had demonstrated the feasibility of implementing incentive procedures and showed a significant positive impact on patients’ engagement in treatment and drug use, several CTN-affiliated treatment providers became early adopters and promoted the use of the intervention in their communities.4,24,29,30
Another dissemination example is the adoption of buprenorphine for the treatment of opioid withdrawal. The two CTN trials conducted by Ling et al31 have demonstrated that buprenorphine/naloxone is more effective than an alternative clonidine treatment in curbing opioid withdrawal. Compared to clonidine, buprenorphine was found to be five times more effective in outpatient settings and three times more effective in inpatient settings in a 13-day detoxification schedule.4,31 Of note, CTPs that participated in these two studies embraced these findings and almost immediately adopted buprenorphine/naloxone for short-term detoxification in their practices. This experience corroborates the concept that programs directly exposed to the research intervention are more likely to adopt it, relative to those that are not exposed.21
These examples clearly demonstrate that dissemination can be achieved by a) promoting the use of sound interventions that are compatible with the adopter’s values and experience, b) increasing the exposure of providers/clinicians to the research process in order to engage potential early adopters, and c) encouraging early adopters to champion the use of new interventions.22,32 Additional information from the network also has shown that the rate of uptake of research findings into practice relates only minimally to the demonstrated treatment effect size of a given intervention, but is more significantly influenced by cost and other environmental factors, such as state policies regulating treatment and general attitudes toward agonist treatment in addiction.33
Taken together, the CTN has continued to address the dissemination of its research products across varying settings and contexts: eg, why do established interventions lose effectiveness over days, weeks, or months? Why do tested interventions sometimes exhibit unintended effects when transferred to a new setting? How can multiple interventions be effectively packaged to capture cost efficiencies? Ultimately, it is not enough to generate evidence-based interventions; it is just as important to understand how to optimize intervention delivery in practice.34
Expansion of the network to address unmet needs
The CTPs participating in the CTN can reach only a small fraction of the people who need substance abuse treatment. Recent data have indicated that of an estimated 23.5 million Americans who needed treatment for an illicit drug or alcohol use problem in 2009, only 2.6 million had actually received treatment at a specialty facility (hospital, rehabilitation facility, or mental health center) in the past year. Four million persons were treated for substance use or medical problems associated with substance use in emergency departments, private doctors’ offices, prison or jail, or self-help groups.35
NIDA and the CTN believe that broadening the scope of the network’s constituent treatment providers will broaden the scope of its research and benefit patients with substance use disorders. One significant step towards this goal is the clinical trial titled Screening Motivational Assessment and Referral to Treatment in Emergency Departments (SMART-ED). The trial is presently enrolling participants in six hospital emergency departments around the country and will evaluate a screening and brief intervention process to identify individuals with substance use, abuse, or dependence and to provide timely interventions and referral to treatment as indicated.2
The SMART-ED project is built on CTN-affiliated researchers’ collaborations with researchers and clinicians in emergency departments. NIDA has sought to extend and for-malize such outreach efforts. NIDA’s 2009 funding announcement for applications to participate in the CTN requested that prospective nodes engage health service entities outside the traditional substance abuse treatment practice system.36 In the future, this expanded network will facilitate research efforts that span the broad spectrum of treatment settings in which substance abuse patients encounter the healthcare system.
Summary and conclusion
The CTN has faced many challenges since its inception in 1999, beginning with a difficult-to-treat patient population and, initially, a lack of research experience among many of the clinicians participating in the network. Likewise, academic researchers lacked insight into the unique needs of various treatment clinics. These differing areas of expertise and perspectives initially hindered appropriate decision-making regarding what to study in the network.
The academic researchers and the community-based clinicians worked collaboratively to develop innovative research paradigms to address the practical clinical questions posed by clinicians in the CTPs and across the field at large. Rather than forcing experimental designs into standard templates, investigators in the CTN have gone outside the scope of more traditional randomized trial models to adapt research interventions to the needs of community treatment centers. Furthermore, many providers in the network had identified a clinical need and adopted the Seeking Safety intervention to treat drug-abusing patients suffering from PTSD without prior conclusive evidence of its effectiveness. Here, the CTN’s role was to objectively evaluate the effectiveness of treatment interventions after their dissemination. The CTN continues to test new practices for addiction treatment for which efficacy has not been adequately characterized.
The CTN studies have shown that quality clinical trials can be successfully implemented in practice settings unfamiliar with research logistics by taking clinicians’ practical needs and research knowledge level into account. The CTN has promoted the adoption of evidence-based treatment practices by researching questions posed by the treatment community and delivering treatments that do not strain community resources.
