Abstract
Past studies have demonstrated that increases in cortisol secretion are associated with either enhancements or impairments of long-term memory, depending on the subprocess involved. However, working memory is generally studied as a unified system within the cortisol literature. The present study sought to determine if cortisol increases are positively associated with increases in performance in the encoding subprocess of working memory, and whether increases are positively or negatively associated with performance changes in the maintenance subprocess. Thirty-three young men (M = 19.4 years, SD = 0.89) participated in a change detection task, consisting of a condition requiring encoding only and a condition requiring both encoding and maintenance. To elicit a cortisol response, participants completed the Trier Social Stress Test (TSST) between two administrations of the task. Cardiovascular measurements and saliva samples were obtained before the TSST (T1), and mid-way between blocks of the second administration of the change detection task (T2), to measure autonomic and cortisol responses to the TSST evident during the second change detection task. Cortisol increases between T1 and T2 were positively correlated with both encoding (r(32) = 0.503, p = 0.003) and maintenance (r(32) = 0.463, p = 0.007) performance. This is a novel finding as previous studies have shown an impairing effect of cortisol on working memory. The positive relation between cortisol and working memory has likely been obscured in previous tasks, which did not examine these specific subprocesses in isolation from each other. The beneficial role of cortisol in the stress response is discussed.
Keywords: attentional blink, change detection task, HPA axis, long-term memory, memory consolidation, Trier Social Stress Test
INTRODUCTION
Memory is most commonly conceptualized as having three information storage systems (sensory, working and long-term), each consisting of subprocesses that act on information. According to gateway models, working memory can act as a gateway between sensory input and long-term memory (Atkinson & Shiffrin, 1968; Baddeley et al., 1984; Baddeley, 1996). According to these models, disruptions in working memory can impact consolidation into long-term memory; thus, the quality of working memory processing is associated with the quality of long-term memory formation (Blumenfeld & Ranganath, 2006). Various studies indicate that cortisol secretion impairs working memory (Lupien et al., 1999; Elzinga & Roelofs, 2005; Schoofs et al., 2008; Schoofs et al., 2009) yet enhances consolidation into long-term memory (Abercrombie et al., 2003). As information must be processed in working memory before it is consolidated into long-term memory, a question remains about how it is possible that cortisol can impair working memory function, yet enhance long-term memory formation.
We may begin to resolve this apparent paradox by examining memory subprocesses. Previous research shows that cortisol can both enhance and impair long-term memory, depending upon the subprocess assessed. Specifically, it is well documented that cortisol impairs retrieval of long-term memory (de Quervain et al., 1998; de Quervain et al., 2000). For example, participants whose cortisol secretion increased after exposure to stress (responders) showed impaired retrieval (compared to participants in the control condition) of information presented 24 hours prior to stress exposure (Buchanan & Tranel, 2008). Cortisol is considered to temporarily inhibit retrieval from the hippocampus during stress through its occupation of mineralocorticoid and glucocorticoid receptors in the hippocampus (de Kloet et al., 1999).
At the same time, cortisol is known to enhance long-term memory consolidation (Roozendaal, 2000; Buchanan & Lovallo, 2001; Roozendaal, 2002; Abercrombie et al., 2003). For example, compared to placebo, participants receiving corticosteroid administration prior to the acquisition of learning show better recall one week later (Buchanan & Lovallo, 2001). Stress is considered to produce these enhancing effects on consolidation through glucocorticoid occupation of glucocorticoid receptors in the amygdala (Roozendaal, 2000). Stress hormones are considered to promote the acquisition of new information while at the same time blocking the retrieval of and interference from old information (de Kloet et al., 1999). Indeed, Henckens and colleagues (2009) found that decreased hippocampal activation during consolidation predicted better retrieval after stress. Hence, while stress hormone levels are elevated, the brain prioritizes the formation of new memories while inhibiting the recall of old information. Once cortisol levels return to normal, memory for events that occurred during stress are enhanced. Thus, cortisol has differential effects on long-term memory, enhancing one subprocess while at the same time inhibiting another.
This cortisol-induced shift in cognitive processes is also observed in studies examining rigid versus flexible cognition (Arnsten, 2009; Plessow et al., 2012). For example, Plessow and colleagues (2011) found that participants exposed to a social stressor showed reduced cognitive flexibility in a selective attention task compared to a control group. Participants in the control condition adjusted their strategy on the selective attention task based upon whether the previous trial contained compatible or incompatible stimuli by increasing goal shielding only after incompatible trials, thus demonstrating a flexible strategy. By contrast, tressed participants showed increased goal shielding regardless of whether the previous trial contained compatible or incompatible stimuli. Hence stress seems to promote the use of a rigid fixed strategy over a more flexible one. Stress may reduce flexible cognitive processing through the action of cortisol in the prefrontal cortex (Arnsten, 2009).
Similarly to long-term memory, different subprocesses are also involved in working memory. Subprocesses of working memory include encoding, maintenance and manipulation, each of which contributes to active processing in the face of internal and external distractions. Encoding is the transfer of information from the senses into the working memory store and requires conscious, or top-down, direction of attention to relevant stimuli. Accordingly, attention during encoding assists in determining item relevance (Ranganath et al., 2004). However, maintenance is moderated by memory load (Ranganath et al., 2004) and can occur passively without conscious effort. Its role in working memory is to keep information online and resistant to decay. Manipulation differs from encoding and maintenance in that it involves the flexible processing and transposition of information. Despite these different subprocesses, working memory is generally studied as a unified system within the cortisol literature. It may be the case that, as with long-term memory, cortisol has differential effects on working memory subprocesses as well.
Studies on the effects of cortisol on working memory have produced mixed results. Although many studies indicate that cortisol impairs working memory (see above), several opposing studies show that working memory is unaffected by cortisol (Kuhlmann et al., 2005; Schoofs et al., 2009; Henckens et al., 2011). A few studies even found that pharmacological administration of cortisol can enhance working memory depending on the dose (Lupien et al., 1999) or if working memory is assessed several hours later (Henckens et al., 2011). Known sources of variability include time of day (Het et al., 2005), whether sympathetic nervous system activity is accounted for (Elzinga & Roelofs, 2005), and whether cortisol secretion is manipulated through stress tasks or pharmacological manipulation. Because different working memory tasks assess different subprocesses of working memory, the type of task used may contribute additional variability regarding the effects of cortisol on working memory. If cortisol has bidirectional effects on working memory subprocesses, it may be necessary to isolate subprocesses within working memory to achieve the homogeneity of findings found within long-term memory studies.
