Abstract
Increasing endogenous ciliary neurotrophic factor (CNTF) expression with a pharmacological agent might be beneficial after stroke as CNTF both promotes neurogenesis and, separately, is neuroprotective. P2X7 purinergic receptor inhibition is neuroprotective in rats and increases CNTF release in rat CMT1A Schwann cells. We, first, investigated the role of P2X7 in regulating CNTF and neurogenesis in adult mouse subventricular zone (SVZ). CNTF expression was increased by daily intravenous injections of the P2X7 antagonist Brilliant Blue G (BBG) in naïve C57BL/6 or Balb/c mice over 3 days. Despite the ∼40–60 % increase or decrease in CNTF with BBG or the agonist BzATP, respectively, the number of proliferated BrdU+SVZ nuclei did not change. BBG failed to increase FGF2, which is involved in CNTF-regulated neurogenesis, but induced IL-6, LIF, and EGF, which are known to reduce SVZ proliferation. Injections of IL-6 next to the SVZ induced CNTF and FGF2, but not proliferation, suggesting that IL-6 counteracts their neurogenesis-inducing effects. Following ischemic injury of the striatum by middle cerebral artery occlusion (MCAO), a 3-day BBG treatment increased CNTF in the medial penumbra containing the SVZ. BBG also induced CNTF and LIF, which are known to be protective following stroke, in the whole striatum after MCAO, but not GDNF or BDNF. However, BBG treatment did not reduce the lesion area or apoptosis in the penumbra. Even so, this study shows that P2X7 can be targeted with systemic drug treatments to differentially regulate neurotrophic factors in the brain following stroke.
Keywords: Ciliary neurotrophic factor, Mice, Neurogenesis, Neuroprotection, P2X7 purinergic receptor, Stroke
Introduction
We are interested in upregulating CNTF expression in the CNS with systemic pharmacological small molecule treatments. CNTF is a good target because it is almost exclusively produced in the CNS and PNS by astrocytes and Schwann cells, respectively [1, 2]. CNTF is a potent survival-promoting factor for oligodendrocytes [3, 4] and a variety of neurons [5–9]. Exogenous [10] and endogenous [11] CNTF are protective following stroke in rodents, where it is increased in astrocytes [12]. In the adult CNS, CNTF promotes neurogenesis in the SVZ under normal [13, 14] and stroke-induced conditions [15], where it is produced by astrocytes [11, 14]. Increasing neuroprotection during the acute phase following stroke and promoting neurogenesis over more protracted postinjury periods with the same agent might be a good treatment strategy.
Extracellular ATP is a potent inducer of astrocytic calcium signaling [16], and P2X7 receptors can mediate neurodegeneration [17], possibly by cytotoxic calcium influx and inflammation [18–21]. Astrocytes have P2X7 receptors [22, 23], and their stimulation can induce glutamate release [24] and a decrease in glutamate uptake [25] possibly contributing to excitotoxicity. Indeed, the P2X7-specific inhibitor BBG [26] is neuroprotective in rats after MCAO-induced stroke [27], spinal cord injury [28, 29], and in a mouse model of Huntington's disease [30]. Therefore, one would expect that BBG would also be effective in mice after stroke although this remains to be tested. P2X7 −/− mice do not have a reduced infarct size after stroke [31], but it is possible that they have undergone developmental compensation by other genes. The protective effects of BBG might be related to an increase in neuroprotective factors such as CNTF, which can rescue striatal neurons after stroke [10] and in Huntington models [32–34]. CNTF release is increased following P2X7 siRNA silencing or pharmacological inhibition in cultured rat CMT1A mutant Schwann cells, which have reduced CNTF expression [35, 36]. Whether astroglial CNTF can be regulated through P2X7 receptors is unknown.
P2X7 receptors have been reported in reactive microglia, astrocytes, and neurons, but this has been brought into question due to unreliable antibodies that have cross-reactivity with an unknown protein [37, 38]. Electrophysiological data confirm that astrocytes can have functional P2X7 receptors [39, 40], raising the possibility that they might regulate CNTF directly. Ependymal cells lining the ventricles also have functional P2X7 receptors, as shown with a reporter gene [41], and electrophysiology [42]. Ependymal cells regulate adult SVZ neurogenesis [43–46].
Here, we investigated the role of the P2X7 receptor in regulating CNTF expression in the adult mouse SVZ and its validity as a pharmacological target of systemic treatments to promote neurogenesis and/or neuroprotection under naïve and stroke conditions.
Materials and Methods
Experimental Animals
A total of 135 male C57BL/6 and Balb/c mice (10–12 weeks of age; 20–28 g; The Jackson Laboratory, Bar Harbor, ME, USA) were used for the in vivo experiments. Cells for tissue cultures were obtained from 2- to 3-day-old postnatal mice. Deep anesthesia for surgeries was achieved by an intraperitoneal injection of Avertin (0.4 mg 2,2,2-tribromoethanol in 0.02 ml of 1.25 % 2-methyl-2-butanol in saline per gram body weight; Sigma-Aldrich, St. Louis, MO, USA). Care and use of experimental animals were carried out according to the guidelines of the University of Louisville Institutional Animal Care and Use Committee and the National Institutes of Health guidelines.
BBG and BzATP Intravenous Injections
For intravenous injections, a midline incision was made in the ventral neck area and one of the jugular veins was exposed by blunt dissection. BzATP [47] and BBG [26] are known as highly selective agonists and antagonists for the P2X7 receptor [16], respectively. For example, BBG has 1,000-fold higher selectivity compared to other P2X receptors [26]. BzATP, for example, fails to activate salivary glands of P2X7 null mice even though they express multiple isoforms of P2X and P2Y nucleotide receptors [48]. A total of 0.1 ml of saline as control vehicle, 35 mg/kg BBG [approximately 1 mg per injection; cat. # B8522, Sigma-Aldrich], or 35 mg/kg BzATP [cat. # B6396, Sigma-Aldrich] was injected daily over 3 days in naïve mice, or starting immediately after middle cerebral artery occlusion (MCAO) (see below). This dose was within the maximally effective range in our preliminary experiment for CNTF expression in naïve mice. Others have shown that i.p. injections of 30 mg/kg BBG twice a day for 3 days is protective after MCAO in rats [27] and that i.v. injection of 10 or 50 mg/kg once a day was equally protective after spinal cord injury in rats [29]. We chose the i.v. route because the drugs would rapidly reach the brain that is important for neuroprotection. Therefore, our dosing was well within the acute neuroprotective range as shown in rats. We chose to inject into the jugular vein due to the viscosity of the BBG solution that made it more difficult to inject in the tail veins.
The skin wound was closed using metal sutures. At the end of the 3-day period, i.e., 24 h following the last injection, i.e., 72 h following the first injection, the mice were processed for RNA extraction or for histology.
