Abstract
The fate of the fragmented DNA (fDNA) observed in neuronal nuclei in Alzheimer brain is unknown. However, its fate is suggested as fDNA is found in the cytoplasm of adjacent activated microglia. After a brief incubation with fDNA, approximately 70% of microglia had fDNA in their cytoplasm, were activated, and overexpressed interleukin-1β. Microglial activation enhanced uptake whereas blocking scavenger receptors suppressed this uptake. These results suggest that the brain rids itself of fDNA from dying neurons through microglial uptake, activation, and overexpression of IL-1. Such overexpression of IL-1 in Alzheimer brain has been linked to Alzheimer pathogenesis.
Keywords: Alzheimer’s disease, Cytokines flow cytometry, fDNA, Microglia, Scavenger receptor, TUNEL
1. Introduction
Neuronal cell dysfunction and loss in specific brain regions is one of the major features underlying the cognitive decline of Alzheimer’s disease (AD). Increasing data suggest that such neurons, undergoing cell death either by a form of apoptosis or necrosis, manifest significant DNA fragmentation (Anderson et al., 1996; Sheng et al., 1998b; Smale et al., 1995; Velez-Pardo et al., 2001). The number of neurons having fragmented DNA (fDNA) correlates with the formation of neurofibrillary tangles in those neurons (Sheng et al., 1998b). Moreover, the number of DNA-damaged neurons found within or very near plaques increases as plaques mature from simple diffuse Aβ deposits to the more complex neuritic plaques, which are diagnostic of AD. Interestingly, there are virtually no neurons, with or without fDNA, associated with nonimmunogenic dense-core nonneuritic plaques (Sheng et al., 1998a). Such DNA-damaged neurons are also found in an experimental model of AD (Sheng et al., 2001). Although a variety of initiating factors of cell death have been extensively studied, very little is known regarding either the mechanisms by which the brain rids itself of fDNA from dying neurons, or regarding potential cascades triggered by these mechanisms.
Resident resting microglia are activated in response to either natural or induced neuronal cell death (Egensperger et al., 1996; Thanos, 1991), suggesting that activated microglia play an essential role in ridding the brain of such debris. Multiple scavenger microglial receptors are proposed mediators of such clearance as they promote microglial adhesion to fibrillar Aβ-containing surfaces (El Khoury et al., 1996) and ingestion of Aβ (Paresce et al., 1996).
Here we provide evidence of microglial involvement in the clearance of fDNA in AD. To address questions concerning the disposition of fDNA in AD, we demonstrated that microglia most often contain fDNA in their cytoplasm, rather than their nuclei, in Alzheimer brain and further verified that microglia in culture are activated with over-expression of interleukin-1β (IL-1β) as they internalize fDNA via specific identifiable processes.
2. Materials and methods
2.1. Tissue processing
Ten patients with postmortem neuropathological confirmation of Alzheimer’s disease according to the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) criteria (Mirra et al., 1991) and five neurologically normal controls of similar ages were used in this study. The right hippocampus and adjacent mesial temporal cortex at the level of lateral geniculate nucleus were processed as previously described (Sheng et al., 1998b) for Tdt-mediated dUTP nick end labeling (TUNEL) and immunohistochemical studies.
2.2. Cell cultures
Primary cultures of rat microglia were generated from the cortical tissues of neonatal (0—3 days) Sprague—Dawley rats, as previously described (Barger and Basile, 2001). The N9 mouse microglial cell line (Corradin et al., 1993) was maintained as previously described (Li et al., 2001). Microglial cells were seeded on culture dishes (Ф, 35 or 60 mm) for RNA assays, or grown on chambered slides (Nunc, Naperville, IL) for fluorescence microscopy.