The CTN is now expanding the network to include research sites that are part of US mainstream medical care. This will allow the treatment community to reach new populations of people with substance use disorders who have not typically sought treatment in specialty care clinics devoted to substance abuse treatment. The ongoing SMART-ED study explores the value of reaching out to untreated individuals with addiction problems by engaging them in hospital emergency departments. At this time, there is little evidence of efficacy to support this strategy in substance use other than alcohol, and therefore, this study is an attempt by the CTN to expand opportunities to offer existing treatments to the segment of the drug-abusing population that utilizes the mainstream healthcare system. The challenges yet to be faced in this effort seem large, but not as large as the potential for improvements in public health.
We have sought to characterize our experiences in a few aspects of this unique venture that might generalize to other efforts of clinical research, translation, and dissemination. Fundamental to the successes of the network has been a willingness to adapt to the street-level realities of clinical research in this field while keeping an eye on the goals of the program, the expertise of the people in the network, and expert advice offered by many outside the network. We eagerly look forward to the many challenges to be faced in the future of NIDA’s CTN.
Footnotes
Disclosure
The authors are employees of the Center for the Clinical Trials Network of the National Institute on Drug Abuse, National Institutes of Health, the funding agency for the National Drug Abuse Treatment Clinical Trials Network. The opinions in this manuscript are those of the authors and do not represent the official position of the US government.
References
- 1.Lamb SJ, Greenlick MR, McCarty D. Bridging the gap between practice and research: forging partnerships with community-based drug and alcohol treatment. Washington, DC: National Academy Press; 1998. [PubMed] [Google Scholar]
- 2.National Drug Abuse Research Clinical Trials Network Available from: http://drugabuse.gov/ctn/index.phpAccessed December 20, 2010
- 3.National Drug Abuse Treatment Clinical Trials Network – Dissemination Library November2009Available from: http://ctndisseminationlibrary.org/default.htmAccessed December 20, 2010
- 4.Tai B, Straus MM, Liu D, Sparenborg S, Jackson R, McCarty D. The first decade of the National Drug Abuse Treatment Clinical Trials Network: bridging the gap between research and practice to improve drug abuse treatment. J Subst Abuse Treat. 2010;38(Suppl 1):S4–S13. doi: 10.1016/j.jsat.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Addiction Technology Transfer Center Network Available from: http://www.attcnetwork.org/index.aspAccessed December 20, 2010
- 6.Wells EA, Saxon AJ, Calsyn DA, Jackson TR, Donovan DM. Study results from the Clinical Trials Network’s first 10 years: where do they lead? J Subst Abuse Treat. 2010;38(Suppl 1):S14–S30. doi: 10.1016/j.jsat.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Najavits L. Seeking safety: a treatment manual for PTSD and substance abuse. New York: Guilford Press; 2002. [Google Scholar]
- 8.Schafer I, Najavits LM. Clinical challenges in the treatment of patients with posttraumatic stress disorder and substance abuse. Curr Opin Psychiatry. 2007;20(6):614–618. doi: 10.1097/YCO.0b013e3282f0ffd9. [DOI] [PubMed] [Google Scholar]
- 9.Rubin A, Springer DW. Treatment of traumatized adults and children. Hoboken, NJ: Wiley; 2009. [Google Scholar]
- 10.Hien DA, Wells EA, Jiang H, et al. Multisite randomized trial of behav-ioral interventions for women with co-occurring PTSD and substance use disorders. J Consult Clin Psychol. 2009;77(4):607–619. doi: 10.1037/a0016227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hien DA, Jiang H, Campbell AN, et al. Do treatment improvements in PTSD severity affect substance use outcomes? A secondary analysis from a randomized clinical trial in NIDA’s Clinical Trials Network. Am J Psychiatry. 2010;167(1):95–101. doi: 10.1176/appi.ajp.2009.09091261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Back SE. Toward an improved model of treating co-occurring PTSD and substance use disorders. Am J Psychiatry. 2010;167(1):11–13. doi: 10.1176/appi.ajp.2009.09111602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilson AM, Ryan KM, Joranson DE, Dahl JL. A reassessment of trends in the medical use and abuse of opioid analgesics and implications for diversion control: 1997–2002. J Pain Symptom Manage. 2004;28(2):176–188. doi: 10.1016/j.jpainsymman.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 14.SAMHSA, Office of Applied Studies . The NSDUH Report: trends in nonmedical use of prescription pain relievers: 2002 to 2007. Rockville, MD: DHHS; 2009. [Google Scholar]