Ischemic Stroke by Temporary Middle Cerebral Artery Occlusion
Focal cerebral ischemia was induced by MCAO for 15 min followed by reperfusion. We chose 15 min so that the lesions would be moderately severe to not injure the SVZ [15] and to give the BBG drug an opportunity to be effective. After the mice were anesthetized, a needle probe of Laser Doppler Flowmetry (Moor VMS-LDF, VP10M200ST, P10d, Moor Instruments Ltd., Wilmington, DE, USA) was glued directly on the superior portion of the temporal bone to monitor cerebral blood flow. A midline skin incision in the neck area with the mouse in the supine position was made, and the left external carotid artery (ECA) was exposed and isolated from small artery branches with a bipolar coagulator (N.S. 237, Codman & Shurtleff, Inc., Randolph, MA, USA). The ECA was ligated with 5–0 silk suture. Microvascular clips were temporarily placed on the common carotid artery (CCM) and the internal carotid artery (ICM), and an arteriotomy was made in the ECA. Next, a 0.21-mm diameter suture (6021PK5Re, DOCOL Co., Redlands, CA, USA) was inserted into the ECA and advanced to the carotid bifurcation along the ICA and to the origin of the MCA. Cerebral blood flow decreased immediately after MCA occlusion to about 20 % of baseline. The suture was withdrawn 15 min after MCA occlusion to restore blood flow of the MCA territory. Sham-operated mice received the same surgery, except suture insertion. Body temperature was maintained at 37.0±0.5 °C with a heating pad during the operation and the recovery periods. The wound was closed in layers using silk and metal sutures. This procedure produces consistent injuries involving the striatum and overlying cortex but does not injure the most medial part of the striatum and the SVZ (Fig. 4a; [15]).
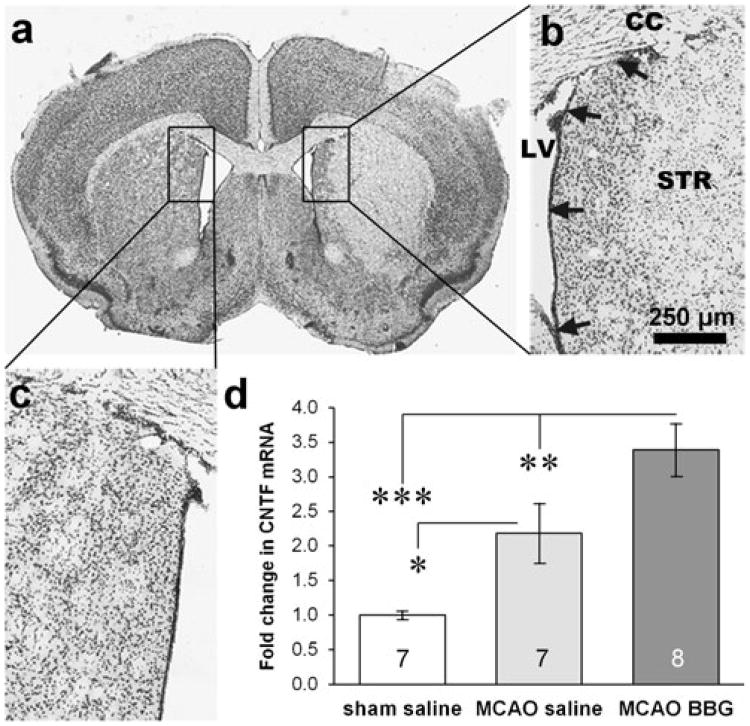
Fig. 4.
P2X7 inhibition stimulates CNTF in the SVZ after stroke. a, b In a cresyl violet-stained coronal section of a C57BL/6 mouse brain, the ischemic injury caused by a unilateral MCAO can be seen in the striatum (STR) and cortex but not the SVZ (arrows). CC corpus callosum, LV lateral ventricle. Scale bar, 250 μm. c The uninjured side is shown for comparison. d MCAO increased CNTF in C57BL/6 mice, while intravenous injections of BBG over 3 days following MCAO increased CNTF mRNA even more in the ipsilateral SVZ as measured by qPCR (n=7 sham, 7 saline MCAO, 8 BBG MCAO). Data are mean ±SEM and calculated as fold of sham-operated control. *=p<0.05, **=p<0.01, ***=p<0.001
Real-time Quantitative Reverse Transcription PCR
Total RNA was isolated from freshly dissected 0.5-mm-wide SVZ strips or the rest of the striatum of mice, 3 days after MCAO, obtained from 1-mm-thick slices using a commercial kit (74104, Qiagen, Valencia, CA, USA), and was used as template for reverse transcription (RT), which runs with total RNA (1.0 μg), 1.0 μl of 500 ng/μl random primers (C118A, Promega, San Luis Obispo, CA, USA), 1.25 μl of 10 mM dNTP mix (U151A, Promega, San Luis Obispo, CA, USA), 5 μl 5× RT buffer, 1.75 μl RNase-free water, and 1 μl of 200 U/μl MMLV-RT (M170A, Promega, San Luis Obispo, CA, USA). RT reactions were performed at 70 °C for 5 min and 37°C for 1 h. Real-time RT-PCR was performed using primer sets specific for mouse CNTF and GAPDH gene sequences (CNTF: mM0046373_m1 FAM, P2X7 receptor: mM00440582_m1 FAM, and GAPDH: 4352339E VIC, Applied Biosystems, Foster City, CA, USA). PCR reactions were performed using the TaqMan® Gene Expression Master Mix kits (4369016, Applied Biosystems, Foster City, CA, USA) as follows: 40 cycles at 95 °C for 15 s and 60 °C for 60 s after 10 min at 95 °C to activate AmpliTaq Gold DNA polymerase in an ABI 7900 Thermal Cycler (Applied Biosystems, Foster City, CA, USA). After quantitative reverse transcription PCR (qPCR), the numbers of cycles used to reach a given FAM fluorescence intensity for the CNTF fragment were subtracted from (normalized to) that for the GAPDH fragment to calculate the relative abundance of CNTF mRNA. We used the 2−ΔΔCt method to calculate changes compared to sham-operated mice.
Bromodeoxyuridine Immunostaining
Bromodeoxyuridine (BrdU) (50 mg/kg per injection; cat. # B5002, Sigma-Aldrich) was injected intraperitoneally twice a day during the 3 days before histological processing. Sixteen hours after the last BrdU injection, the mice were perfused transcardially with ice-cold phosphate-buffered saline (PBS, 0.1 M, pH 7.4) and 4 % paraformaldehyde in phosphate buffer (PB, 0.1 M, pH 7.4). Afterwards, the brains were fixed overnight in paraformaldehyde and cryoprotected in 30 % sucrose in phosphate buffer for 24 h. Coronal 30-μm-thick sections through the brains were cut on a sliding freezing microtome (SM 2000R, Leica, Bannockburn, IL, USA). Starting at a random point along the rostrocaudal axis of the brain, every sixth section through the SVZ was immunostained for BrdU (MAB3510, mouse IgG, clone BU-1, 1:30,000; Millipore, Billerica, MA, USA). Briefly, sections were incubated in 50 % formamide in 2× SSC at 65 °C for 2 h, rinsed in fresh 2× SSC, incubated in 2 N HCl at 37 °C for 30 min, neutralized in 0.1 M boric acid, pH 8.5, for 10 min. This was followed by incubation in 10 % normal horse serum for 30 min to block nonspecific staining in primary antibodies overnight and biotinylated horse anti-mouse IgG (1:800; BA2001, Vector Laboratories, Burlingame, CA, USA) for 1 h, all dissolved in PBS containing 5 % horse serum. This was followed by incubation in avidin–biotin complex conjugated with peroxidase for 1 h (1:600 in PBS, PK6100, Vector Laboratories). Chromogen reaction was performed with 0.04 % 3,3′-diaminobenzidine (cat. # D5637, Sigma-Aldrich) solution containing 0.06 % nickel ammonium sulfate and 1 % hydrogen peroxide in 0.05 M tris buffer–HCl. Sections were then rinsed in 0.1 M phosphate buffer, mounted on glass slides, and coverslipped in Permount (SP15, Fisher Scientific, Fair Lawn, NJ, USA).