Cortisol-induced impairments on working memory are understood to occur due to glucocorticoid receptor occupation in the prefrontal cortex (PFC). The PFC is responsible for flexible and deliberate cognitive processes that are characteristic of certain aspects of working memory, such as manipulation. However, the encoding and maintenance subprocesses of working memory may not be as sensitive to the effects of stress because they are less dependent on the PFC. For instance, the posterior parietal cortex (PPC) plays a key role in visual working memory (Todd & Marois, 2005a) and the PPC and inferior temporal regions are involved in the maintenance of visual information (Munk et al., 2002; Pessoa et al., 2002, Todd & Marois, 2005b). Additionally, stress studies have shown that cortisol is associated with increased activity in the PFC and PPC during maintenance (Weerda et al., 2010). Because maintenance is less dependent on the PFC, and is a less flexible aspect of working memory, it may not be subject to stress-induced working memory impairments.
Considering the reported positive effects of cortisol on long-term memory consolidation, one might expect the encoding subprocess of working memory to be similarly resistant to cortisol-induced impairments, as both encoding and consolidation processes share the feature of memory traces gaining entrance into a memory system. Indeed, the findings from several stress studies indicate that cortisol may have an enhancing effect on encoding (Al’Absi et al., 2002; Schwabe & Wolf, 2010; Weerda et al., 2010). For instance, the ability of cortisol to reduce attentional blink (Schwabe & Wolf, 2010), i.e. the failure to encode stimuli presented rapidly after a previous stimulus (Raymond et al., 1992), indicates that cortisol may enhance encoding by increasing the rate at which information can be encoded into working memory. Additionally, results from a functional magnetic resonance imaging (fMRI) study (Weerda et al., 2010) and an event-related potential (ERP) study (Monk & Nelson, 2002) indicate that increased cortisol levels are associated with increased or altered hippocampal activity during encoding. Furthermore, amygdala activity at encoding is associated with subsequent long-term memory formation (Cahill et al., 1996; Hamann et al., 1999). Given these findings, the fundamental role of cortisol in memory may be to promote the encoding of novel information.
The present study sought to determine if cortisol has specific effects on separate subprocesses of working memory by using a change detection task designed by Woodman and Vogel (2005) consisting of a condition requiring only encoding and a condition requiring encoding and maintenance. To elicit a cortisol response, participants completed the Trier Social Stress Test (TSST) between two administrations of the working memory task. Due to the large amount of evidence indicating that encoding may be resistant to or even enhanced by cortisol, we hypothesised that the degree of cortisol reactivity would be positively associated with encoding performance in both conditions.
Due to the commonly found impairing effect of cortisol on working memory, we predicted that despite the hypothesized positive association with encoding performance, cortisol reactivity may be negatively associated with the maintenance component of the second working memory condition. It may be the case that cortisol has an impairing effect on maintenance while enhancing encoding.
Alternatively, cortisol may have an enhancing effect on both encoding and maintenance. Both encoding and maintenance are associated with the quality of long-term memory formation (Brewer et al., 1998), and it is well established that cortisol enhances long-term memory formation. Enhancement by cortisol of long-term memory may be linked to enhancements during encoding and maintenance. Furthermore, because encoding and maintenance require less cognitive flexibility than the manipulation component of working memory, these processes may not be subject to cortisol-induced impairments; as some research indicates that cortisol may inhibit the more flexible cognitive processes and encourage more rigid thought patterns (Plessow et al., 2011; Schwabe et al., 2009). If this is the case, cortisol reactivity may be positively associated with both encoding and maintenance performance in the two conditions.
METHODS
Participants
Thirty-three New Mexico State University, USA, undergraduate students participated for course credit or financial compensation. Participants were recruited at New Mexico State University through announcements. Two additional participants took part in the study, but their data were excluded due to computer error during the working memory task (n = 1) or due to a past psychiatric diagnosis (n = 1). The mean participant age was 19.4 years (SD = 0.89). Only men were asked to participate in order to control for the possible confounding influence of the menstrual cycle and oral contraceptives on the endocrine system (Kirschbaum et al., 1999). In order to prevent saliva sample contamination or hormonal alteration, participants were asked to refrain from drinking, eating, exercising or smoking within the hour prior to the appointment. Approval for the study was given by the New Mexico State University Institutional Review Board and the study procedures conformed to the Declaration of Helsinki. All participants provided written informed consent.
General Procedure
Experimental sessions began at either 13:00h or 15:00 h. The afternoon phase was chosen because cortisol concentrations rise considerably in the morning and are more stable in the afternoon, making identification of increases in response to a stressor more straightforward (Pruessner et al., 1997; Dickerson & Kemeny, 2004). Participants began each session with a 30 min rest phase. After the initial rest phase, participants completed a working memory task twice: at T1, which occurred before the TSST, and at T2, which followed the TSST. Physiological and subjective measures were also taken at T1 and T2.
Trier Social Stress Test (TSST)
The TSST is a psychosocial stress task that has been found to reliably produce cortisol and physiological responses (Kirschbaum et al., 1993). The TSST produces changes in the sympathetic-adrenal-medullary axis, the hypothalamic-pituitary-adrenal axis, the immune system and a wide variety of other biological parameters (Kirschbaum et al., 1993). While the TSST produces a wide range of physiological responses, we were primarily interested in the role of cortisol in the stress response. The cortisol response to the TSST has shown substantial variability (Kudielka et al., 2009), with some participants showing an increase in cortisol and others a decrease. Accordingly, some studies comparing memory performance between participants exposed to the TSST to a control group have not found differences in overall memory performance between the two groups, and have instead made comparisons in performance based upon cortisol reactivity to the task (Domes et al., 2002, Elzinga & Roelofs, 2005, Nater et al, 2007). Here we did not include a control condition and all participants took part in the TSST, as we were interested in examining how the degree of cortisol reactivity is associated with changes in working memory performance occurring after the stressor.
In the present study, the TSST began with a 5 min anticipatory phase in which the participants prepared a speech about their qualifications for a hypothetical job, and ended with a 10 min stress phase. During the stress phase, participants gave their 5 min speech facing two confederates and a video camera, and then completed a mental arithmetic task for 5 min.
Working Memory Task
The working memory task was a change detection task presented on a 33x28 cm computer screen. The design was modeled after Woodman and Vogel’s (2005) task, which consisted of two conditions: (a) Encoding, and (b) Encoding During Maintenance (Figure 1).
Figure 1.