2.3. Cell treatments
To examine whether microglia are capable of internalizing fDNA, we extracted genomic DNA mice (C57BL/6SJL) or rats (Sprague-Dawley) using a DNA Mini kit (Qiagen, Valencia, CA). The genomic DNA was sonicated in a Sonic Dismembrator (Fisher Model 60) and the fDNA was fluorescein-labeled using Fluorescein High-Prime labeling kit (Boehringer-Mannheim Biochemica, Indianapolis, IN) according to the manufacturer’s instruction. The labeled fDNA was purified using QIAquick Nucleotide removal kit (Qiagen). For cell sorting experiments, microglial cell cultures were incubated with fluorescein-labeled fDNA for 2 h (FCS assay and fluorescence microscopy), or up to 24 h (cytokine expression assay). Some experiments were performed in the presence of interferon-γ (IFNγ; Pepro-Tech, Rocky Hill, NJ; 10 ng/ml), fucoidan (40 ng/ml; Sigma), or antiscavenger receptor antibody (2F8; Serotec, Raleigh, NC; 1 µg/ml). In preparation for direct fluorescence microscopy or TUNEL detection to verify microglial phagocytosis of unlabelled fDNA, cultures on chamber slides were treated with either fluorescein-labeled fDNA or unlabeled fDNA, fixed for 30 min in 4% paraformaldehyde, and rinsed three times with phosphate-buffered saline (PBS).
2.4. Fluorescence-activated cell sorting (FACS) for analysis of fDNA phagocytosis
A total of 5 × 104 N9 microglia in 1 ml of DMEM were incubated with 1 µg of fluorescein-labeled fDNA for 2 h either alone or in the presence of fucoidan, IFN-γ, or IL-1β in a 5% CO2/95% air atmosphere. The cells were then washed twice with PBS and resuspended in 0.5 ml of PBS for FACS.
2.5. TUNEL labeling
TUNEL was performed as previously described (Sheng et al., 1998b) using an in situ Cell Death Detection Kit-POD (Boehringer-Mannheim Biochemica), and either directly examined using fluorescence microscopy or indirectly assayed by incubating with anti-fluorescein-POD for 30 min and visualizing with metal-enhanced diaminobenzidine substrate by light microscopical analysis.
2.6. Fluorescence microscopy and confocal microscopy
For fluorescence microscopy and digital image collection, a Nikon fluorescence microscope equipped with a charge-coupled device (CCD) camera was used. Laser confocal microscopy was employed to define the localization of fluorescein-labeled fDNA in the microglia.