Histology for Cell Death
To demarcate the astrocyte-free infarct area, every sixth brain section through the injured striatum of 3 days after MCAO was immunostained with rabbit anti-GFAP antibodies (1:1,000; AB5804 Millipore, Billerica, MA, USA) and Alexa fluor 488-conjugated goat anti-rabbit secondary antibodies (1:500; A11008 Invitrogen, Eugene, OR, USA).
To detect cells undergoing apoptosis in the cortical penumbra, adjacent tissue sections were stained for terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL; cat. # 17–141, Millipore, Billerica, MA, USA) and counterstained with Hoechst to detect cells with condensed or fragmented nuclei. Staining was performed according to the manufacturer's protocol. Briefly, after washing in PBS, sections were incubated with proteinase K for 30 min at 37 °C and reaction was stopped by washing sections with PBS. This was followed by incubation in TdT end-labeling cocktail for 1 h at 37 °C. To remove the TdTend-labeling cocktail and stop the reaction, sections were immersed in TB buffer and PBS and followed by incubation in blocking buffer for 20 min at room temperature. Sections were immunostained with avidin–FITC solution for 30 min at 37 °C and then washed in PBS. For counterstaining, sections were stained with Hoechst for 5 min, washed in PB, and coversliped with antifade Gel Mount aqueous mounting media (SouthernBiotech, Birmingham, AL, USA).
BrdU-Positive Nuclei Counts and Stroke Injury Measurements
The number of BrdU-positive nuclei in the SVZ was counted using an unbiased optical fractionator stereological method (Stereologer; Systems Planning and Analysis, Alexandria, VA, USA) [49] and a motorized Leica DMIRE2 microscope. The reference space was defined as a 50-μm-wide strip of the entire lateral of all the lateral ventricles, encompassing dorsoventrally the ventral tip and the dorsolateral triangular regions of the lateral ventricle, and rostrocaudally from the genu of the corpus callosum to the caudal end of the decussation of the anterior commissure. BrdU-positive nuclei were counted within the reference space in software-defined frames. The total number of BrdU-positive cells in a brain was calculated by the software as: n=number of nuclei counted×1/section sampling fraction× 1/area sampling fraction×1/thickness sampling fraction.
The injury area in vehicle or BBG-treated mice was measured in GFAP-immunostained sections using ImageJ (Wayne Rasband, NIH) and the area calculated as a percentage of the entire ipsilateral (MCAO) or contralateral half of the brain. The distance of the injury rim to the SVZ was also measured to indicate the extent of the injury in the medial penumbra, where a 3-day treatment with BBG had increased CNTF in naïve mice or after MCAO.
TUNEL+cells were counted manually in the penumbra of three sections from vehicle or BBG-treated mice, 3 days after MCAO. The analyzed penumbra of the MCAO injury was defined as spanning the width of the primary somatosensory cortex area [50], using the GFAP-dense region in adjacent sections as a guide to define the penumbra and using a 63× oil objective (Fig. 5b). In addition, apoptotic cells were counted in the medial striatum next to the SVZ. Only TUNEL+nuclei with clear signs of apoptotic condensation or fragmentation were counted.
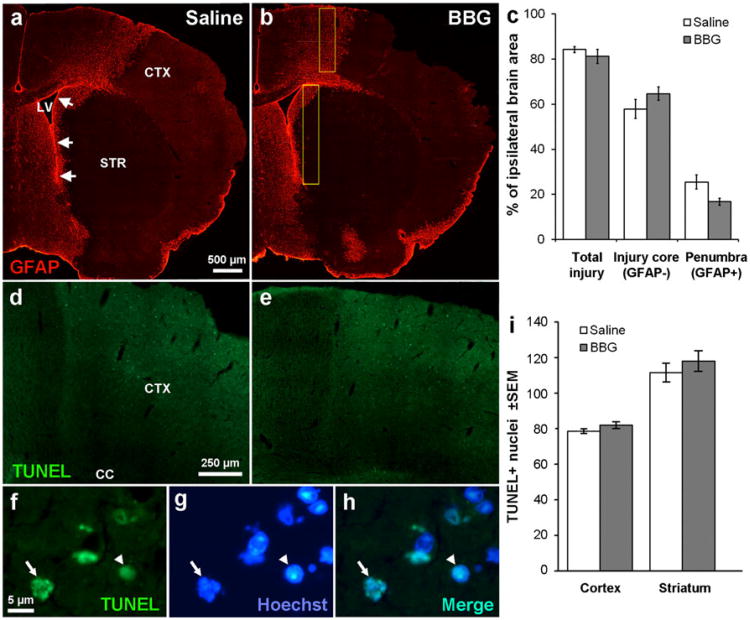
Fig. 5.
P2X7 inhibition is not neuroprotective after stroke. a MCAO in C57BL/6 mice caused severe loss of cells as shown in the lack of GFAP immunofluorescent staining in the injury core, involving both the striatum (STR) and cortex (CTX). The medial penumbra towards the SVZ (arrows) and neighboring lateral ventricle (LV) shows abundant GFAP+staining. b The injury appeared similar in mice treated for 3 days with BBG. c Measurements of the injury area were calculated as a percentage of the whole ipsilateral brain and show no significant effect of a BBG treatment over 3 days following MCAO on the area of the injured core or the penumbra. N=6 saline, 4 BBG. TUNEL staining shows many apoptotic cells in the penumbral region of the cortex in both saline- (d) and BBG (e)-injected mice after MCAO. Apoptotic nuclei were defined by TUNEL staining (f) and their typical condensed (arrowhead) or fragmented (arrow) appearance as seen in Hoechst staining (g) as confirmed by double staining (h). i Apoptotic cells were counted in the primary somatosensory area and medial striatum (yellow boxes in b) with a 63× oil objective and were not significantly different between vehicle and BBG-treated mice. N=5 saline, 4 BBG
P2X7 In Situ Hybridization
Because the location of P2X7 receptors in the SVZ is unknown, we performed in situ hybridization. Two C57BL/6 mice were terminally anesthetized and transcardially perfused with ice-cold PBS followed by 4 % paraformaldehyde. Brains were removed and postfixed for 24 h at 4 °C and then embedded in paraffin wax and 7-μm coronal sections prepared on a microtome. Sections were dewaxed by immersion in xylene and rehydrated through a series of alcohol:H2O washes (100, 90, 70, and 50 % ethanol). Sections were further fixed in paraformaldehyde for 10 min, washed in PBS, and endogenous peroxidases quenched by incubation in 0.3 % H2O2 for 30 min. Sections were acetylated in acetic anhydride for 10 min (0.5 %), washed in PBS:Tween (PBST, 0.5 %) followed by incubation with proteinase K (10 μg/ml, 30 min, 37 °C; cat. # P2308, Sigma-Aldrich). Proteinase K was deactivated by washing in glycine (5 min, 2 mg/ml in PBS) followed by washes in 2× saline sodium citrate (SSC). Sections were prehybridized for 2 h at 50 °C in hybridization buffer (50 % formamide, 5× SSC, 1× Denhardts, 20 % dextran sulfate, and 0.1 mg/ml salmon sperm (cat. # 15632–011, Invitrogen). Hybridization was then performed for 24 h at 50 °C with 500 ng/ml of 3′ end-labeled digoxigenin (DIG) probe previously labeled using the DIG oligonucleotide 3′ end labeling kit (cat. # 03353575910, Roche, Indianapolis, IN, USA). P2X7 receptor oligonucleotide probes were 5′-GACTTTGTTTGTCTCATACTGCAAGACATCGTTC, CCAAAGCAAAGCTAATGTAGGAAAAGACGATCATG, and GTCTGCACTTGGCCTTCTGACTTGACATAGTTTGT-3′ (Invitrogen, US) and located outside P2X7 receptor mutation site P451L. Positive control probes were targeted to proteolipid protein (PLP), found throughout the brain. Washes were performed in 1× SSC for 20 min followed by washing in 0.5× SSC at hybridization temperature. Probes were blocked for 1 h in heat-inactivated sheep serum (5 % in PBST) at room temperature, incubated in 1:500 anti-DIG-POD (cat. # 01120773 3910, Roche, Indianapolis, IN, USA) in 0.5 % sheep serum overnight at 4 °C, and labeled for detection with the TSA-Plus Rhodamine system (cat. # NEL744E001KT, PerkinElmer, San Jose, CA, USA) according to manufacturer's protocol.
