Abstract
Promotion of apoptosis in cancer cells could potentially lead to the regression and improved prognosis of hormone-refractory prostate cancer. Xanthohumol (XN), a prenylated chalcone-derived from hops, has shown strong antitumorigenic activity towards diverse types of cancer cells. In the present study, the growth-inhibitory and apoptosis-inducing activity of XN was tested in hormone-sensitive and hormone-refractory human prostate cancer cells lines. Cell growth/viability assay (MTS) demonstrated that prostate cancer cells are highly sensitive to XN at a concentration range of 20-40 μM. The primary mode of tumor cell destruction was apoptosis as demonstrated by the binding of annexin V-FITC, cleavage of PARP-1, activation of procaspases -3, -8, and -9, mitochondrial depolarization and release of cytochrome c from mitochondria. Induction of apoptosis by XN was associated with the inhibition of prosurvival Akt, NF-κB and mTOR signaling proteins and NF-κB-regulated anti-apoptotic Bcl-2 and survivin. These studies provide a rationale for clinical evaluation of XN for the treatment of hormone-refractory metastatic prostate cancer.
Keywords: Xanthohumol, prostate cancer, apoptosis, prosurvival signaling proteins
Carcinoma of the prostate (CaP) is the most commonly diagnosed cancer and the second leading cause of cancer-related mortality in men in the United States. Current therapies, such as androgen deprivation, radical prostatectomy, local radiotherapy or brachytherapy, while effective against localized disease, are mostly ineffective against metastatic disease (1, 2). Effective chemotherapeutic agents for prostate cancer are unavailable at present. Since the incidence of CaP increases with advancing age, prostate cancer is expected to become an increasingly greater problem as life expectancy improves. Novel treatment modalities are therefore needed to treat hormone-resistant tumors and to prevent the progression of hormone-sensitive prostate cancer to hormone-refractory stage.
Epidemiological and laboratory studies suggest that diet plays an important role in prevention of human diseases. Long-term consumption of plant-derived foods including vegetables, fruits, beans, and nuts has been linked to a low incidence of cancer, coronary heart disease and inflammatory diseases (3, 4). The disease-preventing effects of plant-derived foods have been attributed to the presence of polyphenolic phytochemicals with strong anti-inflammatory and antioxidant activity as well as their ability to modulate a range of cell signaling pathways involved in cellular proliferation, differentiation, survival and apoptosis (5). Indeed, there has been an increasing use of dietary supplements to prevent and/or treat prostate cancer.
Hops or ‘hop cones’, the female inflorescence of the hop plant, Humulus lupus L., are widely used in beer brewing to add bitterness and flavor to beer. Hop extract contains polyphenolic acids, prenylated chalcones, flavonoids, catechins and proanthocyanidins. Xanthohumol (3-[3,3-dimethyl allyl]-2,4,4-trihydroxychalcone; XN) is the principal prenylated flavonoid found in hop resin (6). Recent reports have shown the potential health benefits of XN (7, 8). XN has been shown to inhibit the growth of a wide variety of human cancer cell lines including breast, colon, prostate, ovarian and blood cancers by inhibiting proliferation and inducing apoptosis (9-12). Others have shown the inhibition of tumor cell invasion and angiogenesis and the activity of topoisomerase 1 and aromatase by XN (13-16).
In the present study, the response of both hormone-sensitive and hormone-refractory prostate cancer cell lines to XN was investigated. The results demonstrated that XN inhibited the growth of prostate cancer cells by inducing apoptosis. XN induced apoptosis through the activation of procaspases -3, -8 -9 and mitochondrial depolarization. Further, induction of apoptosis was associated with the inhibition of prosurvival Akt, NF-κB and mTOR signaling molecules and antiapoptotic Bcl2 and survivin.
Materials and Methods
Reagents
XN was purchased from Alexis Biochemicals (San Diego, CA, USA). Anti-caspase-3, -caspase-8, and -caspase-9 antibodies were purchased from BD Pharmingen (San Diego, CA, USA). Anti-NF-κΒ (p65), anti-Bcl-2, anti-Bcl-xL, and anti-survivin antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). 96 AQueous One Solution Proliferation Assay System was from Promega (Madison, WI, USA).
A 100 mM solution of XN was prepared in DMSO and all test concentrations were prepared by diluting the stock solution in tissue culture medium.