2.7. Reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted from cultured cells using TriReagent™ RNA (Molecular Research Center, Cincinnati, OH). RT-PCR was performed as previously described (Li et al., 2000, 2001). The primer sequences for rat IL-1β were as follows: upstream, 5’ TGA CTC GTG GGA TGA TGA CG 3’; downstream, 5’ CTG GAG ACT GCC CAT TCT CG 3’. Equal volumes of reaction mixture from each sample were loaded onto 1.5% agarose gels, and fluorescent images were digitally captured for analysis of intensity with NIH Image software. Levels of IL-1β mRNA were normalized relative to G3PDH mRNA in the same sample.
2.8. Statistical analysis
Data were analyzed using an unpaired t test, and values were considered significantly different when the two-tailed p value was <0.05. Results are expressed as mean± S.E.M.
3. Results
3.1. TUNEL positivity is preferentially distributed to the cytoplasm, not nuclei, of microglia in Alzheimer brains
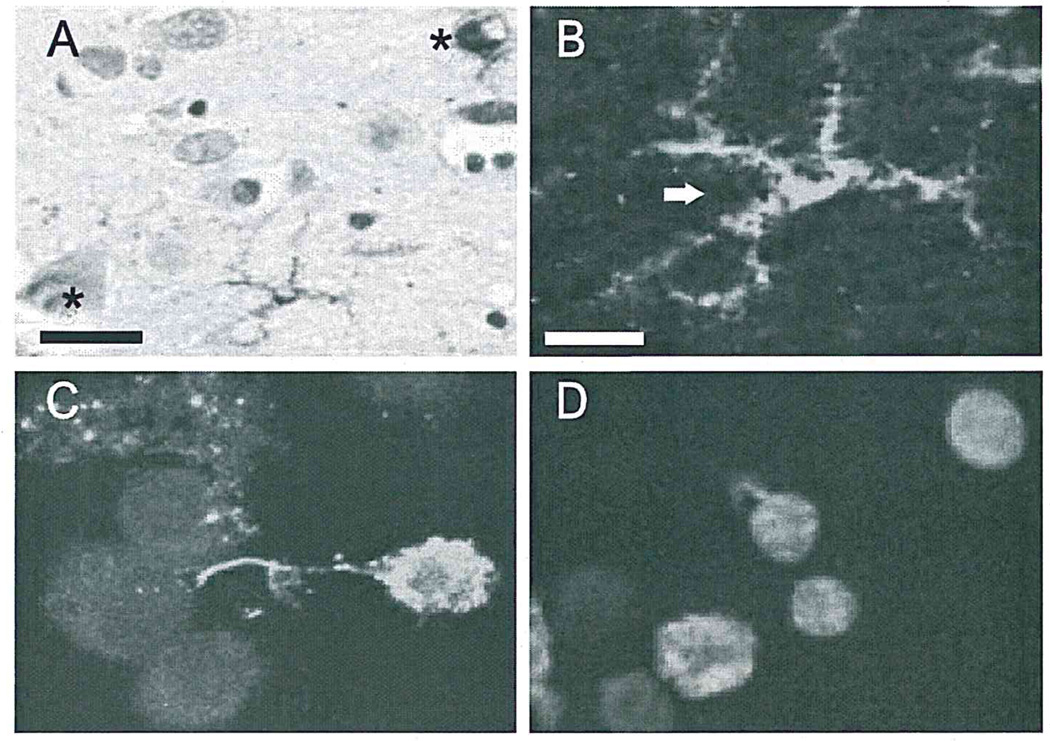
Consistent with previous reports on tissue sections from Alzheimer patients (Sheng et al., 1998a,b), the majority of TUNEL positivity is in the nuclei of neurons. We found that in addition to neuronal TUNEL, microglial cells exhibited TUNEL positivity. Some microglia were TUNEL-positive in both cytoplasm and nuclei, but in most, TUNEL positivity was restricted to the cytoplasm (Fig. 1A and B). These microglia were usually found either adjacent to neurons with TUNEL-positive nuclei or to Aβ plaques. Such views suggest that the DNA fragments in microglial cytoplasm were from nuclei of nearby dying neurons. The total number of TUNEL-positive microglia was significantly less in control than in AD (20.3 ± 5.53 vs. 3.75 ± 2.88 microglia/ mm2; p < 0.05).
Fig. 1.
Microglia take up DNA fragments in Alzheimer brain and in vitro. (A and B) TUNEL positivity in cytoplasm (arrow), but not nucleus, in microglia, and in nuclei (asterisk), but not cytoplasm, of neurons in Alzheimer temporal lobe. (A) and (B) are from the same field at different magnifications (A, bar = 25 µm; B, bar= 10 µm). (C) Confocal microscopy demonstrates fluorescein-labeled fDNA within the cytoplasm of N9 microglia. (D) TUNEL-positive fDNA was seen in N9 microglia exposed to unlabeled fDNA, confirming microglial phagocytosis of fDNA.