Astrocyte–Neuron Coculture
To test whether P2X7 agonist and antagonists could have direct effects on astrocytes, primary astrocyte–neuron cocultures were prepared as described elsewhere [51] with some modifications [11]. In short, brains from postnatal 2–3-day C57BL/6 mice were dissociated, cells triturated, and plated on 10-cm dishes. Cells were grown to confluence and then maintained for an additional 6–7 days before astrocytes were collected and plated into six-well plates at 30,000 cells/ml and grown for a further 5–7 days. Primary mouse neuron cultures were isolated from P0-1 C57BL/6 mice cortices and, after dissociation, the neurons were plated onto five to seven DIV astrocyte cultures in 0 % serum medium. BBG (50 μM; cat. # B8522, Sigma-Aldrich) and BzATP (50 μM; cat. # B6396, Sigma-Aldrich) were applied to culture medium for 24 h before RNA was isolated for RT-qPCR. These concentrations were above the IC50 of known BBG binding to P2X7 [26] and the lowest concentrations that did not cause cell loss. Parallel cultures were fixed and immunostained with GFAP for assessment of astrocyte morphology.
Statistical Analysis
All qPCR and histological analyses were done with investigators blinded to the treatment. Statistical analyses were performed with either the Student's t test or, if more than two groups, ANOVA using Excel software (Microsoft, Seattle, WA, USA). A value of p<0.05 was considered to be statistically significant. All data are presented as a group average+SEM.
Results
P2X7 Receptors Inhibit Endogenous CNTF Expression in the Adult Mouse SVZ
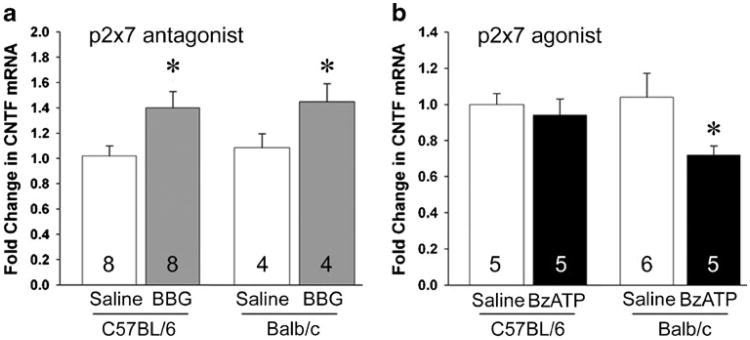
Naïve mice were injected i.v. once a day for 3 days and analyzed 24 h after the last injection, i.e., 72 h after the first one. CNTF mRNA expression was increased by BBG in the SVZ of C57BL/6 mice (1.40±0.13 versus saline, 1.00± 0.08; Fig. 1a). To determine whether P2X7-induced calcium influx might be involved in inhibiting CNTF expression, we compared C57BL/6 mice, which have a P2X7 point mutation that reduces, but not abolishes, calcium signaling [52–54], to Balb/c mice. BBG-treated Balb/c mice also had an increase in CNTF mRNA in the SVZ (1.36±0.13 versus saline, 1.00±0.10; Fig. 1a). The P2X7 agonist BzATP decreased CNTF mRNA in Balb/c mice (0.67± 0.04 versus saline, 1.00±0.12; Fig. 1b) but had no effect in C57BL/6 mice (0.94±0.09 versus saline, 1.00±0.06; Fig. 1b). These data suggest that P2X7 receptor can suppresses CNTF expression in the SVZ through calcium signaling and at least one other signaling pathway. Importantly, BBG induced CNTF expression, which raised the possibility that it could both induce neurogenesis and act as a neuroprotectant.
Fig. 1.
CNTF expression in the adult mouse SVZ is downregulated by the P2X7 receptor. a Intravenous injections of the P2X7 antagonist BBG over 3 days increased CNTF mRNA measured by qPCR in the naïve SVZ in C57BL/6 mice (n=8 per group). CNTF expression was also increased in BBG-injected Balb/c mice (n=4 per group). b The P2X7 receptor agonist, BzATP, did not change CNTF expression in C57BL/6 mice (n=5 per group) but reduced CNTF mRNA in Balb/c mice (n=6 saline, n=5 BzATP). Data are mean±SEM and calculated as fold of control. *=p<0.05
P2X7 Receptors Do Not Regulate CNTF Expression in Cultured Astrocytes
In the SVZ, as in most of the brain, only astrocytes produce CNTF and we wanted to determine whether the P2X7 drug effects were direct or not. Like others [39], we detect specific signal in cultured primary cortical astrocytes with a ΔCT value of approximately 8.5 compared to GAPDH. Finding any signal is supportive of genuine P2X7 receptors as the TaqMan FAM/BHQ probes provide an additional level of specificity above and beyond the specific primer sets. However, treatment of the neuron–astrocyte cocultures for 24 h with BBG or BzATP did not affect the levels of CNTF mRNA nor the morphology of the astrocytes (data not shown). The drug concentrations ranged from 0.1 to 200 μM, with 0.1 and 50 μM BBG being around the maximum effectiveness on rat and human P2X7 receptors, respectively [26]. This suggests that the P2X7 receptors do not directly regulate CNTF gene expression in astrocytes.