Cell lines
Hormone-sensitive LNCaP (AR+) and hormone-refractory PC-3 (AR–) and DU145 (AR–) human prostate cancer cell lines were obtained from American Type Culture Collection (ATCC), Rockville, MD, USA. Hormone-refractory (AR+) prostate cancer cell line C4-2 derived from LNCaP cell line was obtained from Dr. Svend Freytag, Henry Ford Hospital, Detroit, MI, USA. All cell lines were cultured at 37°C in a humidified atmosphere consisting of 5% CO2 and 95% air, and maintained by subculturing cells twice a week as previously described (17).
Cell viability assay
Cells (1×104) were seeded into each well of a 96-well plate in 100 μl of tissue culture medium. After 24 h incubation to allow cells to adhere, cells were treated with XN at concentrations of 5 μM to 40 μM for 72 h. Cell viability was then determined by the colorimetric MTS assay using CellTiter 96 AQueous One Solution Proliferation Assay System from Promega (Madison, WI, USA) as previously described (17).
Annexin V-FITC binding
Induction of apoptosis was assessed by the binding of annexin V-FITC to phosphotidylserine, which is externalized to the outer leaflet of the plasma membrane early during induction of apoptosis. Briefly, untreated cells and cells treated with XN for 72 h were resuspended in the binding buffer provided in the annexin V-FITC apoptosis detection kit II (BD Biosciences, Pharmingen) and reacted with 5 μl of annexin V-FITC reagent and 5 μl of propidium iodide (PI) for 30 min at room temperature in the dark. Stained cells were analyzed by fluorescent activated cell sorting (FACS) on a FACScan flow cytometer (Becton Dickinson).
Mitochondrial depolarization assay
The effect of XN on mitochondrial potential was determined using mitochondrial potential sensor JC-1 (Molecular Probes, Invitrogen, San Diego, CA, USA). A total of 1×106 control cells or cells treated with XN for 72 h were loaded with JC-1 (10 μg/ml) for 10 minutes at 22°C and analyzed by flow cytometry. In normal cells, dye is aggregated in mitochondria, fluoresces red, and is detected in the FL2 channel. In cells with altered mitochondrial potential, the dye fails to accumulate in the mitochondria, remains as monomers in the cytoplasm, fluoresces green, and is detected in the FL1 channel.
Measurement of cytochrome c release
To determine whether XN induces the release of cytochrome c from mitochondria, C4-2 cells were treated with 5 to 40 μM XN for 72 h. Using ApoAlert Cell Fractionation Kit (Clontech, Laboratories Inc., CA, USA), mitochondrial fractions were prepared from treated and untreated control cells and fractionated (10 μg) on a 12% SDS-PAGE gel. After transfer of proteins, membranes were probed with cytochrome c antibody.
Extraction of nuclear proteins
Nuclear extracts were prepared as described previously (17). Following treatment with XN for 72 h, cells were washed three times with PBS and incubated on ice for 15 minutes in hypotonic buffer A (10 mM HEPES, pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 0.5 mM PMSF, and 0.6% NP40). Cells were vortexed gently for lysis and nuclei were separated from the cytosol by centrifugation at 12,000 × g for 1 minute. Nuclei were resuspended in buffer C (20 mM HEPES, pH 7.9, 25% glycerol, 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 0.5 mM PMSF) and shaken for 30 minutes at 4°C. Nuclear extracts were obtained by centrifugation at 12,000 ×g and protein concentration measured by Bradford assay (Bio Rad, Richmond, CA, USA). NF-κB in nuclear extracts was detected by Western blotting as described below.
Western blotting
Total cellular proteins were isolated by detergent lysis (1% Triton-X 100 (v/v), 10 mM Tris-HCl (pH 7.5), 5 mM EDTA, 150 mM NaCl, 10% glycerol, 2 mM sodium vanadate, 5 μg/ml leupeptin, 1 μg/ml aprotinin, 1 μg/ml pepstatin A, and 10 μg/ml 4-2-aminoethyl-benzenesulfinyl fluoride). Lysates were clarified by centrifugation at 14,000 ×g for 10 min at 4°C and protein concentrations were determined by Bradford assay. Protein samples (50 μg) were separated on 10-14% SDS-polyacrylamide gels. Proteins resolved on the gels were transferred to nitrocellulose membranes and probed with protein specific antibodies to NF-κB (1:500), p-Akt (1:500), p-mTOR (1:1000), Bcl-2 (1:500), Bcl-xL (1:1000), survivin (1:500) or β-actin (1:500) followed by HRP-conjugated secondary antibody as described previously (17).