3.2. Microglial uptake of fDNA is via scavenger receptor
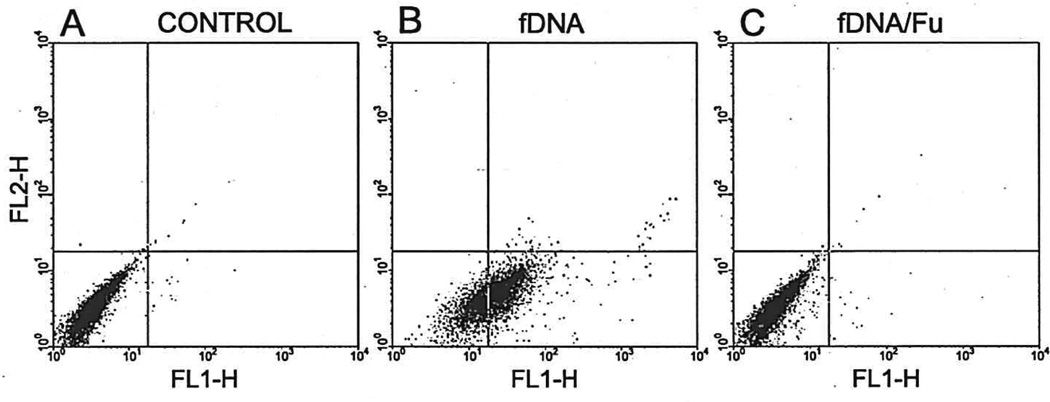
The cytoplasm of 72% of N9 microglia took up fDNA, following a 2-h incubation with fluorescein-labeled fDNA (1 µg/ml). Some N9 cells showed punctate fluorescence labeling first associated with the membrane and then within the cytoplasm (Fig. 1C). This phagocytosis was independent of fluorescein labeling as shown by TUNEL immunoreactivity following incubation with unlabeled fDNA (Fig. 1D). Untreated sister cultures, or those treated with intact DNA, showed neither fluorescence nor TUNEL positivity (data not shown). Microglia pretreated with either IL-1β (10 ng/ml) or interferon-γ (10 ng/ml), before incubation with fDNA, markedly enhanced the uptake capacity of microglia, suggesting that microglial activation state influences fDNA uptake (data not shown). Conversely, pretreatment with either antiscavenger receptor A antibody or fucoidan, a general inhibitor of scavenger receptors, decreased the uptake of fDNA. As shown by FAC analysis (Fig. 2), 72% of the fDNA-treated N9 microglia internalized the DNA fragments. Fucoidan (fDNA/Fu) completely blocked (Fig. 2C)—and antibody against class A type scavenger receptors partially blocked (data not shown)—this phagocytosis. This suggests that microglial uptake of fDNA was mediated by multiple scavenger receptor types.
Fig. 2.
Microglial uptake of DNA fragments is mediated by a scavenger receptor. (A) Untreated sister microglial cultures. (B) fDNA treatment of N9 microglia. (C) fDNA treatment of N9 in the presence of fucoidan (Fu), a general inhibitor of scavenger receptors analyzed by flow cytometry.
3.3. Microglia are activated by internalization of fragmented DNA
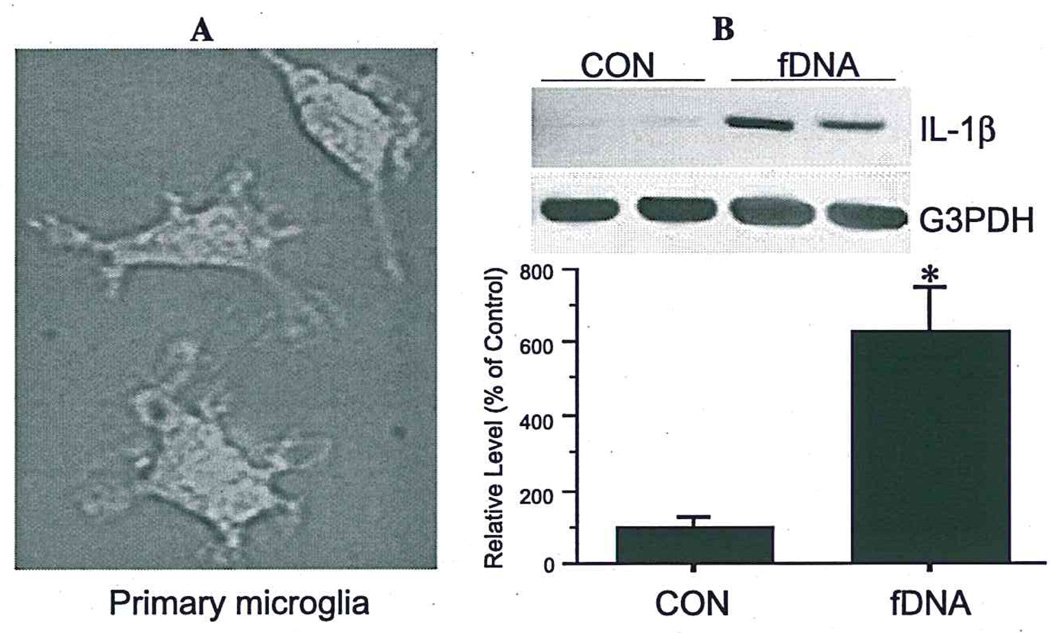
Treatment of primary microglia with fluorescein-labeled fDNA fragments resulted in significant uptake; 75% of cells were fluorescence-labeled after a 2-h incubation compared to untreated sister cultures (Fig. 3A). Such uptake of fDNA resulted in activation as shown by increased expression of IL-1β mRNA (5.3-fold) (Fig. 3B). Non-fDNA did not alter microglial IL-1β production (data not shown).