P2X7 Receptors are Present, But At Very Low Levels, in the Adult Mouse SVZ
To help identify whether other cells might mediate the drug effects on astroglial CNTF in vivo, we tried a number of different methods to detect the cell type expressing P2X7 in the adult mouse SVZ. We tested several commercial antibodies that showed no specific signal before we realized that they were unreliable as discussed in the “Introduction”. We designed a specific P2X7 probe set and performed in situ hybridization but were unable to find any signal (data not shown). Our PLP-positive control probe gave intense staining of many cells through the brain as expected, whereas the control sense probe revealed no staining. P2X7 mRNA levels measured by qPCR were fourfold higher in naïve SVZ tissue than in the striatal extracts that lack the SVZ (data not shown). Together with the culture data, this suggests that P2X7 is present in the SVZ, probably in the ependymal cells that line the medial side of the SVZ [41, 42].
P2X7 Receptor Modulation Does Not Affect Neurogenesis in the Adult Mouse SVZ
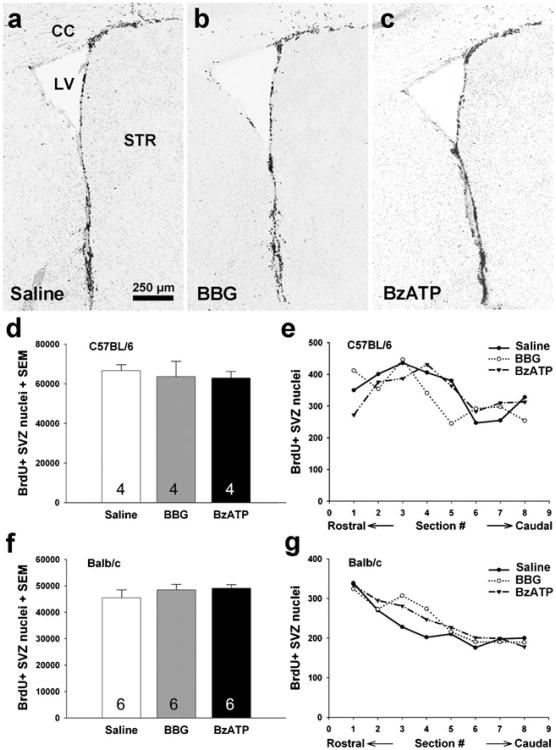
Endogenous CNTF increases the rate of neurogenesis as shown before by CNTF antibodies, knockout mice, and CNTF injections over 3-day treatment periods [13, 14]. Thus, we tested whether the changes in CNTF caused by the 3-day P2X7 drug treatments would also promote neurogenesis. BrdU-positive cells were counted in the SVZ using unbiased stereology after systemic injections of BrdU and BBG or BzATP over 3 days (72 and 24 h after the first and last drug injections, respectively). In C57BL/6 mice, neither BBG nor BzATP caused any change in the number of BrdU-positive nuclei compare to the saline-injected group (saline, 66,580±3,088; BBG, 63,607±7,725; BzATP, 62,871±3,368; Fig. 2a–d). To rule out the possibility that the drugs were more effective in certain regions of the SVZ, the datasets were analyzed for the numbers of events (nuclear counts) per coronal section along the rostrocaudal extent of the SVZ. This showed that the drugs were ineffective throughout the SVZ (Fig. 2e). In Balb/c mice, where both BBG and BzATP had effects on CNTF levels, no changes in the numbers of BrdU-positive nuclei were observed either (saline, 45,419±3,058; BBG, 48,487±2,061; BzATP, 49,098±1,366; Fig. 2f. The differences in the total numbers between C57BL/6 and Balb/c mice indicate a strain-dependent difference in the proliferation rate, as also found by others [55].
Fig. 2.
SVZ neurogenesis is not affected by P2X7 antagonist or agonist injections. BrdU-positive cells of are shown in coronal sections through the SVZ of adult C57BL/6 mice treated with saline (a), BBG (b), and BzATP (c). CC corpus callosum, LV lateral ventricle, STR striatum. Scale bar: 250 μm. The number of BrdU-positive SVZ cells counted by unbiased stereology was not affected by 3-day BBG or BzATP injections compared to vehicle injections in C57BL/6 mice (d, e, n=4 each group) or Balb/c mice (f, g, n=6 each group)
P2X7 Inhibition Fails to Induce Proneurogenic Growth Factor Conditions
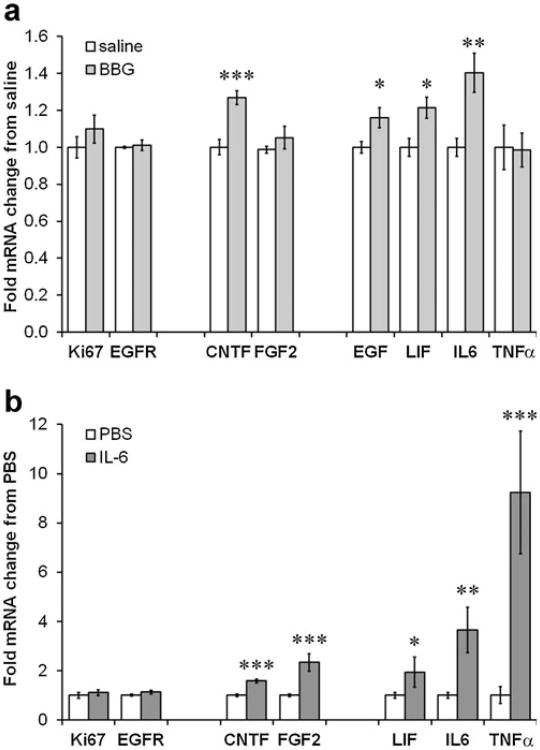
We had previously shown that stroke-induced neurogenesis was dependent on CNTF with downstream induction of FGF2 [15], known to promote neurogenesis as opposed to EGF [56]. LIF [57], IL-6 [58], and TNFα [59, 60] can counteract progenitor proliferation. In the SVZ of naïve mice treated for 3 days with BBG, which induced CNTF mRNA (Fig. 1a), FGF2 expression was not increased, whereas the antineurogenic growth factors were increased by ∼20–40 % (Fig. 3a). As expected, within the same tissue extracts, expression of proliferation markers, specifically EGFR of the rapidly proliferating C cell progenitor, was not increased either (Fig. 3a).
Fig. 3.
P2X7 inhibition induces counteracting growth factors in the SVZ. a A 3-day injection of BBG in naïve C57BL/6 mice did not induce the expression of the general proliferation marker, Ki67, or the C cell marker EGFR. Expression of CNTF mRNA was increased, but not of FGF2, which is known to drive neuroblast formation. EGF, LIF, and IL-6, which are known to reduce neurogenesis, are increased by BBG (n=6 saline, 8 BBG). b Injection of 1 μg IL-6 directly into the striatum next to the SVZ in naïve C57BL/6 mice caused an increase in mRNA for CNTF, FGF2, LIF, and IL-6 itself in the SVZ after 24 h (n= 7 PBS, 4 IL-6). Data are mean±SEM and calculated as fold of control. *=p<0.05, **=p<0.01, ***=p<0.001. All data in Fig. 3 were obtained by qPCR
We had previously shown that recombinant IL-6 injections into the neighboring striatum induces CNTF expression in the naïve SVZ as measured by qPCR 24 h later [11]. In the same SVZ extracts, we found that IL-6 also increased expression of FGF2 (2.2-fold), LIF (1.9-fold), IL-6 (3.6-fold), and TNFα (9.2-fold), but not the proliferation marker EGFR (Fig. 3b). This may indicate a neutral outcome of the proneurogenic (FGF2) and NSC self-renewal (IL-6 and LIF) effects. It is possible that BBG-induced IL-6 has similar effects.