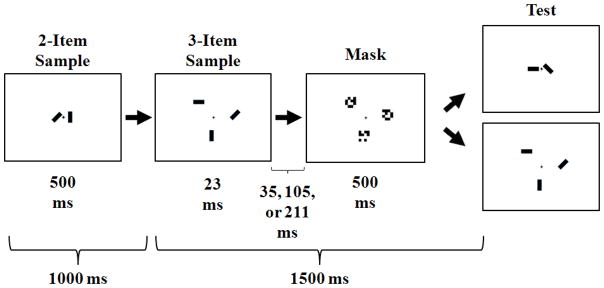
Procedure used in the Encoding condition (last 1500 ms) and the Encoding During Maintenance condition (entire 2500 ms). In the Encoding During Maintenance condition, participants were tested on the 2-item array on 50% of the trials to measure maintenance, and the 3-item array on 50% of the trials to measure encoding. This figure is redrawn from Woodman and Vogel (2005).
The Encoding condition consisted of a sample array composed of 3 rectangles at different orientations, a mask array, and a test array. There were 3 fixed locations and 4 possible orientations for the rectangle stimuli. The mask array consisted of black and white checkerboard squares. Each trial began with a fixation cross presented for 500 ms. Next, the sample array was presented for 23 ms, followed by a mask array presented for 500 ms. The inter-stimulus interval (ISI) between the sample array and mask array varied between three intervals: 35 ms, 105 ms or 211 ms (Figure 1). The test array was identical to the stimulus array on half the trials. The participant pressed the “x” key to indicate the test array was identical to the stimulus array, and the “z” key to indicate the test array was different from the stimulus array. The test array was presented for 5,000 ms or until the participant pressed a key.
The Encoding During Maintenance condition was identical to the Encoding condition, except that the Encoding During Maintenance condition began with the addition of a 2-item array that was not masked. The lack of a mask allowed uninterrupted encoding of the 2-item array, and participants were required to maintain this array in working memory throughout the rest of the trial. At test, half the trials required a response to a 2-item array (to assess maintenance) and the other half of the trials required a response to a 3-item array (to assess encoding). Because the type of response trial varied randomly, participants did not know which array they would be tested on for any given trial.
Participants alternated between blocks of trials in the Encoding and Encoding During Maintenance conditions, with the beginning condition counterbalanced across participants. In both conditions, the time between the onset of the Encoding stimulus array and the offset of the test array was 1500 ms. There were 6, 24-trial blocks in each condition at both T1 and T2. Each block began with the presentation of articulatory suppression stimuli that the participant was to repeat aloud throughout that block (“ABCD,” “WXYZ,” “1234” or “6789”). This methodology is commonly used to prevent the transfer of visually presented information to an auditory code.
Physiological Measures
All physiological and subjective measures were obtained twice. The first round of assessments took place after the 30 min rest phase, which was 30 min before the start of the TSST (T1). The second assessment took place 15 min after completion of the TSST (T2), which was also the mid-point of the second working memory assessment (after completion of 3 of the 6 working memory blocks). As T2 assessments occurred 15 min after cessation of the TSST, and Sympathetic Nervous System (SNS) measures may no longer be elevated at this time; however this sampling point was chosen because it is most representative of what is occurring during the entire working memory task.
Salivary cortisol concentration
Saliva samples were collected from each participant twice by having participants place a cotton dental roll in their mouth. Saliva was collected at T1 to determine baseline cortisol concentrations. Peak cortisol concentrations were expected at T2 (Dickerson & Kemeny, 2004), which was the mid-point of the working memory task. Samples were centrifuged and stored at −20°C within 2 hours of collection. Samples were shipped to Salimetrics, Inc. for radioimmunoassay analysis. All samples were analyzed twice, and the average of the two measurements was used in analyses. The average intra-assay coefficient of variation was 0.03%. Salimetrics reports an average inter-assay coefficient of variation of 5.1% (Salimetrics, 2012). Salivary cortisol concentrations were not normally distributed, which was confirmed with Shapiro-Wilk tests, consequently they were log-transformed for normality. Only log10 values were included in analyses.
Sympathetic activation
Blood pressure and pulse were assessed at T1 and T2 using an Omron Premium blood pressure monitor and a Nellcor OxiMax N-65 Handheld Pulse Oximeter.
Subjective Measures
Possible changes in self-reported emotion were also assessed at T1 and T2. Participants rated how anxious, nervous, angry and insecure they were on a scale from 0 to 10. Subjective measures were obtained in order to account for the possible influence of emotion and arousal on working memory performance. To assess a possible association between cortisol response and motivation to perform well on the working memory task, a single motivation question was included at the end of the session, specifically, “How hard did you try to successfully answer the computer memory task (with the rectangles)?” on a 5-point scale.
Statistical Analysis
Performance on the working memory task was measured in terms of change-detection accuracy, and followed Woodman and Vogel’s (2005) data analysis. Following this quantitative approach, hits and false alarms were converted to K, which represents the number of items encoded into working memory. K values were entered into Cowan’s (2001) revision of Pashler’s (1988) approach to change detection. The revised formula controls for guessing: K = {[SS * (HR − FAR)] / (1-FAR)} * 1 − FAR, where SS is set size (the number of rectangles in the array). Therefore, the maximum possible K value for encoding task responses in either condition was 3 and the maximum possible K value for maintenance task responses was 2.
Salivary cortisol, blood pressure, pulse, and subjective measures across T1 and T2 time intervals were submitted to separate paired comparison t-tests. To determine if there were changes in working memory performance between T1 and T2 assessments, three separate analyses of variance (ANOVAs) were conducted on working memory measures in each condition. To determine if cortisol secretion was the best predictor of working memory performance after the stress manipulation, three separate regression analyses were conducted, one for each working memory condition, with working memory performance at T2 as the dependent variable and changes between log10T1 and log10T2 in cortisol, pulse, blood pressure and a single subjective measure as predictor variables in each analysis. To test the main hypotheses that increases in cortisol secretion in response to the stress manipulation would be associated with changes in encoding and maintenance performance, we conducted correlations between cortisol change (log10T2 – log10T1) and differences in K values for both encoding and maintenance measures between T1 and T2 for all conditions. All reported p-values are two-tailed, and the alpha level for significance was set at p < 0.05.
RESULTS
Stress Induction
Changes in endocrine, autonomic, and subjective measures were associated with the administration of the TSST, and are shown in Table 1. Significant overall group increases in salivary cortisol concentration, diastolic blood pressure, anger, and insecurity occurred between T1 and T2. Interestingly, pulse rate significantly decreased in this interval. While Kudielka and colleagues (2004) found heart rate returned to baseline levels within 5-min of cessation of the TSST, consistent with our finding, post-stress heart rate has also been shown to rebound significantly to below baseline levels after psychological stress in order to reset baroreflex sensitivity (Mezzacappa et al., 2001).