Fig. 3.
Microglia are activated by internalization of DNA fragments. Fluorescein-labeled fDNA uptake (A) significantly increases IL-1β mRNA expression in primary rat microglia (B). Values are expressed as mean ± S.E.M. of four samples (*p< 0.05).
4. Discussion
The data presented here show fragmented DNA in the cytoplasm, not nucleus, of activated microglia located adjacent to neurons with nuclear fDNA and to Aβ plaques in AD brain, suggesting that microglia take up DNA fragments from nearby damaged or dying neurons. Our in vitro experiments identify mechanisms involved in microglial internalization of fDNA. This uptake was via multiple scavenger receptor classes, concomitantly activating microglia and inducing excessive expression of IL-1, a cytokine known to regulate several neurodegenerative cascades, including phosphorylation of tau, decreased production of synaptophysin (Li et al., 2003), and alterations in neurotransmitter systems and cell survival (Barger and Basile, 2001; Giulian et al., 1995; Li et al., 2000). Each of these IL-1-driven events is important in Alzheimer pathogenesis (Griffin and Mrak, 2002).
Our finding of activated microglia containing cytoplasmic fDNA close to damaged neurons or within amyloid plaques suggests that these cells are beneficial in that they clear cellular debris, but may also be deleterious in that overexpression of cytokines during this clearance contributes to neurodegenerative cascades and thus further neuronal degeneration, exacerbating an already existing problem. This suggestion is supported by our previous in vitro studies (Barger and Harmon, 1997; Li et al., 2000, 2001) and our previous studies in AD where the number of activated microglia, overexpressing IL-1, dramatically increases with both the accumulation of neurofibrillary tangles in neurons (Sheng et al., 1997) and the progression of plaques from nonneuritic diffuse Aβ deposits to neuritic Aβ plaques diagnostic of Alzheimer’s disease (Griffin et al., 1995). In view of the progressive neuronal dysfunction and loss, together with the progressive accumulation of neuritic Aβ plaques in AD, it is clear that the plethora of activated microglia available to clear away this debris is insufficient to counterbalance either the degenerative changes in neurons or the over-production and deposition of Aβ. We propose that failure of microglia to stem the tide of neuronal degeneration and Aβ deposition in AD is the result of the concomitant overexpression of cytokines, such as IL-1, which not only set in motion neurodegenerative cascades but also induce further microglial activation. The inability of microglia to sufficiently clear debris in AD, including fDNA as shown here, may be responsible, at least in part, for the apparently continuous activation of microglia, overexpressing IL-1, in AD, thus ensuring a constant source of cytokines and other factors that foster neurodegeneration (Barger and Harmon, 1997; Giulian et al., 1995; Li et al., 2000; Meda et al., 1995; Paresce et al., 1996). Our finding that some microglia had TUNEL-positive cytoplasm as well as TUNEL positive-nuclei suggests that activation of microglia, together with the act of taking up fDNA, leads to the death of microglia. This observation supports the finding that microglial activation and apoptosis are correlated (Lee et al., 2001).
Our in vitro results, demonstrating that microglia take up fDNA via scavenger receptors, may explain why there is elevated expression of scavenger receptors on activated microglia in the vicinity of senile plaques in AD (Christie et al., 1996). Our finding that IFNγ enhances microglial uptake of fDNA may be due to IFNγ-induced over-expression of scavenger receptors on microglia (Grewal et al., 1997), or to activation of a potential IFNγ-binding site in the scavenger receptor A promoter (Grewal et al., 2001). Both scavenger receptors classes A and B are present on microglia, but not on astrocytes or neurons. Since we show that fucoidan, a nonselective scavenger receptor inhibitor, completely blocks microglial uptake of fDNA while an antibody against class A scavenger receptor alone only partially blocks this uptake, several receptors are likely participants in fDNA uptake by microglia.