P2X7 Inhibition Further Increases Stroke-Induced CNTF in the SVZ/Medial Striatum
In naïve mice, the 3-day BBG induced CNTF expression in the SVZ tissue (Fig. 1). This contains a part of the medial striatum, i.e., the penumbra of a 15-min MCAO injury (Fig. 4b). In mice, endogenous CNTF is neuroprotective after MCAO [11]. We, therefore, tested whether the P2X7 mechanisms regulating CNTF would still be intact following MCAO. CNTF levels increased 2.2-fold over sham-operated mice in the SVZ tissue after systemic i.p. injection with vehicle over 3 days starting immediately following an MCAO in C57BL/6 and analyzed 24 h after the last injection (Fig. 4d). In a previous study, a 15-min MCAO induced CNTF expression only 23 % in the SVZ at 12 days, whereas the 30-min MCAO induced CNTF by more than fourfold [15]. One potential reason for this apparent discrepancy is that the current mice were younger and weighed less, potentially creating larger lesions, as also suggested by the histological sections. Also, we do not know what the CNTF levels at the 3-day time point were in our previous study. Injections of BBG caused a further increase in CNTF expression (3.4-fold versus sham; Fig. 4d). Together, these data suggest that CNTF expression is regulated normally following a mild MCAO injury that does not cause overt cell loss in the SVZ.
P2X7 Inhibition Differentially Induces Growth Factors and Does Not Rescue Brain Cells After Stroke
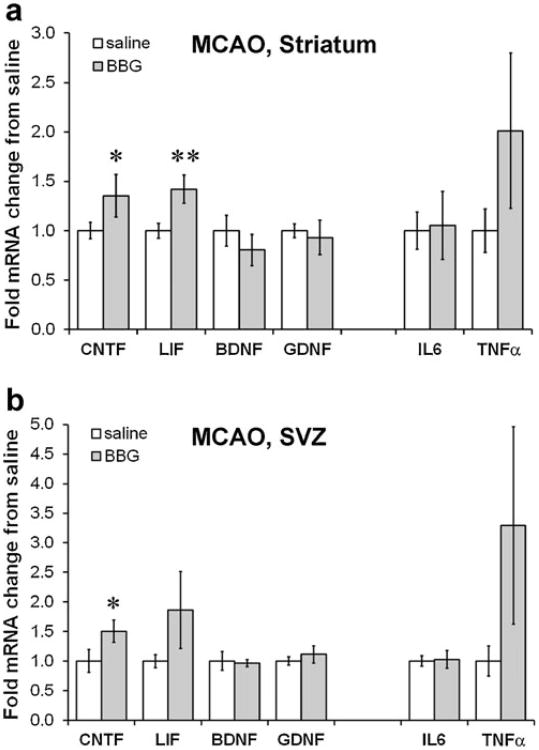
Despite the increase in CNTF caused by the BBG treatment, a separate set of treated mice with an MCAO did not show any evidence of neuroprotection (Fig. 5a, b), as quantified by the total injury area, the GFAP-negative injury core, or the injured GFAP+penumbra in tissue sections (Fig. 5c). There also was no significant difference in the number of apoptotic cells in the cortical penumbra of adjacent sections between the BBG and vehicle treatment, as determined by counts of TUNEL+nuclei with condensed or fragmented nuclei (Fig. 5d–i). The distance of the medial striatal penumbra to the SVZ was also not different between vehicle and BBG-treated mice (1,570±266 μm versus 564±92 μm; p=0.19). To exclude the possibility that BBG did not reach or affect the striatum after MCAO, we measured expression of various neurotrophic factors. Striatal CNTF mRNA was increased ∼40 % by BBG treatment after MCAO, as was LIF mRNA (Fig. 6a), which is neuroprotective following stroke [61]. The expression of two other neurotrophic factors, BDNF and GDNF, was not affected by BBG (Fig. 6a). IL-6 and TNFα, both markers of inflammation, were also not significantly affected by the BBG treatment following MCAO (Fig. 6a). The same measurements in the SVZ of the MCAO mice showed that only CNTF was significantly increased by BBG treatment (Fig. 6b). As expected, BBG did not affect the proliferation markers Ki67 and EGFR in the SVZ after MCAO (data not shown).
Fig. 6.
P2X7 inhibition after stroke increases some neurotrophic factors. a qPCR measurements of growth factor mRNA expression in the injured striatum show that CNTF and LIF are significantly increased by BBG injections over 3 days following MCAO in C57BL/6 mice. N=8 saline, 5 BBG. p<0.05, **=p<0.01. b BBG treatment induced CNTF in the ipsilateral SVZ following MCAO as measured by qPCR but had no significant effects on the other growth factors, in some cases, due to high variability. N=4 saline, 6 BBG. Data are mean± SEM and calculated as fold of saline control. *=p<0.05
Discussion
P2X7 Receptor Signaling May Selectively Inhibit Expression of CNTF Family Members
This study identifies several important new aspects of the P2X7 receptor. In the naïve SVZ, BBG stimulated CNTF, LIF, and IL-6 expressions, but not that of FGF2 and TNFα. Following MCAO, BBG increased expression of CNTF and LIF in the striatum, but not of GDNF and BDNF. This shows an interesting selectivity of the P2X7 receptor in inhibiting growth factor expression. Astroglial CNTF is regulated by neuronal contact, but not by glutamate and GABA [11], whereas BDNF and NGF are regulated by these neurotransmitters [62–64]. We have found that CNTF and IL-6 injections into the striatum induces CNTF expression [11]. This feed-forward mechanism is also present in the retina [65]. Given their common signaling via the gp130 receptor [66], this suggests that the downstream STAT3 transcription factor is involved. We have recently found that CNTF is repressed by inhibitory phosphorylation of serine-727 residue on STAT3 [67]. Another intracellular signaling pathway that regulates CNTF expression in astrocytes involves inhibitory cAMP [14, 51, 68], and we have shown that the cAMP-reducing D2 dopamine agonist quinpirole increases CNTF expression in the adult mouse SVZ [14]. It remains to be determined whether and how the P2X7 receptor would affect these STAT3 and cAMP pathways in the CNS.
While the effects of BBG on CNTF expression in both Balb/c and C57BL/6 mice suggest a common signaling pathway, the agonist data reveal an additional signaling pathway. BzATP reduced CNTF expression in Balb/c, but not in C57BL/6 mice. C57BL/6 mice have a natural P2X7 receptor mutation (P451L) that reduces, although not abolishes, the sensitivity to ATP or BzATP and decreases the calcium influx in several types of cells, including astrocytes [52–54]. Thus, CNTF inhibition by P2X7 receptors may also be mediated by calcium-dependent signaling, but this remains to be tested.