Table 1.
Mean values of all stress measures at T1 and T2 assessments. Raw salivary cortisol concentrations rounded to three decimal places. All other physiological measures rounded to nearest whole number. Subjective measures are on a scale from 1–10.
| T1 | T2 | |
|---|---|---|
| Cortisol (μg/dL) | 0.158 (SEM = 0.01) | 0.233 (SEM = 0.02)** |
| Systolic Blood Pressure (mmHg) | 121 (SEM = 2.0) | 122 (SEM = 2.1) |
| Diastolic Blood Pressure | 75 (SEM = 1.5) | 78 (SEM = 1.7)* |
| Pulse (beats /min, bpm) | 71 (SEM = 1.6) | 67 (SEM = 1.6)** |
| Anger | 0.41 (SEM D = 0.21) | 1.09 (SEM = 0.34)* |
| Anxiousness | 2.24 (SEM = 0.45) | 3.12 (SEM = 0.49)m |
| Insecurity | 0.70 (SEM = 0.28) | 1.76 (SEM = 0.47)** |
| Nervousness | 1.73 (SEM = 0.41) | 2.00 (SEM = 0.46) |
SEM = Standard Error of the Mean. T1 and T2 measures were compared using paired t-tests.
N = 33.
p < 0.01,
p < 0.05,
p < 0.10
A Pearson r was computed between the difference in salivary cortisol concentration between T1 and T2 and the subjective motivation measure, and results showed that these variables were not correlated, r(29) = 0.198, p = 0.30.
Working Memory Analyses Without Cortisol Considerations
As the possible range of K values differed for the encoding (0–3) and maintenance (0–2) conditions, a single ANOVA involving the full experimental design was not possible. Therefore an ANOVA for the encoding conditions is reported first, followed by an ANOVA for the maintenance condition.
For the analysis of encoding into working memory performance, a 2 X 2 X 3 ANOVA was performed, using time (T1 and T2 working memory assessments), working memory condition (Encoding and Encoding During Maintenance), and ISI (35, 105, 211 ms) as repeated measures. There was no main effect of time, indicating that performance did not change with repeated administration of the working memory task. This result is consistent with research showing that visual working memory does not generally improve with training (Olson & Jiang, 2004). Performance in the Encoding condition was significantly higher (M = 1.28, SD = 0.64) than encoding task performance in the Encoding During Maintenance Condition (M = 0.77, SD = 0.58; F(1, 32) = 57.07, p < 0.001, ηp2 = 0.64). This difference is expected because the working memory store is partially filled by the 2-item array in the Encoding During Maintenance condition, which limits subsequent encoding. Overall, encoding performance also improved with increases in ISI, F(2, 64) = 23.81, p < 0.001, ηp2 MSE = 0.43. However, there were no significant interactions. To examine potential improvements in maintenance processes due to repeated task administrations, a separate 2 X 3 repeated measures ANOVA was conducted on maintenance performance in the Encoding During Maintenance condition, using time (T1 and T2) and ISI (35, 105, 211 ms) as variables in the analysis. As in the previous analysis on encoding performance, the results for maintenance showed no main effect of time, indicating a lack of improvement due to repeated administrations of the task. Moreover, there was no main effect of ISI on maintenance task performance, and no interaction with time. The lack of an ISI effect is to be expected as participants were always given 500 ms to encode the unmasked 2-item array to test information maintenance. The ISI refers to the subsequently presented masked 3-item array used to test information encoding during maintenance.
Regression Analyses on Working Memory Using Cortisol and SNS Predictor Variables
To determine if cortisol secretion was the best predictor of working memory performance after the stress manipulation, three separate regression analyses were conducted, one for each working memory condition, with working memory performance at T2 as the dependent variable and changes between log10T1 and log10T2 in salivary cortisol concentration, pulse rate, blood pressure and the subjective measure insecurity as predictor variables in each analysis (Insecurity was chosen because it was the only subjective measure to change significantly from T1 to T2). In each of the three linear regression analyses, the sole significant predictor of working memory performance at T2 was cortisol change between T1 and T2. Results are as follows. For the Encoding condition, Model R = 0.485, with change in cortisol from T1 to T2 being the only significant predictor of encoding in this condition (β = 0.456), t(32) = 2.671, p = 0.013. For the Encoding During Maintenance condition, Model R = 0.509, with change in cortisol from T1 to T2 being the only significant predictor of encoding in this condition (β = 0.460), t(32) = 2.734, p = 0.011. For the Maintenance condition, Model R = 0.476, with change in cortisol from T1 to T2 being the only significant predictor of maintenance performance (β = 0.434), t(32) = 2.527, p = 0.018.
Correlations Between Changes in Working Memory and Cortisol Response
Encoding condition
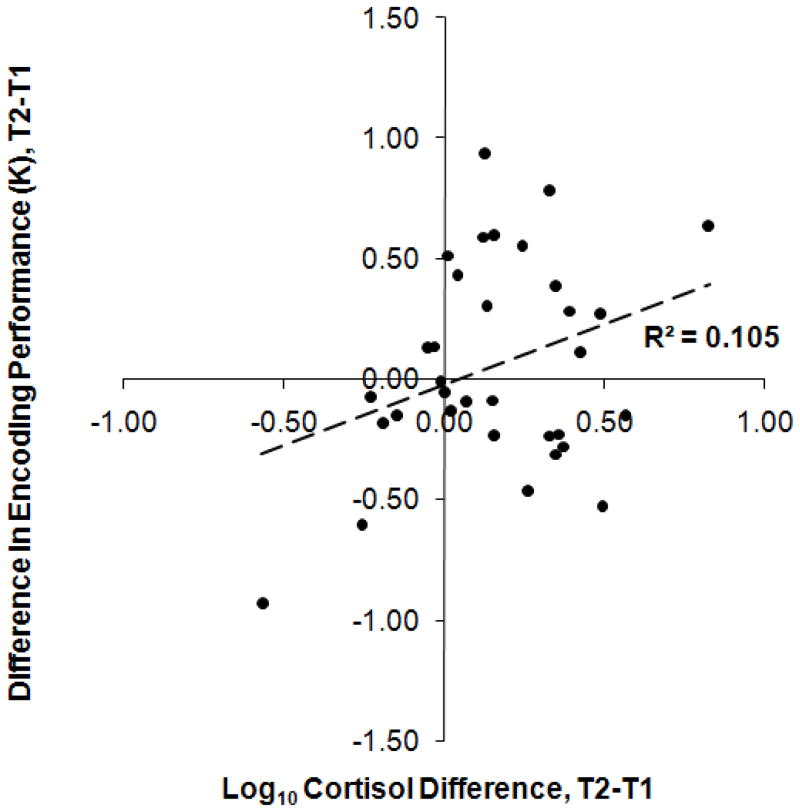
Pearson r correlations between cortisol change and encoding performance change at each ISI were computed. These were: 0.108, 0.390, and 0.103 for the 35-, 105-, and 211-ms conditions, respectively. A Fisher r-to-z transformation was applied to assess the significance of the difference between the correlations that differed the most in this set, z = 1.19, p = 0.234. Since these correlation values were not significantly different from each other, results were collapsed across ISI in order to provide a more stable estimate. A Pearson r was computed between cortisol change and encoding performance change between T1 and T2, r(32) = 0.324, p = 0.065, revealing a marginally significant positive correlation in this condition. A scatterplot for these data is shown in Figure 2.