Statistical analysis
Data are expressed as means±S.D.
Results
XN inhibits the growth of prostate cancer cells
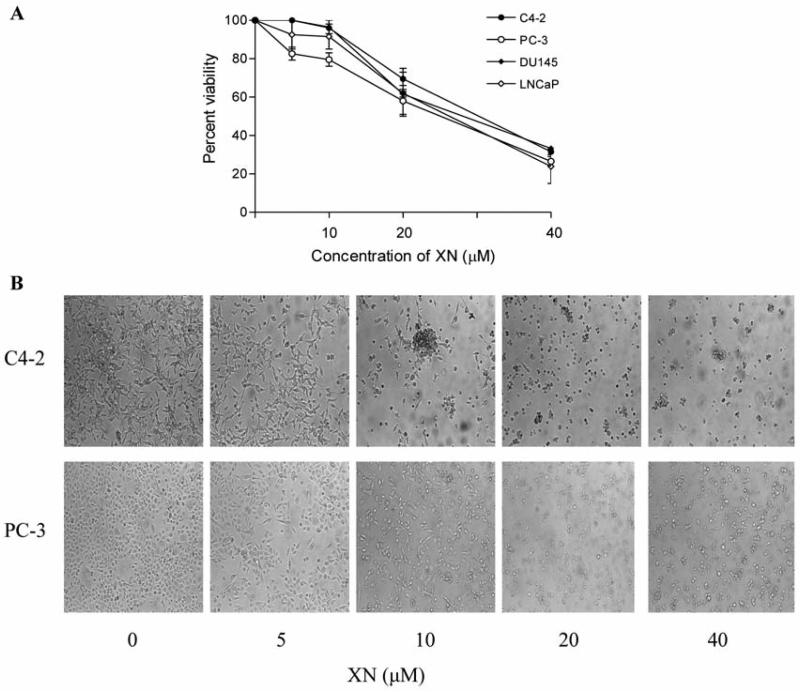
To test the effect of XN on proliferation of prostate cancer cells, LNCaP, C4-2, PC-3 or DU145 cells were treated with XN (5 to 40 μM) for 72 h and viability determined by MTS assay. As shown in Figure 1A, XN was ineffective in inhibiting the growth of any of the prostate cancer cell lines at 5 and 10 μM. Moderate growth inhibition was observed at 20 μM XN (LNCaP=41%; C4-2=30%; PC-3=42%, DU145=38%). However, at 40 μM, XN potently inhibited the growth of all cell lines (LNCaP=81%; C4-2=69%; PC-3=74%, DU145=67% inhibition). These data indicate that XN moderately to significantly inhibited the growth of prostate cancer cells at a concentration range of 20 to 40 μM.
Figure 1.
Effect of XN on the viability of prostate cancer cells. A: 1×104 LNCaP, C4-2, PC-3 and DU145 cells were separately seeded in each well of a microtiter plate in 0.1 ml of culture medium. Cells were allowed to adhere for 24 h before treating with XN at concentrations of 0 to 40 μM in triplicates for 72 h. Cell viability was measured by MTS assay using CellTiter AQueous assay system from Promega. Similar results were obtained in 3 independent experiments. B: Morphological changes in cell cultures (C4-2 and PC-3 cells) treated or not with XN for 72 h as visualized by light microscopy. Similar results were obtained in two independent experiments.
Microscopic examination of cancer cell cultures (C4-2 and PC-3) showed partial rounding of cells at 10 μM XN and complete detachment and significant cell death (trypan blue dye exclusion) at higher concentrations of XN (20-40 μM) after treatment for 72 h (Figure 1B).