Potential Indirect Effects of P2X7 Receptors on CNTF Expression
Published and our current data suggest that P2X7 receptor levels in the adult mouse brain are very low, yet functionally involved in regulating CNTF expression. Others have concluded that the currently available antibodies are not reliable and that levels of P2X7 protein in the brain, if present, are too low to be detected by current methods [37, 38, 41]. We also did not detect P2X7 receptors using in situ hybridization, consistent with the lack of expression seen in the Allen Mouse Brain Atlas. Others have detected P2X7 by in situ hybridization in rat SVZ, and in neurons, astrocytes, and microglia [69]. Their results are also consistent with a low abundance, as they used 35 PCR cycles for Northern blotting and 35S-labeled cRNA probes on tissue sections exposed for 25 days. Others have found neuronal mRNA labeling in the spinal cord and medulla using 35 PCR cycles and DIG-labeled in situ hybridization probes [70]. It is possible that rats have greater P2X7 receptor expression levels than mice.
What cells might mediate the regulation of CNTF expression by P2X7 receptors? In the mouse SVZ, as in most of the CNS, astrocytes produce CNTF [14]. Functional P2X7 receptors have been found in astrocytes [39, 40, 71]. Astrocytes in cortical slices of wild type, but not two lines of P2X7−/− mice, show functional responses to BBG and BzATP, as assessed by whole-cell patch clamping [40]. Our cultured astrocytes expressed P2X7 receptors, as shown by qPCR, but the P2X7 drugs did not affect CNTF expression. We have shown that our astrocyte cultures can respond to drugs, such as D2 agonists or integrin signaling antagonists, by increasing CNTF transcription [14, 67]. This suggests that the known P2X7 receptors of intracellular signaling pathways [72, 73] do not include those involved in CNTF transcription in astrocytes. It also suggests that the drug effects in vivo are indirect through other cells. One candidate is the ependymal SVZ neighbors that are the only cell type expressing detectable levels of P2X7 receptor mRNA in the mouse ventricle lining, as shown by βGal staining in P2X7−/− mice with a lacZ insertion in the locus [37, 41]. Our data showing that P2X7 receptor levels are fourfold higher in the SVZ, which contains the ependymal cells, than in striatal tissue are consistent with this idea. These ependymal P2X7 receptors are functional as shown by electrophysiology and mRNA can be detected by RT-PCR after retrieval from the recorded cells [42]. Thus, it is possible that P2X7 regulates synthesis and/or release of molecules by ependymal cells that, in turn, regulate astroglial CNTF expression in the SVZ. However, it remains to be determined whether this indirect mechanism exists.
The mRNA of P2X7 receptors was increased in the striatum but not in the SVZ after a 15-min MCAO stroke (data not shown). This injury causes cell loss in the striatum, but there was no overt cell loss in the SVZ. This suggests that P2X7 receptors are increased in reactive astrocytes or microglia in response to the ischemic injury and loss of neurons as proposed by others [74, 75]. These cells could mediate the effects of BBG on CNTF expression in the striatum after the MCAO injury.
P2X7 Inhibition May Fail to Induce Neurogenesis by Coinducing Counteracting Growth Factors
Surprisingly, despite the changes in CNTF levels in the SVZ in response to the P2X7 agonist or antagonist treatments, we did not see an effect on neurogenesis as measured by BrdU incorporation over 3 days or by qPCR for proliferation markers. We previously found that CNTF mediates stroke-induced neurogenesis and that this appears to involve downstream stimulation of FGF2 expression [15]. FGF2 treatment promotes SVZ neurogenesis, i.e., the formation of new neuroblasts from progenitor cells [56]. This is consistent with our data showing that FGF2 in the SVZ is not induced by inhibiting P2X7 receptor with BBG. However, we also find that BBG induces IL-6 and that direct IL-6 injections induce both CNTF and FGF2 expression in the naïve SVZ. This suggests that there are P2X7-related mechanisms that counteract the effects of CNTF on neurogenesis and/or the induction of FGF2. Our data show that BBG induced several growth factors known to i6nduce NSC self-renewal to the expense of neuroblast formation, including EGF [56], LIF [57], and IL-6 [58]. Interestingly, BBG did not affect expression of TNFα that is also known to reduce neurogenesis [59, 60].
The P2X7 receptor, therefore, seems to have other activities that affect neurogenesis, competing with its regulation of CNTF expression. To the best of our knowledge, no publications on the role of P2X7 in developmental or adult neurogenesis exist [17, 76]. The P2Y type purinergic receptors are involved in adult neurogenesis and these are found on the progenitors [17]. In cultured astrocytes, P2Y and P2× receptors have opposite effects, for example, in the ability of FGF2 to promote their proliferation [77]. Another potential role of P2X7 receptor is its stimulation of microglial proliferation [78] and microglia may release factors needed for neurogenesis [79]. Thus, P2X7 inhibition by BBG could inhibit such activation of neurogenic support by microglia, counteracting the increases in CNTF. The role of microglia might be further complicated by their dual role in neurogenesis seen in inflammatory conditions [80].
P2X7 Inhibition Does Not Reduce Cell Loss or Apoptosis Following Stroke in Mice
Others have shown neuroprotective effects of BBG against MCAO-induced stroke in adult Wistar rats using a similar dose of BBG [27]. We do not know why rats and mice respond differently, but this result suggests that they have different P2X7-related mechanisms that contribute to MCAO-induced cell loss. P2X7 is known to contribute directly to neuronal death [17]. However, our finding that BBG is ineffective in reducing cell loss in the striatum or apoptosis in the penumbra at 3 days after MCAO is consistent with the finding that P2X7−/− mice have similar infarct sizes after experimental stroke as wild type mice [31]. Thus, it is possible that P2X7 does not play a role in stroke-induced neuronal loss in mice.
CNTF treatments into the brain over 4 weeks reduce infarct size and enhance recovery following ischemic stroke in rats [10], and endogenous CNTF appears to be neuroprotective after MCAO [11]. Injection of LIF is also neuroprotective following stroke [61]. Therefore, it is surprising that BBG did not have neuroprotective effects despite the increases in CNTF and LIF. One possibility is that the treatments with CNTF and LIF protein were effective due to the much higher doses relative to the BBG-induced increases of endogenous protein in the brain. Alternatively, the BBG-induced increases may have been too late compared to the natural endogenous response after the stroke. On the other hand, despite our interpretation of a previous study showing increased injury size in CNTF−/− mice after MCAO [11], acute increases in CNTF may not be protective under some circumstances like this one. Whether or not this protective potential might be related to the injury severity remains to be determined.
P2X7 plays a role in the microglial response that is a component of the inflammatory response of CNS injury [17]. However, our results suggest that BBG did not affect inflammation as measured by TNFα expression in naïve mice or over the 3 days after stroke. This may also explain why we do not see protective effects and are consistent with the findings that we and others have been unable to reduce inflammation over the first week after ischemic stroke [11, 81]. Alternatively, BBG injections may have reduced the beneficial effects of microglia, as suggested by the finding that P2X7 inhibition can increase neuronal loss at 3 days after MCAO [82], thus perhaps, counteracting the beneficial effects of BBG-increased CNTF. It remains to be tested whether shorter BBG treatments would circumvent this potential complex and detrimental interaction.