Figure 2.
Participants’ change in performance from T1 to T2 in the Encoding condition as a function of their change in log10 salivary cortisol concentration between T1 and T2. R2 is reported, (n = 33), p = 0.065.
Encoding in the Encoding During Maintenance condition
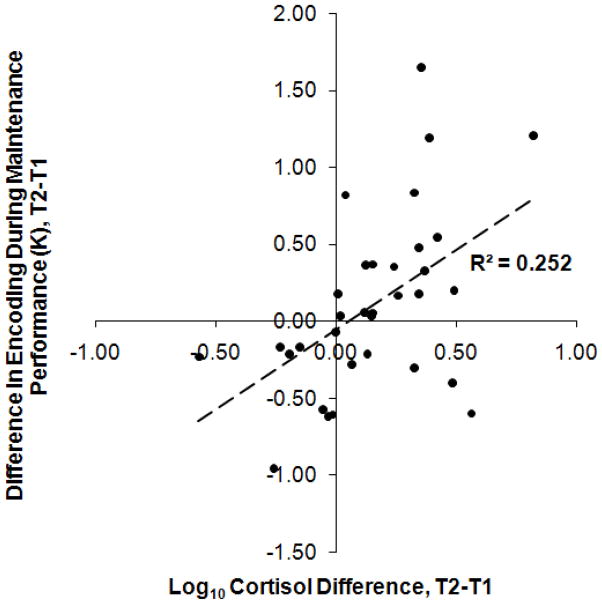
Pearson r correlations between cortisol change and encoding performance change at each ISI in this condition were: 0.435, 0.329, and 0.266 for the 35-, 105-, and 211-ms conditions, respectively. A Fisher r-to-z transformation was applied to the two correlations with the greatest difference, and results showed z = 0.75, p = 0.453; consequently, K value differences were collapsed across ISI. A Pearson r showed that increases in cortisol between T1 and T2 were significantly correlated with increases between T1 and T2 in K values for encoding in this condition, r(32) = 0.503, p = 0.003. (Figure 3).
Figure 3.
Participants’ change in performance from T1 to T2 in the Encoding During Maintenance condition as a function of their change in log10 salivary cortisol concentration between T1 and T2. R2 is reported, (n = 33), p = 0.003.
Maintenance in the Encoding During Maintenance condition
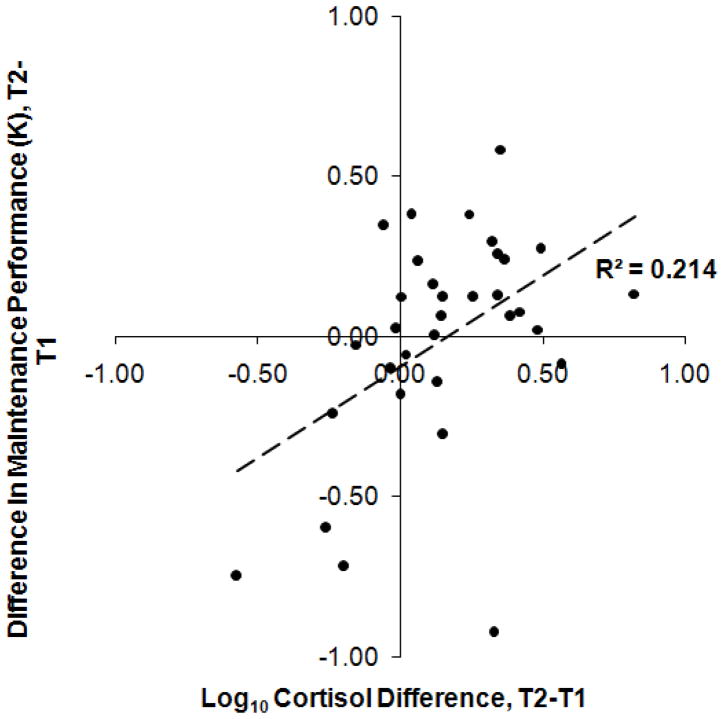
Due to the fact that participants were shown an unmasked array to test information maintenance, results for changes in maintenance across T1 and T2 were collapsed across ISI condition. A Pearson r revealed a significant positive correlation between increases in cortisol between T1 and T2 and increases in K values for maintenance performance between T1 and T2 in the Encoding During Maintenance condition, r(32) = 0.463, p = 0.007. (Figure 4).
Figure 4.
Participants’ change in performance from T1 to T2 in the Maintenance condition as a function of their change in log10 salivary cortisol concentration between T1 and T2. R2 is reported, (n = 33), p = 0.007.
DISCUSSION
The present study found that cortisol increases were associated with improvements in both the encoding and maintenance subprocesses of working memory. The TSST was associated with significant cortisol secretion in most participants and this cortisol increase was positively associated with improvement in working memory performance from T1 to T2 in all three conditions. This finding is novel because previous studies indicate that cortisol typically has either no effect (Kuhlmann et al., 2005; Henckens et al., 2011) or an impairing effect (Young et al., 1999; Wolf et al., 2001; Oei et al., 2006; Schoofs et al., 2008) on working memory. One possible reason for these results is that glucocorticoids have often been found to have an inverted U-shaped dose-response effect on memory (Conrad et al., 1999; de Kloet et al., 1999; Abercrombie et al., 2003; Diamond et al., 2007). That is, memory performance (typically consolidation) is often improved with low to moderate increases in glucocorticoid secretion, but impaired when glucocorticoid concentrations are either too high or too low. This dose-response curve may be due to the differing levels of receptor saturation in the hippocampus: when hippocampal mineralocorticoid receptors, which have a greater affinity than glucocorticoid receptors (GRs) for glucocorticoids, are relatively saturated, animals show a greater readiness to respond adaptively in learning situations. When glucocorticoid concentrations increase greatly, however, GRs become more fully saturated, and this saturation of GRs is associated with impaired memory consolidation (de Kloet et al., 1999). It may be that the cortisol increase elicited by the TSST in the present study was an appropriate amount (not too small; not too large) to improve performance. Alternatively, the conflict between the results of the present study and those of previous studies of effects of cortisol on working memory may be due to the tendency for previous studies to rely on global assessments of working memory (e.g. mental arithmetic) that also require executive processes and retrieval. The present study is novel in its examination of the effect of cortisol on isolated subprocesses of working memory.