XN induces apoptosis in prostate cancer cells
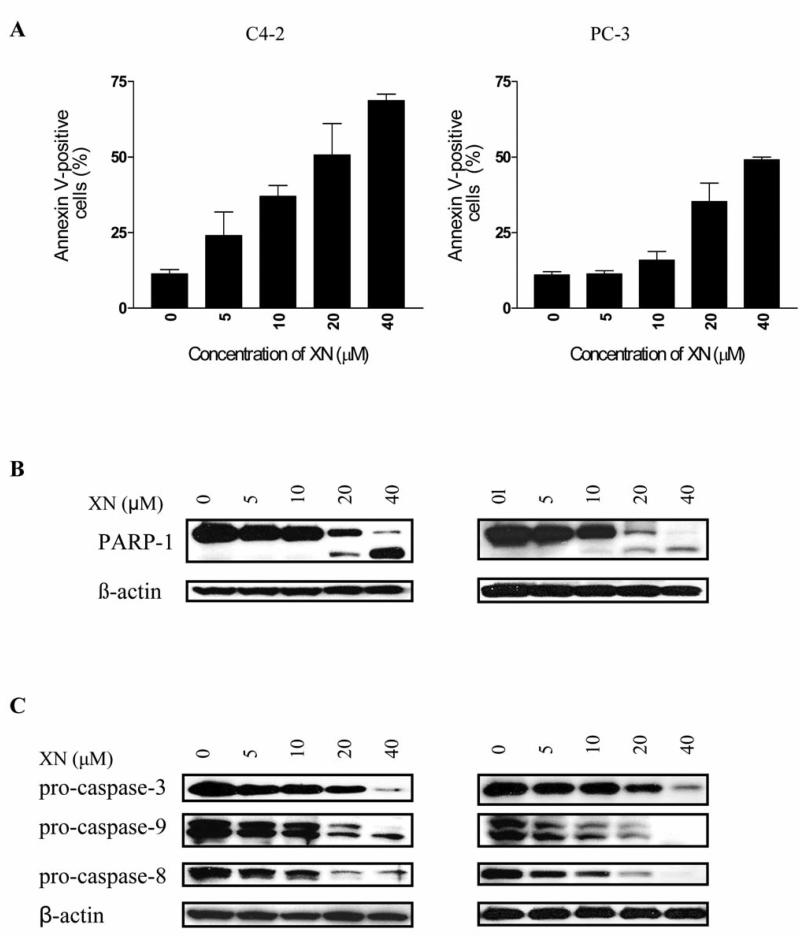
Whether XN kills prostate cancer cells by inducing apoptosis was investigated by the binding of annexin V-FITC and cleavage of PARP-1 and procaspases-3, -8 and -9. As shown in Figure 2A, a small percentage of untreated PC-3 and C4-2 cells bound annexin V-FITC (~11%). There was a dose-dependent increase in the percentage of annexin V-FITC-binding C4-2 cells (e.g., 24%, 37%, 50% and 69% at 5, 10, 20 and 40 μM XN, respectively). In the case of PC-3 cells, an appreciable increase in annexin V-FITC-binding cells was observed following treatment with XN at 20 μM (35%), which increased to 49% at 40 μM XN. In addition, the native PARP-1 (110 kDa) protein was almost completely cleaved at 20 and 40 μM XN with the appearance of 89 kDa cleavage product in both cell lines (Figure 2B).
Figure 2.
Treatment with XN induces apoptosis in prostate cancer cells. A: XN increases annexin V-FITC binding. C4-2 and PC-3 cells were treated with XN at concentrations of 5 to 40 μM for 72 h. Cells were then reacted with 5 μl of annexin V-FITC reagent for 30 min at room temperature. The percentage of annexin V-FITC-positive tumor cells was determined by flow cytometry. Results are presented as percentage of annexin V-FITC-binding cells. B: XN cleaves PARP-1. Cells were treated with XN as described above and cleavage of PARP-1 was analyzed by immunoblotting. C: XN cleaves procaspases -3, -8 and -9. C4-2 and PC-3 cells were treated with XN at concentrations of 5 to 40 μM 72 h and cleavage of procaspases-3, -8 and -9 was probed by Western blotting. Each experiment was repeated at least two times.
Further evidence that XN kills prostate cancer cells by inducing apoptosis was provided by the effect of XN on procaspases 3, -8, and -9. C4-2 and PC-3 cells treated with XN showed almost complete processing of procaspases -3, -8, and -9 at 20 and 40 μM XN (Figure 2C) as determined from the decrease or disappearance of the native (basal) procaspases in treated cells. Some reduction in the levels of procaspases was also measurable at 10 μM XN. Together, these data demonstrate that XN kills prostate cancer cells by inducing apoptosis.