- 15.Weiss RD, Potter JS, Copersino ML, et al. Conducting clinical research with prescription opioid dependence: defining the population. Am J Addict. 2010;19(2):141–146. doi: 10.1111/j.1521-0391.2009.00017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiss RD, Potter JS, Provost SE, et al. A multi-site, two-phase, Prescription Opioid Addiction Treatment Study (POATS): rationale, design, and methodology. Contemp Clin Trials. 2010;31(2):189–199. doi: 10.1016/j.cct.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCarty D, Dilonardo J, Argeriou M. State substance abuse and mental health managed care evaluation program. J Behav Health Serv Res. 2003;30(1):7–17. doi: 10.1007/BF02287809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hohmann AA, Shear MK. Community-based intervention research: coping with the “noise” of real life in study design. Am J Psychiatry. 2002;159(2):201–207. doi: 10.1176/appi.ajp.159.2.201. [DOI] [PubMed] [Google Scholar]
- 19.Santa Ana EJ, Martino S, Ball SA, Nich C, Frankforter TL, Carroll KM. What is usual about “treatment-as-usual”? Data from two multisite effectiveness trials. J Subst Abuse Treat. 2008;35(4):369–379. doi: 10.1016/j.jsat.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roman PM, Abraham AJ, Rothrauff TC, Knudsen HK. A longitudinal study of organizational formation, innovation adoption, and dissemination activities within the National Drug Abuse Treatment Clinical Trials Network. J Subst Abuse Treat. 2010;38(Suppl 1):S44–S52. doi: 10.1016/j.jsat.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ducharme LJ, Knudsen HK, Roman PM, Johnson JA. Innovation adoption in substance abuse treatment: exposure, trialability, and the Clinical Trials Network. J Subst Abuse Treat. 2007;32(4):321–329. doi: 10.1016/j.jsat.2006.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogers EM. Diffusion of innovations. 5th ed. New York: Free Press; 2003. [Google Scholar]
- 23.Sox HC. Evaluating off-label uses of anticancer drugs: time for a change. Ann Intern Med. 2009;150(5):353–354. doi: 10.7326/0003-4819-150-5-200903030-00110. [DOI] [PubMed] [Google Scholar]
- 24.Stitzer ML, Petry NM, Peirce J. Motivational incentives research in the National Drug Abuse Treatment Clinical Trials Network. J Subst Abuse Treat. 2010;38(Suppl 1):S61–S69. doi: 10.1016/j.jsat.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carroll KM, Rounsaville BJ. Bridging the gap: a hybrid model to link efficacy and effectiveness research in substance abuse treatment. Psychiatr Serv. 2003;54(3):333–339. doi: 10.1176/appi.ps.54.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sindelar JL, Olmstead TA, Peirce JM. Cost-effectiveness of prize-based contingency management in methadone maintenance treatment programs. Addiction. 2007;102(9):1463–1471. doi: 10.1111/j.1360-0443.2007.01913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peirce JM, Petry NM, Stitzer ML, et al. Effects of lower-cost incentives on stimulant abstinence in methadone maintenance treatment: a National Drug Abuse Treatment Clinical Trials Network study. Arch Gen Psychiatry. 2006;63(2):201–208. doi: 10.1001/archpsyc.63.2.201. [DOI] [PubMed] [Google Scholar]
- 28.Petry NM, Peirce JM, Stitzer ML, et al. Effect of prize-based incentives on outcomes in stimulant abusers in outpatient psychosocial treatment programs: a national drug abuse treatment clinical trials network study. Arch Gen Psychiatry. 2005;62(10):1148–1156. doi: 10.1001/archpsyc.62.10.1148. [DOI] [PubMed] [Google Scholar]
- 29.Kellogg SH, Burns M, Coleman P, Stitzer M, Wale JB, Kreek MJ. Something of value: the introduction of contingency management interventions into the New York City Health and Hospital Addiction Treatment Service. J Subst Abuse Treat. 2005;28(1):57–65. doi: 10.1016/j.jsat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Stitzer M, Kellogg S. Large-scale dissemination efforts in drug abuse treatment clinics. In: Higgins ST, Silverman K, Heil SH, editors. Contingency Management in Substance Abuse. 1st ed. New York, NY: Guilford Press; 2008. pp. 241–260. [Google Scholar]
- 31.Ling W, Jacobs P, Hillhouse M, et al. From research to the real world: buprenorphine in the decade of the Clinical Trials Network. J Subst Abuse Treat. 2010;38(Suppl 1):S53–S60. doi: 10.1016/j.jsat.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berwick DM. Disseminating innovations in health care. JAMA. 2003;289(15):1969–1975. doi: 10.1001/jama.289.15.1969. [DOI] [PubMed] [Google Scholar]