Because increases in cortisol from T1 to T2 were associated with improvement in working memory performance on all three tasks, cortisol did not appear to have bidirectional effects on the working memory subprocesses tested, as it does with long-term memory. It therefore seems unlikely that enhancements occurred due to a shift in cognitive resources from one subprocess to another. However, because information must first be encoded before it is maintained, superior maintenance performance may simply reflect the advantageous nature of cortisol response at encoding.
The exact cause of improvements in working memory performance among participants whose salivary cortisol concentration increased can only be speculated. Examining the cognitive processes underlying change detection can help map out the nature of effects of cortisol on working memory. Research on change detection indicates that focused attention is the fundamental requirement for detection of change (Rensink et al., 1997). As attention increases, the threshold for change detection decreases. Perhaps cortisol secretion helps to focus attention, enabling greater detection of change. This is in line with a recent study that has shown that cortisol reduces the attentional blink, i.e. the failure to encode a second stimulus when it is presented rapidly after a first (Schwabe & Wolf, 2010). Attentional blinks of a T2 stimulus occur when presented within 500 ms of a T1 stimulus and are considered to occur because attention is still directed at the T1 stimulus. Detection of T2 stimuli can only occur after the processing of the T1 stimulus is complete. This indicates that cortisol may decrease the amount of time needed to consolidate information into working memory into a stable form.
Interestingly, the subjective state of stress increases the attentional blink of T2 stimuli, and this is considered to occur because anxious mood states slow down the consolidation of information (Jefferies et al., 2008). However, when studies measure cortisol in addition to the subjective state of stress, a different picture emerges. Stress-induced cortisol secretion actually decreases the attentional blink (Schwabe & Wolf, 2010). Therefore, as shown in the present study, cortisol secretion may counteract the attentional deficits induced by stressful situations. Regression analyses revealed that although the subjective measures of anger and insecurity increased in response to the TSST, they were not predictive of working memory performance. Only participants who secreted cortisol in response to the TSST showed enhanced performance.
Stress-induced cortisol secretion may enhance encoding even as it inhibits memory retrieval: as it prepares the brain for acquisition of new information, it prevents the retrieval of old information (Roozendaal, 2002). Indeed, research with rodents has repeatedly shown a facilitatory effect of glucocorticoids on new learning, either when the glucocorticoid increase immediately precedes (Diamond et al., 2007) or immediately follows (Roozendaal, 2002) the to-be-learned information. Conversely, increased concentrations of glucocorticoids are typically associated with the impairment of long-term memory retrieval (Roozendaal, 2002). These results parallel those found with human participants: that stress-induced cortisol increases are associated with impaired retrieval of previously-learned information (Buchanan & Tranel, 2008), and pre-learning administration of cortisol is associated with improved consolidation of information (Buchanan & Lovallo, 2001; Abercrombie et al., 2003). In this way, the effect of cortisol on cognition depends on the information processing stage. It could be that the present task showed an enhancing effect of cortisol because it relied so minimally upon retrieval, instead, assessing the stage of processing when information is first coming in.
Limitations and Future Directions
One limitation of the present study is that, to control for hormonal confounds, only men served as participants. It is therefore unknown whether a sample of female participants would yield the same results. A further limitation of the present study is that, while the design included pre- and post-stressor measures, it did not include a non-stressor control condition. Without a control condition, the causal role of stress on working memory is unclear; that is, there are factors that may potentially coincide with cortisol response to stressors that could be causally related to working memory advantages. Stress-induced cortisol secretion occurs along with a cascade of other physiological events. The beneficial effects of cortisol seen in this study may have been due to an interaction between cortisol and noradrenergic activity such as norepinephrine release. However, previous studies that did measure other stress hormones did not find them to be significant factors. For example, Roelofs et al. (2005) found that salivary alpha-amylase levels (an indirect measure of plasma norepinephrine levels) did not differ between low cortisol responders and high cortisol responder groups. Furthermore, Schoofs et al. (2008) found that salivary alpha-amylase did not differ between stress and control groups 10 min or 25 min after initiation of treatment condition; however, it was significantly higher in the stress group 1 min after the stressor.
A final limitation of the study is the timing of stress measures. In the present study sympathetic activation measures (blood pressure, and heart rate) were not significant predictors of working memory. The T2 physiological assessments took place at the midpoint of the working memory task (after completion of 3 of the 6 blocks of trials). As such, this time point represents the state of the participant during the working memory task, not their immediate reaction to the TSST. It is likely that physiological measurements obtained during and immediately after cessation of the TSSTwould have provided different results. Future studies should obtain physiological measures at multiple points to provide a more complete picture of the stress response.
Conclusion
The novel finding that increases in cortisol secretion are positively associated with both the encoding and maintenance subprocesses of working memory is in line with earlier studies showing cortisol modulation of long-term memory. Both encoding (Brewer et al., 1998; Ranganath et al., 2003) and maintenance (Schon et al., 2004; Ranganath et al., 2005) are associated with the quality of long-term memory formation. Therefore, the present findings are not only compatible with previous studies, but they indicate a possible mechanism for enhancement by cortisol of long-term memory consolidation. It may be that enhancements during working memory encoding and maintenance create richer and more coherent representations that are subsequently transferred to long-term memory. Therefore, one interesting question that arises is the degree to which enhancement by cortisol of long-term memory is the byproduct of advantages of a cortisol response exhibited during working memory processing. Future studies should investigate this issue. In sum, the belief that stress-induced cortisol secretion negatively impacts working memory needs to be reexamined. These results indicate that the particular subprocesses recruited by working memory tasks play a role in the valence of effects of cortisol on working memory.
Acknowledgments
We would like to thank Ryan Foullon, Daniel Gambacorta, Cynthia Killough and Matt Rambert for their assistance in this project.
Declaration of Interest
The project described was supported by Award Number SC1HD060887 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver Institute of Child Health & Human Development or the National Institutes of Health. The authors report no conflict of interest.