XN induces mitochondrial depolarization and release of cytochrome c
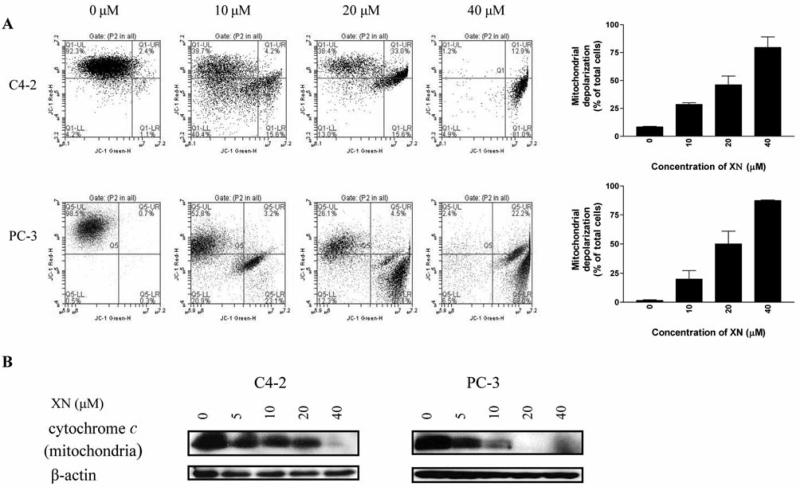
The cleavage of procaspase-9 suggested the involvement of mitochondrion in induction of apoptosis in prostate cancer cells by XN. To further confirm this, a JC-1 probe was used to evaluate mitochondrial depolarization in C4-2 and PC-3 cells treated with XN. There was a significant change in mitochondrial potential after treatment with XN for 72 h. The percentage of cells with green fluorescence increased from 8% at 0 XN to 28%, 46% and 79 % at 10, 20 and 40 μM XN, respectively (Figure 3A). The mitochondrial integrity in PC-3 cells was identically affected by XN (percentage of cells with green fluorescence: 1% 20%, 50% and 87% at 0, 10, 20 and 40 μM XN, respectively).
Figure 3.
XN induces mitochondrial depolarization and release of cytochrome c. A: C4-2 and PC-3 cells were treated with XN at 0 to 40 μM for 20 h; 1×106 cells were resuspended in 1 ml culture medium and loaded with mitochondrial potential sensor JC-1 (10 μg/ml) for 10 minutes at 22°C. Cells were analyzed by flow cytometry for fluorescence emission. Data are shown as flow cytographs of cells fluorescing red (FL2 channel) or green (FL1 channel). Histograms show percentage of cells with loss of mitochondrial potential difference. B: C4-2 and PC-3 cells were treated with 0 to 40 μM XN for 72 h and mitochondrial cytochrome c levels were analyzed by Western blotting as described in Materials and Methods. Similar results were obtained in two separate experiments.
XN also caused the release of cytochrome c from mitochondria. As shown in Figure 3B, treatment of C4-2 and PC-3 cells with XN for 72 h reduced the level of mitochondrial cytochrome c in a dose-dependent manner with complete depletion occurring at 40 μM XN in both cell lines.
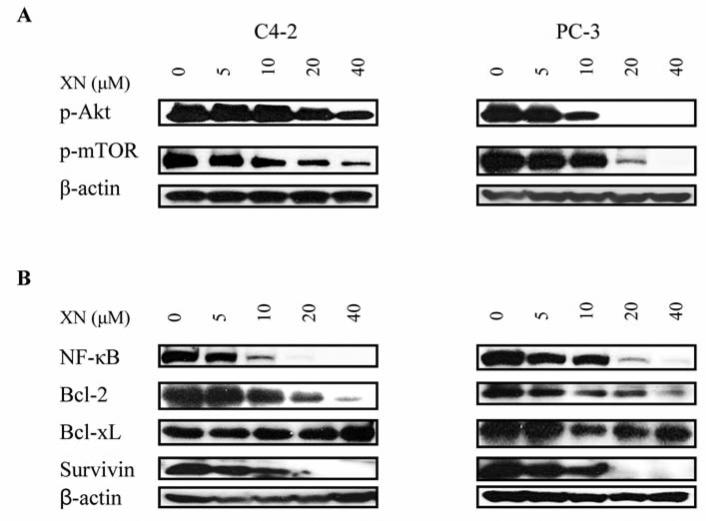
XN inhibits prosurvival Akt, mTOR and NF-κB signaling proteins and antiapoptotic Bcl-2 and survivin
Akt, NF-κB and mTOR signaling pathways confer survival advantage and resistance to anticancer therapies. The effect of XN on these signaling proteins was investigated in C4-2 and PC-3 cells. XN significantly inhibited p-Akt in C4-2 cells at 20 and 40 μM (50 to 70% reduction), but it completely inhibited p-Akt in PC-3 cells at these concentrations (Figure 4A). XN also significantly to completely inhibited p-mTOR and NF-κB in both cell lines at concentrations of 10 μM and above. In addition, XN reduced the levels of antiapoptic proteins in both cell lines (Figure 4B). The levels of Bcl-2 and survivin were markedly reduced at 10 to 40 μM XN. However, Bcl-xL was mostly unaffected by XN in C4-2 cells but was reduced in PC-3 cells at concentrations of 10 to 40 μM. Together, these data suggested that prosurvival Akt, mTOR and NF-κB and antiapoptotic Bcl-2, survivin and perhaps Bcl-xL are targets of XN in prostate cancer cells.