- 33.Saxon AJ, McCarty D. Challenges in the adoption of new pharmaco-therapeutics for addiction to alcohol and other drugs. Pharmacol Ther. 2005;108(1):119–128. doi: 10.1016/j.pharmthera.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 34.Woolf SH, Johnson RE. The break-even point: when medical advances are less important than improving the fidelity with which they are delivered. Ann Fam Med. 2005;3(6):545–552. doi: 10.1370/afm.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.SAMHSA . Results from the 2009 National Survey on Drug Use and Health: Volume 1: summary of national findings. Rockville, MD: DHHS; 2010. NSDUH Series H-38A. [Google Scholar]
- 36.The National Drug Abuse Treatment Clinical Trials Network (U10) National Institute on Drug Abuse. National Institutes of Health; 2009. [Google Scholar]
- 37.Ling W, Amass L, Shoptaw S, et al. A multi-center randomized trial of buprenorphine-naloxone versus clonidine for opioid detoxification: findings from the National Institute on Drug Abuse Clinical Trials Network. Addiction. 2005;100(8):1090–1100. doi: 10.1111/j.1360-0443.2005.01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ling W, Hillhouse M, Domier C, et al. Buprenorphine tapering schedule and illicit opioid use. Addiction. 2009;104(2):256–265. doi: 10.1111/j.1360-0443.2008.02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ball SA, Martino S, Nich C, et al. Site matters: multisite randomized trial of motivational enhancement therapy in community drug abuse clinics. J Consult Clin Psychol. 2007;75(4):556–567. doi: 10.1037/0022-006X.75.4.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carroll KM, Ball SA, Nich C, et al. Motivational interviewing to improve treatment engagement and outcome in individuals seeking treatment for substance abuse: a multisite effectiveness study. Drug Alcohol Depend. 2006;81(3):301–312. doi: 10.1016/j.drugalcdep.2005.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reid MS, Fallon B, Sonne S, et al. Smoking cessation treatment in community-based substance abuse rehabilitation programs. J Subst Abuse Treat. 2008;35(1):68–77. doi: 10.1016/j.jsat.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 42.Woody GE, Poole SA, Subramaniam G, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008;300(17):2003–2011. doi: 10.1001/jama.2008.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hubbard RL, Leimberger JD, Haynes L, et al. Telephone enhancement of long-term engagement (TELE) in continuing care for substance abuse treatment: a NIDA clinical trials network (CTN) study. Am J Addict. 2007;16(6):495–502. doi: 10.1080/10550490701641678. [DOI] [PubMed] [Google Scholar]
- 44.Winhusen T, Kropp F, Babcock D, et al. Motivational enhancement therapy to improve treatment utilization and outcome in pregnant substance users. J Subst Abuse Treat. 2008;35(2):161–173. doi: 10.1016/j.jsat.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hien DA, Wells EA, Jiang H, et al. Multisite randomized trial of behavioral interventions for women with co-occurring PTSD and substance use disorders. J Consult Clin Psychol. 2009;77(4):607–619. doi: 10.1037/a0016227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Booth RE, Campbell BK, Mikulich-Gilbertson SK, et al. Reducing HIV-Related Risk Behaviors Among Injection Drug Users in Residential Detoxification. AIDS Behav. 2010;10(1):3–8. doi: 10.1007/s10461-010-9751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Calsyn DA, Hatch-Maillette M, Tross S, et al. Motivational and skills training HIV/sexually transmitted infection sexual risk reduction groups for men. J Subst Abuse Treat. 2009;37(2):138–150. doi: 10.1016/j.jsat.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tross S, Campbell AN, Cohen LR, et al. Effectiveness of HIV/STD sexual risk reduction groups for women in substance abuse treatment programs: results of NIDA Clinical Trials Network Trial. J Acquir Immune Defic Syndr. 2008;48(5):581–589. doi: 10.1097/QAI.0b013e31817efb6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carroll KM, Martino S, Ball SA, et al. A multisite randomized effectiveness trial of motivational enhancement therapy for Spanish-speaking substance users. J Consult Clin Psychol. 2009;77(5):993–999. doi: 10.1037/a0016489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winhusen TM, Somoza EC, Brigham GS, et al. Impact of attention-deficit/hyperactivity disorder (ADHD) treatment on smoking cessation intervention in ADHD smokers: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2010 May 18; doi: 10.4088/JCP.09m05089gry. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]