References
- Abercrombie HC, Kalin NH, Thurow ME, Rosenkranz MA, Davidson RJ. Cortisol variation in humans affects memory for emotionally laden and neutral information. Behav Neurosci. 2003;117(3):505–516. doi: 10.1037/0735-7044.117.3.505. [DOI] [PubMed] [Google Scholar]
- Al’Absi M, Hugdahl K, Lovallo WR. Adrenocortical stress responses and altered working memory performance. Psychophysiology. 2002;39:95–99. doi: 10.1017/S0048577202001543. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Stress signaling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009 Jun;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson RC, Shiffrin RM. Human memory: a proposed system and its control processes. Psychol Learn Motiv. 1968;2:89–195. [Google Scholar]
- Baddeley A. The fractionation of working memory. Proc Natl Acad Sci USA. 1996 Nov;93:13468–13472. doi: 10.1073/pnas.93.24.13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A, Lewis V, Eldridge M, Thomson N. Attention and retrieval from long-term memory. J Exp Psychol Gen. 1984;113(4):518–540. [Google Scholar]
- Blumenfeld RS, Ranganath C. Dorsolateral prefrontal cortex promotes long-term memory formation through its role in working memory organization. J Neurosci. 2006 Jan 18;26(3):916–925. doi: 10.1523/JNEUROSCI.2353-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JD. Making memories: brain activity that predicts how well visual experience will be remembered. Science. 1998 Aug 21;281:1185–1187. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Lovallo WR. Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology. 2001;26(3):307–317. doi: 10.1016/s0306-4530(00)00058-5. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Tranel D. Stress and emotional memory retrieval: effects of sex and cortisol response. Neurobiol Learn Mem. 2008;89:134–141. doi: 10.1016/j.nlm.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, Fallon J, Alkire MT, Tang C, Keator D, Wu J, McGaugh JL. Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proc Natl Acad Sci USA. 1996 Jul;93:8016–8021. doi: 10.1073/pnas.93.15.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Lupien SJ, McEwen BS. Support for a bimodal role for Type II adrenal steroid receptors in spatial memory. Neurobiol Learn Mem. 1999;72(1):39–46. doi: 10.1006/nlme.1998.3898. [DOI] [PubMed] [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: a reconsideration of mental storage capacity. Behav Brain Sci. 2001;24(1):87–114. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Oitzl MS, Joels M. Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998 Aug 20;394(6695):787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Roozendaal B, Nitsch RM, McGaugh JL, Hock C. Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Nat Neurosci. 2000 Apr;3(4):313–314. doi: 10.1038/73873. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Campbell AM, Park CR, Halonen J, Zoladz PR. The temporal dynamics model of emotional memory processing: a synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes-Dodson Law. Neural Plast. 2007:1–33. doi: 10.1155/2007/60803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Reichwald U, Hautzinger M. Hypothalamic-pituitary-adrenal axis reactivity to psychological stress and memory in middle-aged women: high responders exhibit enhanced declarative memory performance. Psychoneuroendocrinology. 2002 Oct;27(7):843–853. doi: 10.1016/s0306-4530(01)00085-3. [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Roelofs K. Cortisol-induced impairments of working memory require acute sympathetic activation. Behav Neurosci. 2005;119(1):98–103. doi: 10.1037/0735-7044.119.1.98. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Eli TD, Grafton ST, Kilts CD. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nat Neurosci. 1999;2:289–303. doi: 10.1038/6404. [DOI] [PubMed] [Google Scholar]
- Henckens MJ, van Wingen GA, Joëls M, Fernández G. Time-dependent corticosteroid modulation of prefrontal working memory processing. Proc Natl Acad Sci USA. 2011 Apr 5;108(14):5801–5806. doi: 10.1073/pnas.1019128108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Het S, Ramlow G, Wolf OT. A meta-analytic review of the effects of acute cortisol administration on human memory. Psychoneuroendocrinology. 2005;30:771–784. doi: 10.1016/j.psyneuen.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Jefferies LN, Smilek D, Eich E, Enns JT. Emotional valence and arousal interact in attentional control. Psychol Sci. 2008;19(3):290–295. doi: 10.1111/j.1467-9280.2008.02082.x. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61(2):154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, Wust S. Why do we respond so differently? reviewing determinants of human salivary cortisol response to challenge. Psychoneuroendocrinology. 2009;34:2–18. doi: 10.1016/j.psyneuen.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. Differential heart rate reactivity and recovery after psychosocial stress (TSST) in healthy children, younger adults, and elderly adults: the impact of age and gender. Int J Behav Med. 2004;11(2):116–121. doi: 10.1207/s15327558ijbm1102_8. [DOI] [PubMed] [Google Scholar]
- Kuhlmann S, Piel M, Wolf OT. Impaired memory retrieval after psychosocial stress in healthy young men. J Neurosci. 2005 Mar 16;25(11):2977–2982. doi: 10.1523/JNEUROSCI.5139-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, Gillin CJ, Hauger RL. Working memory is more sensitive than declarative memory to the acute effects of corticosteroids: a dose-response study in humans. Behav Neurosci. 1999;113(3):420–430. doi: 10.1037//0735-7044.113.3.420. [DOI] [PubMed] [Google Scholar]
- Mezzacappa ES, Kelsey RM, Katkin ES, Sloan RP. Vagal rebound and recovery from psychological stress. Psychosom Med. 2001;63:650–657. doi: 10.1097/00006842-200107000-00018. [DOI] [PubMed] [Google Scholar]
- Monk CS, Nelson CA. The effects of hydrocortisone on cognitive and neural function: a behavioral and event-related potential investigation. Neuropsychopharmacol. 2002;26(4):505–519. doi: 10.1016/S0893-133X(01)00384-0. [DOI] [PubMed] [Google Scholar]
- Munk MH, Linden DE, Muckli L, Lanfermann H, Zanella FE, Singer W, Goebel R. Distributed cortical systems in visual short-term memory revealed by event-related functional magnetic resonance imaging. Cereb Cortex. 2002;12:886–876. doi: 10.1093/cercor/12.8.866. [DOI] [PubMed] [Google Scholar]