Figure 4.
XN inhibits prosurvival p-Akt, NF-κB and p-mTOR and antiapoptotic Bcl-2, Bcl-xL and survivin. C2-4 and PC-3 cells were treated with XN at 0 to 40 μM for 72 h. After treatment, cell lysates were prepared and analyzed by Western blotting using anti-p-Akt antibody, anti-NF-κB (p65), anti-p-mTOR antibodies (A) or anti-Bcl-2, ant-Bcl-xL, anti-survivin antibody (B) or anti-β-actin antibody (loading control).
Discussion
Natural products have been used in traditional medicine for centuries to treat a variety of human diseases. Hop cones are used as sedatives, antispasmodic, bitter stomachic and microbicidal in folk medicine. Recent laboratory studies have shown that flavonones and chalcones present in hops have numerous biological effects, including chemopreventive, antiproliferative, antioxidant and anti-inflammatory activities (8, 9, 18, 19). XN inhibited the growth of a wide variety of human cancer cell lines including breast, colon, prostate, ovarian and blood cancer by inhibiting proliferation and inducing apoptosis (9-12).
In the present study, XN inhibited the growth of both hormone-sensitive and hormone-refractory human prostate cancer cells. Furthermore, hormone refractory AR– (PC-3) and AR+ (C4-2) cells were both equally sensitive to XN. XN was most effective at a concentration range of 20 to 40 μM. The growth inhibitory effect of XN was attributed to the induction of apoptosis as determined by the increased binding of annexin V-FITC to treated cells. In addition, XN induced the cleavage of PARP-1. The growth inhibitory and apoptosis inducing effects of XN in prostate cancer cells observed in this study are consistent with those previously reported in other tumor models, including prostate cancer (10-12, 20). Together, these studies indicate that induction of apoptosis is part of the mechanism by which XN inhibits the growth of cancer cells including prostate cancer.
Two major pathways of apoptotic cell death program, namely death receptor-mediated (extrinsic) and chemical-induced mitochondrial (intrinsic) apoptosis have been identified. In both cases, caspases, a family of cysteine proteases, play an important role in apoptotic cell death (21, 22). XN caused the cleavage of initiator procaspase-8 and the effector procaspase-3 in PC-3 and C4-2 cells, suggesting that the death receptor-signaling pathway of apoptosis is involved in XN-induced apoptosis. Whether XN increases the expression of death receptors DR4 and DR5 has not been determined. XN also induced the cleavage of procaspase-9, mitochondrial depolarization, and release of cytochrome c from mitochondria, indicating that the intrinsic pathway of apoptosis is also activated by XN. It is unclear at this time if induction of both pathways of apoptosis by XN is unique to the prostate cancer cells or common to the induction of apoptosis by XN in all types of cancer cells.
Akt/PBK is a major antiapoptotic pathway which is frequently hyperactivated in most types of cancer (23). Activated p-Akt promotes cell growth and survival by inactivating downstream substrates such as Bad, procaspase-9, and Forkhead transcription factors. Antiapoptotic NF-κB and progrowth mTOR signaling pathways are downstream targets of activated Akt/PBK and control the expression of genes involved cell proliferation, oncogenesis, angiogenesis, and apoptosis (24, 25). XN inhibited p-Akt, NF-κB and p-mTOR in prostate cancer cells, indicating that the inhibition of these prosurvival signaling proteins is essential for induction of apoptosis by XN. NF-κB-regulated Bcl-2, Bcl-xL and survivin are major antiapoptotic proteins that render cancer cells resistant to anticancer agents. The inhibition of Bcl-2 and survivin indicated that they are targets of XN in prostate cancer cells. The inhibition of Akt by XN would explain the inhibition of mTOR, since mTOR is a downstream target of Akt. XN has been shown to prevent the activation of NF-κB in leukemia cells by inhibiting the phosphorylation and degradation of IκBα, an inhibitor of NF-κB (26). It is likely that XN inhibits NF-κB in prostate cancer cells through a similar mechanism. How XN inhibits Akt remains to be determined. Overall, the growth inhibition and induction of apoptosis in prostate cancer cells by XN suggests that XN is a novel agent for therapeutic development to treat hormone-refractory prostate cancer.