In summary, our findings are consistent with the idea that accumulation of debris results in microglial activation in Alzheimer brain. Such activation is at once potentially beneficial, on one hand, in clearing neurotoxic substances and, on the other hand, detrimental, for example, by triggering excessive synthesis and release of microglia-derived neurotoxic factors and cytokines, such as IL-1, which is known to have neurodegenerative consequences that contribute to neuronal dysfunction and loss. Such neuronal dysfunction and loss promote further microglial activation, which, if unchecked, will over time create a self-amplifying cycle of neurodegenerative events that have been linked to Alzheimer pathogenesis (Griffin and Mrak, 2002).
Acknowledgements
This work was supported, in part, by P01AG12411, R01AG17498, R01HD37989, the Alzheimer’s Association, and the Donald W. Reynolds Foundation.
References
- Anderson AJ, Su JH, Cotman CW. DNA damage and apoptosis in Alzheimer’s disease: colocalization with c-Jun immunoreactivity, relationship to brain area, and effect of postmortem delay. J. Neurosci. 1996;16:1710–1719. doi: 10.1523/JNEUROSCI.16-05-01710.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger SW, Basile AS. Activation of microglia by secreted amyloid precursor protein evokes release of glutamate by cystine exchange and attenuates synaptic function. J. Neurochem. 2001;76:846–854. doi: 10.1046/j.1471-4159.2001.00075.x. [DOI] [PubMed] [Google Scholar]
- Barger SW, Harmon AD. Microglial activation by Alzheimer amyloid precursor protein and modulation by apolipoprotein E. Nature. 1997;388:878–881. doi: 10.1038/42257. [DOI] [PubMed] [Google Scholar]
- Christie RH, Freeman M, Hyman BT. Expression of the macrophage scavenger receptor, a multifunctional lipoprotein receptor, in microglia associated with senile plaques in Alzheimer’s disease. Am. J. Pathol. 1996;148:399–403. [PMC free article] [PubMed] [Google Scholar]
- Corradin SB, Mauel J, Donini SD, Quattrocchi E, Ricciardi-Castagnoli P. Inducible nitric oxide synthase activity of cloned murine microglial cells. Glia. 1993;7:255–262. doi: 10.1002/glia.440070309. [DOI] [PubMed] [Google Scholar]
- Egensperger R, Maslim J, Bisti S, Hollander H, Stone J. Fate of DNA from retinal cells dying during development: uptake by microglia and macroglia (Muller cells) Brain Res. Dev. Brain Res. 1996;97:1–8. doi: 10.1016/s0165-3806(96)00119-8. [DOI] [PubMed] [Google Scholar]
- El Khoury J, Hickman SE, Thomas CA, Cao L, Silverstein SC, Loike JD. Scavenger receptor-mediated adhesion of microglia to beta-amyloid fibrils. Nature. 1996;382:716–719. doi: 10.1038/382716a0. [DOI] [PubMed] [Google Scholar]
- Giulian D, Haverkamp LJ, Li J, Karshin WL, Yu J, Tom D, Li X, Kirkpatrick JB. Senile plaques stimulate microglia to release a neurotoxin found in Alzheimer brain. Neurochem. Int. 1995;27:119–137. doi: 10.1016/0197-0186(95)00067-i. [DOI] [PubMed] [Google Scholar]
- Grewal RP, Yoshida T, Finch CE, Morgan TE. Scavenger receptor mRNAs in rat brain microglia are induced by kainic acid lesioning and by cytokines. Neuroreport. 1997;8:1077–1081. doi: 10.1097/00001756-199703240-00003. [DOI] [PubMed] [Google Scholar]
- Grewal T, Priceputu E, Davignon J, Bemier L. Identification of a gamma-interferon-responsive element in the promoter of the human macrophage scavenger receptor A gene. Arterioscler. Thromb. Vase. Biol. 2001;21:825–831. doi: 10.1161/01.atv.21.5.825. [DOI] [PubMed] [Google Scholar]
- Griffin WS, Mrak RE. Interleukin-1 in the genesis and progression of and risk for development of neuronal degeneration in Alzheimer’s disease. J. Leukoc. Biol. 2002;72:233–238. [PMC free article] [PubMed] [Google Scholar]