- Nater UM, Moor C, Okere U, Stallkamp R, Martin M, Ehlert U, Kliegel M. Performance on a declarative memory task is better in high than low cortisol responders to psychosocial stress. Psychoneuroendocrinology. 2007 Jul;32(6):758–763. doi: 10.1016/j.psyneuen.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Oei NY, Everaerd WT, Elzinga BM, van Well S, Bermond B. Psychosocial stress impairs working memory at high loads: an association with cortisol levels and memory retrieval. Stress. 2006 Sep;9(3):133–141. doi: 10.1080/10253890600965773. [DOI] [PubMed] [Google Scholar]
- Olson IR, Jiang Y. Visual short-term memory is not improved by training. Mem Cognition. 2004;32(8):1326–1332. doi: 10.3758/bf03206323. [DOI] [PubMed] [Google Scholar]
- Pashler H. Familiarity and visual change detection. Percept Psychophys. 1988;44:369–378. doi: 10.3758/bf03210419. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Gutierrez E, Bandettini PA, Ungerleider LG. Neural correlates of visual working memory: fMRI amplitude predicts task performance. Neuron. 2002 Aug;35:975–987. doi: 10.1016/s0896-6273(02)00817-6. [DOI] [PubMed] [Google Scholar]
- Plessow F, Fischer R, Kirschbaum C, Goschke T. Inflexibly focused under stress: psychosocial stress increases shielding of action goals at the expense of reduced cognitive flexibility with increasing time lag to the stressor. J Cognitive Neurosci. 2011;23(11):3218–3227. doi: 10.1162/jocn_a_00024. [DOI] [PubMed] [Google Scholar]
- Plessow F, Kiesel A, Kirschbaum C. The stressed prefrontal cortex and goal-directed behavior: acute psychosocial stress impairs the flexible implementation of task goals. Exp Brain Res. 2012;216:397–408. doi: 10.1007/s00221-011-2943-1. [DOI] [PubMed] [Google Scholar]
- Pruessner J, Wolf O, Hellhammer D, Buske-Kirschbaum A, von Auer K, Jobst S, Kaspers F, Kirschbaum C. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci. 1997;61(26):2539–2549. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Cohen MX, Brozinsky CJ. Working memory maintenance contributes to long-term memory formation: neural and behavioral evidence. J Cognitive Neurosci. 2005;17(7):994–1010. doi: 10.1162/0898929054475118. [DOI] [PubMed] [Google Scholar]
- Ranganath C, DeGutis J, D’Esposito M. Category-specific modulation of inferior temporal activity during working memory encoding and maintenance. Cognitive Brain Res. 2004;20(1):37–45. doi: 10.1016/j.cogbrainres.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Johnson MK, D’Esposito M. Prefrontal activity associated with working memory and episodic long-term memory. Neuropsychologia. 2003;41(3):378–389. doi: 10.1016/s0028-3932(02)00169-0. [DOI] [PubMed] [Google Scholar]
- Raymond JE, Shapiro KL, Arnell KM. Temporary suppression of visual processing in an RSVP task: an attentional blink. J Exp Psychol Human. 1992;18(3):849–860. doi: 10.1037//0096-1523.18.3.849. [DOI] [PubMed] [Google Scholar]
- Rensink RA, O’Regan JK, Clark JJ. To see or not to see: the need for attention to perceive changes in scenes. Psychol Sci. 1997;8(5):368–373. [Google Scholar]
- Roelofs K, Elzinga BM, Rotteveel M. The effects of stress-induced cortisol response on approach-avoidance behavior. Psychoneuroendocrinology. 2005;30:665–677. doi: 10.1016/j.psyneuen.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Roozendaal B. Glucocorticoids and the regulation of memory consolidation. Psychoneuroendocrinology. 2000;25(3):213–238. doi: 10.1016/s0306-4530(99)00058-x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B. Stress and memory: opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiol Learn Mem. 2002;78(3):578–595. doi: 10.1006/nlme.2002.4080. [DOI] [PubMed] [Google Scholar]
- Salimetrics. High sensitivity salivary cortisol enzyme immunoassay kit. State College (PA): Salimetrics, LLC; 2012. p. 1. Cortisol determination: Cat No: 1-3002 research kit; Cat No: 1-3102 diagnostic kit. [Google Scholar]
- Schon K, Hasselmo ME, LoPresti ML, Tricarico MD, Stern CE. Persistence of parahippocampal representation in the absence of stimulus input enhances long-term encoding: a functional magnetic resonance imaging study of subsequent memory after a delayed match-to-sample task. J Neurosci. 2004 Dec 8;24(49):11088–11097. doi: 10.1523/JNEUROSCI.3807-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoofs D, Preuß D, Wolf OT. Psychosocial stress induces working memory impairments in an n-back paradigm. Psychoneuroendocrinology. 2008;33(5):643–653. doi: 10.1016/j.psyneuen.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Schoofs D, Wolf OT, Smeets T. Cold pressor stress impairs performance on working memory tasks requiring executive functions in healthy young men. Behav Neurosci. 2009;123(5):1066–1075. doi: 10.1037/a0016980. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Oitzl MS, Richter S, Schachinger H. Modulation of spatial and stimulus-response learning strategies by exogenous cortisol in healthy young women. Psychoneuroendocrinology. 2009;34:358–366. doi: 10.1016/j.psyneuen.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT. Emotional modulation of the attentional blink: is there an effect of stress? Emotion. 2010;10(2):283–288. doi: 10.1037/a0017751. [DOI] [PubMed] [Google Scholar]
- Todd JJ, Marois R. Posterior parietal cortex activity predicts individual differences in visual short-term memory capacity. Cogn Affect Behav Ne. 2005a;5(2):144–155. doi: 10.3758/cabn.5.2.144. [DOI] [PubMed] [Google Scholar]
- Todd JJ, Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature. 2005b;428:751–754. doi: 10.1038/nature02466. [DOI] [PubMed] [Google Scholar]
- Weerda R, Muehlhan M, Wolf OT, Thiel CM. Effects of acute psychosocial stress on working memory related brain activity in men. Hum Brain Mapp. 2010;31(9):1418–1429. doi: 10.1002/hbm.20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf OT, Convit A, McHugh PF, Kandil E, Thorn EL, De Santi S, McEwen BS, de Leon MJ. Cortisol differentially affects memory in young and elderly men. Behav Neurosci. 2001;115(5):1002–1011. doi: 10.1037//0735-7044.115.5.1002. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Vogel EK. Fractionating working memory: consolidation and maintenance are independent processes. Psychol Sci. 2005;16(2):106–11. doi: 10.1111/j.0956-7976.2005.00790.x. [DOI] [PubMed] [Google Scholar]
- Young AH, Sahakian BH, Robbins TW. The effects of chronic administration of hydrocortisone on cognitive function in normal male volunteers. Psychopharmacology. 1999;145:260–266. doi: 10.1007/s002130051057. [DOI] [PubMed] [Google Scholar]