Acknowledgements
This work was supported by NIH grant 1R01 130948.
References
- 1.Garnick MB. Hormonal therapy in the management of prostate cancer: From Higgins to the present. Urology. 1997;49:5–15. doi: 10.1016/s0090-4295(97)00163-5. [DOI] [PubMed] [Google Scholar]
- 2.Hanks GE. Long-term control of prostate cancer with radiation. Urol Clin North Amer. 1996;23:605–616. doi: 10.1016/s0094-0143(05)70339-6. [DOI] [PubMed] [Google Scholar]
- 3.Block G, Patterson B, Subar A. Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr Cancer. 1992;18:1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- 4.Hu FB, Stampfer MJ, Manson JE, Rimm E, Colditz GA, Rosner BA, Hennekens CH, Willett WC. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med. 1997;337:1491–1499. doi: 10.1056/NEJM199711203372102. [DOI] [PubMed] [Google Scholar]
- 5.Surh Y. Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic substances. Mutat Res. 1995;428:305–327. doi: 10.1016/s1383-5742(99)00057-5. [DOI] [PubMed] [Google Scholar]
- 6.Stevens JF, Taylor AW, Deinzer ML. Quantitative analysis of xanthohumol and related prenylflavonoids in hops and beer by liquid chromatography-tandem mass spectrometry. J Chromatogr. 1999;832:97–107. doi: 10.1016/s0021-9673(98)01001-2. [DOI] [PubMed] [Google Scholar]
- 7.Stevens JF, Page JE. Xanthohumol and related prenylflavonoids from hops and beer: to your good health! Phytochemistry. 2004;65:1317–1330. doi: 10.1016/j.phytochem.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 8.Gerhauser C, Alt A, Heiss E, Gamal-Eldeen A, Klimo K, Knauft J, Neumann I, Scherf HR, Frank N, Bartsch H, Becker H. Cancer chemopreventive activity of Xanthohumol, a natural product derived from hop. Mol Cancer Ther. 2002;1:959–969. [PubMed] [Google Scholar]
- 9.Delmulle L, Bellahcene A, Dhooge W, Comhaire F, Roelens F, Huvaere K, Heyerick A, Castronovo V, De Keukeleire D. Anti-proliferative properties of prenylated flavonoids from hops (Humulus lupulus L.) in human prostate cancer cell lines. Phytomedicine. 2006;13:732–734. doi: 10.1016/j.phymed.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Colgate EC, Miranda CL, Stevens JF, Bray TM, Ho E. Xanthohumol, a prenylflavonoid derived from hops induces apoptosis and inhibits NF-kappaB activation in prostate epithelial cells. Cancer Lett. 2006;246:201–209. doi: 10.1016/j.canlet.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Lust S, Vanhoecke B, Janssens A, Philippe J, Bracke M, Offner F. Xanthohumol kills B-chronic lymphocytic leukemia cells by an apoptotic mechanism. Mol Nutr Food Res. 2005;49:844–850. doi: 10.1002/mnfr.200500045. [DOI] [PubMed] [Google Scholar]
- 12.Pan L, Becker H, Gerhauser C. Xanthohumol induces apoptosis in cultured 40-16 human colon cancer cells by activation of the death receptor- and mitochondrial pathway. Mol Nutr Food Res. 2005;49:837–843. doi: 10.1002/mnfr.200500065. [DOI] [PubMed] [Google Scholar]
- 13.Vanhoecke B, Derycke L, Marck VV, Depypere H, Keukeleire DD, Bracke M. Antiinvasive effect of xanthohumol, a prenylated chalcone present in hops (Humulus lupulus L.) and beer. Int J Cancer. 2005;117:889–895. doi: 10.1002/ijc.21249. [DOI] [PubMed] [Google Scholar]