- Griffin WS, Sheng JG, Roberts GW, Mrak RE. Interleukin-1 expression in different plaque types in Alzheimer’s disease: significance in plaque evolution. J. Neuropathol. Exp. Neurol. 1995;54:276–281. doi: 10.1097/00005072-199503000-00014. [DOI] [PubMed] [Google Scholar]
- Lee P, Lee J, Kim S, Lee MS, Yagita H, Kim SY, Kim H, Suk K. NO as an autocrine mediator in the apoptosis of activated microglial cells: correlation between activation and apoptosis of microglial cells. Brain Res. 2001;892:380–385. doi: 10.1016/s0006-8993(00)03257-1. [DOI] [PubMed] [Google Scholar]
- Li Y, Liu L, Kang J, Sheng JG, Barger SW, Mrak RE, Griffin WS. Neuronal-glial interactions mediated by interleukin-1 enhance neuronal acetylcholinesterase activity and mRNA expression. J. Neurosci. 2000;20:149–155. doi: 10.1523/JNEUROSCI.20-01-00149.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu L, Barger SW, Mrak RE, Griffin WS. Vitamin E suppression of microglial activation is neuroprotective. J. Neurosci. Res. 2001;66:163–170. doi: 10.1002/jnr.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu L, Barger SW, Griffin WS. IL-I mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway activity and mRNA expression. J. Neurosci. 2003;23:1605–1611. doi: 10.1523/JNEUROSCI.23-05-01605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda L, Cassatella MA, Szendrei GI, Otvos L, Jr, Baron P, Villalba M, Ferrari D, Rossi F. Activation of microglial cells by beta-amyloid protein and interferon-gamma. Nature. 1995;374:647–650. doi: 10.1038/374647a0. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Paresce DM, Ghosh RN, Maxfield FR. Microglial cells internalize aggregates of the Alzheimer’s disease amyloid beta-protein via a scavenger receptor. Neuron. 1996;17:553–565. doi: 10.1016/s0896-6273(00)80187-7. [DOI] [PubMed] [Google Scholar]
- Sheng JG, Mrak RE, Griffin WS. Glial-neuronal interactions in Alzheimer’s disease: Progressive association of IL-1α+ microglia and S100β+ astrocytes with neurofibrillary tangle stages. J. Neuropathol. Exp. Neurol. 1997;56:285–290. [PubMed] [Google Scholar]
- Sheng JG, Mrak RE, Griffin WS. Progressive neuronal DNA damage associated with neurofibrillary tangle formation in Alzheimer disease. J. Neuropathol. Exp. Neurol. 1998a;57:323–328. doi: 10.1097/00005072-199804000-00003. [DOI] [PubMed] [Google Scholar]
- Sheng JG, Zhou XQ, Mrak RE, Griffin WS. Progressive neuronal injury associated with amyloid plaque formation in Alzheimer disease. J. Neuropathol. Exp. Neurol. 1998b;57:714–717. doi: 10.1097/00005072-199807000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng JG, Mrak RE, Jones RA, Brewer MM, Zhou XQ, McGin-ness J, Woodward S, Bales K, Paul SM, Cordell B, Griffin WS. Neuronal DNA damage correlates with overexpression of interleukin-lbeta converting enzyme in APPV717F mice. Neurobiol. Aging. 2001;22:895–902. doi: 10.1016/s0197-4580(01)00298-6. [DOI] [PubMed] [Google Scholar]
- Smale G, Nichols NR, Brady DR, Finch CE, Horton WE. Evidence for apoptotic cell death in Alzheimer’s disease. Exp. Neurol. 1995;133:225–230. doi: 10.1006/exnr.1995.1025. [DOI] [PubMed] [Google Scholar]
- Thanos S. The relationship of microglial cells to dying neurons during natural neuronal cell death and axotomy-induced degeneration of the rat retina. EurJNeurosci. 1991;3:1189–1207. doi: 10.1111/j.1460-9568.1991.tb00054.x. [DOI] [PubMed] [Google Scholar]
- Velez-Pardo C, Arroyave ST, Lopera F, Castano AD, Jimenez Del Rio M. Ultrastructure evidence of necrotic neural cell death in familial Alzheimer’s disease brains bearing presenilin-1 E280A mutation. J. Alzheimer’s Dis. 2001;3:409–415. doi: 10.3233/jad-2001-3408. [DOI] [PubMed] [Google Scholar]