- 14.Albini A, Dell'Eva R, Vene R, Ferrari N, Buhler DR, Noonan DM, Fassina G. Mechanisms of the antiangiogenic activity by the hop flavonoid xanthohumol: NFkappaB and Akt as targets. FASEB J. 2006;20:527–529. doi: 10.1096/fj.05-5128fje. [DOI] [PubMed] [Google Scholar]
- 15.Lee SH, Kim HJ, Lee JS, Lee IS, Kang BY. Inhibition of topoisomerase 1 activity and efflux drug transporter's expression by xanthohumol from hops. Arch Pharm Res. 2007;30:1435–1439. doi: 10.1007/BF02977368. [DOI] [PubMed] [Google Scholar]
- 16.Monteiro R, Faria A, Azevedo I, Calhau C. Modulation of breast cancer cell survival by aromatase inhibiting hop (Humulus lupus L.) flavonoids. J Steroid Bioche Mol Biol. 2007;105:124–130. doi: 10.1016/j.jsbmb.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 17.Deeb D, Gao X, Jiang H, Arbab AS, Dulchavsky SA, Gautam SC. CDDO-Me induces apoptosis and inhibits Akt, mTOR and NF-κB signaling proteins in prostate cancer cells. Anticancer Res. 2007;27:3035–3044. [PubMed] [Google Scholar]
- 18.Miranda CL, Stevens JF, Helmrich A, Henderson MC, Rodriguez RJ, Yang YH, Deinzer ML, Barnes DW, Buhler DR. Antiproliferative and cytotoxic effects of prenylated flavonoids from hops (Humulus lupulus) in human cancer cell lines. Food Chem Toxicol. 1999;37:271–285. doi: 10.1016/s0278-6915(99)00019-8. [DOI] [PubMed] [Google Scholar]
- 19.Miranda CL, Stevens JF, Ivanov V, McCall M, Frei B, Deinzer ML, Buhler DR. Antioxidant and prooxidant actions of prenylated and nonprenylated chalcones and flavanones in vitro. J Agric Food Chem. 2000;48:3876–3884. doi: 10.1021/jf0002995. [DOI] [PubMed] [Google Scholar]
- 20.Yang J-Y, Della-Fera MA, Rayalam S, Baile CA. Effects of xanthohumol and isoxanthohumol on 3T3-L1 cell apoptosis and adipogenesis. Apoptosis. 2007;12:1953–1963. doi: 10.1007/s10495-007-0130-4. [DOI] [PubMed] [Google Scholar]
- 21.Ashkenazi A, Dixit VM. Death receptors: Signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 22.Sun X-M, MacFarlane M, Zhuang J, Wolf BB, Green DR, Cohen GM. Distinct caspase cascades are initiated in receptor-mediated and chemical-induced apoptosis. J Biol Chem. 1999;274:5053–5060. doi: 10.1074/jbc.274.8.5053. [DOI] [PubMed] [Google Scholar]
- 23.Marte BM, Downward J. PKB/Akt: connecting phosphoinositide3-kinase to cell survival and beyond. Trends Biochem Sci. 1997;22:355–358. doi: 10.1016/s0968-0004(97)01097-9. [DOI] [PubMed] [Google Scholar]
- 24.Mayo MW, Baldwin AS. The transcription factor NF-κB: control of oncogenesis and cancer therapy resistance. Biochim Biophys Acta. 2000;1470:M55–M62. doi: 10.1016/s0304-419x(00)00002-0. [DOI] [PubMed] [Google Scholar]
- 25.Hidalgo M, Rowinsky EK. The rapamycin-sensitive signal transduction pathway as a target for cancer therapy. Oncogene. 2000;19:6680–6686. doi: 10.1038/sj.onc.1204091. [DOI] [PubMed] [Google Scholar]
- 26.Harikumar KB, Kunnumakkara AB, Ahn KS, Anand P, Krishnan S, Guha S, Aggarwal BB. Modification of the cysteine residues in I{kappa}B {alpha} kinase and NF-{kappa}B (p65) by xanthohumol leads to suppression of NF-{kappa}B-regulated gene products and potentiation of apoptosis in leukemia cells. Blood. 2009;113:2003–2013. doi: 10.1182/blood-2008-04-151944. [DOI] [PMC free article] [PubMed] [Google Scholar]