In conclusion, the P2X7 receptor repression of endogenous CNTF and its biological effects in the adult mouse brain appear to be much more complex than that of other CNTF regulators such as astroglial D2 dopamine receptors that induce neurogenesis directly via CNTF [14]. This study also shows that CNTF expression is accessible for induction with systemic pharmacological drugs under physiological as well as pathological conditions and that P2X7 receptors provide an avenue to differentially regulate neurotrophic factors.
Acknowledgments
We wish to thank Rollie Reid (1972–2011), Erin Welsh, Hillary Conway, and Vicky Tran for their excellent technical assistance. Sheila Arnold is thanked for her guidance with the stereology and tissue culture. Dr. Michal Hetman is thanked for the use of his culture facility. Dr. Matthew Qiu is thanked for his kind gift of the PLP probe. This work was supported by NIH grants AG29493 and GM103507, Norton Healthcare, and the Commonwealth of Kentucky Challenge for Excellence.
Footnotes
Compliance with ethics requirements: All institutional and national guidelines for the care and use of laboratory animals were followed.
Conflict of interest: None.
Contributor Information
Seong Su Kang, Kentucky Spinal Cord Injury Research Center, University of Louisville, 511 South Floyd Street, MDR Building, Room 616, Louisville, KY 40292, USA; Department of Neurological Surgery, University of Louisville, Louisville, KY 40292, USA.
Matthew Phillip Keasey, Kentucky Spinal Cord Injury Research Center, University of Louisville, 511 South Floyd Street, MDR Building, Room 616, Louisville, KY 40292, USA; Department of Neurological Surgery, University of Louisville, Louisville, KY 40292, USA.
Theo Hagg, Email: theo.hagg@louisville.edu, Kentucky Spinal Cord Injury Research Center, University of Louisville, 511 South Floyd Street, MDR Building, Room 616, Louisville, KY 40292, USA; Department of Neurological Surgery, University of Louisville, Louisville, KY 40292, USA; Department of Pharmacology and Toxicology, University of Louisville, Louisville, KY 40292, USA.
References
- 1.Stockli KA, Lottspeich F, Sendtner M, Masiakowski P, Carroll P, Gotz R, et al. Molecular cloning, expression and regional distribution of rat ciliary neurotrophic factor. Nature. 1989;342(6252):920–3. doi: 10.1038/342920a0. [DOI] [PubMed] [Google Scholar]
- 2.Ip NY. The neurotrophins and neuropoietic cytokines: two families of growth factors acting on neural and hematopoietic cells. Ann N Y Acad Sci. 1998;840:97–106. doi: 10.1111/j.1749-6632.1998.tb09553.x. [DOI] [PubMed] [Google Scholar]
- 3.Cagnon L, Braissant O. CNTF protects oligodendrocytes from ammonia toxicity: intracellular signaling pathways involved. Neurobiol Dis. 2009;33(1):133–42. doi: 10.1016/j.nbd.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 4.Cao Q, He Q, Wang Y, Cheng X, Howard RM, Zhang Y, et al. Transplantation of ciliary neurotrophic factor-expressing adult oligodendrocyte precursor cells promotes remyelination and functional recovery after spinal cord injury. J Neurosci. 2010;30(8):2989–3001. doi: 10.1523/JNEUROSCI.3174-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sendtner M, Kreutzberg GW, Thoenen H. Ciliary neurotrophic factor prevents the degeneration of motor neurons after axotomy. Nature. 1990;345(6274):440–1. doi: 10.1038/345440a0. [DOI] [PubMed] [Google Scholar]
- 6.Hagg T, Varon S. Ciliary neurotrophic factor prevents degeneration of adult rat substantia nigra dopaminergic neurons in vivo. Proc Natl Acad Sci USA. 1993;90(13):6315–9. doi: 10.1073/pnas.90.13.6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emerich DF, Thanos CG. Intracompartmental delivery of CNTF as therapy for Huntington's disease and retinitis pigmentosa. Curr Gene Ther. 2006;6(1):147–59. doi: 10.2174/156652306775515547. [DOI] [PubMed] [Google Scholar]
- 8.Simon CM, Jablonka S, Ruiz R, Tabares L, Sendtner M. Ciliary neurotrophic factor-induced sprouting preserves motor function in a mouse model of mild spinal muscular atrophy. Hum Mol Genet. 2010;19(6):973–86. doi: 10.1093/hmg/ddp562. [DOI] [PubMed] [Google Scholar]
- 9.Hellstrom M, Harvey AR. Retinal ganglion cell gene therapy and visual system repair. Curr Gene Ther. 2011;11(2):116–31. doi: 10.2174/156652311794940746. [DOI] [PubMed] [Google Scholar]
- 10.Kumon Y, Sakaki S, Watanabe H, Nakano K, Ohta S, Matsuda S, et al. Ciliary neurotrophic factor attenuates spatial cognition impairment, cortical infarction and thalamic degeneration in spontaneously hypertensive rats with focal cerebral ischemia. Neurosci Lett. 1996;206(2–3):141–4. doi: 10.1016/s0304-3940(96)12450-2. [DOI] [PubMed] [Google Scholar]
- 11.Kang SS, Keasey MP, Cai J, Hagg T. Loss of neuron–astroglial interaction rapidly induces protective CNTF expression after stroke in mice. J Neurosci. 2012;32(27):9277–87. doi: 10.1523/JNEUROSCI.1746-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park CK, Ju WK, Hofmann HD, Kirsch M, Ki Kang J, Chun MH, et al. Differential regulation of ciliary neurotrophic factor and its receptor in the rat hippocampus following transient global ischemia. Brain Res. 2000;861(2):345–53. doi: 10.1016/s0006-8993(00)02045-x. [DOI] [PubMed] [Google Scholar]
- 13.Emsley JG, Hagg T. Endogenous and exogenous ciliary neurotrophic factor enhances forebrain neurogenesis in adult mice. Exp Neurol. 2003;183(2):298–310. doi: 10.1016/s0014-4886(03)00129-8. [DOI] [PubMed] [Google Scholar]
- 14.Yang P, Arnold SA, Habas A, Hetman M, Hagg T. Ciliary neurotrophic factor mediates dopamine D2 receptor-induced CNS neurogenesis in adult mice. J Neurosci. 2008;28(9):2231–41. doi: 10.1523/JNEUROSCI.3574-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang SS, Keasey MP, Arnold SA, Reid R, Geralds J, Hagg T. Endogenous CNTF mediates stroke-induced adult CNS neurogenesis in mice. Neurobiol Dis. 2013;49:68–78. doi: 10.1016/j.nbd.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fields RD, Burnstock G. Purinergic signalling in neuron–glia interactions. Nat Rev Neurosci. 2006;7(6):423–36. doi: 10.1038/nrn1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neary JT, Zimmermann H. Trophic functions of nucleotides in the central nervous system. Trends Neurosci. 2009;32(4):189–98. doi: 10.1016/j.tins.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Le Feuvre R, Brough D, Rothwell N. Extracellular ATP and P2X7 receptors in neurodegeneration. Eur J Pharmacol. 2002;447(2–3):261–9. doi: 10.1016/s0014-2999(02)01848-4. [DOI] [PubMed] [Google Scholar]
- 19.Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, et al. Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem. 2001;276(1):125–32. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- 20.Bernardino L, Balosso S, Ravizza T, Marchi N, Ku G, Randle JC, et al. Inflammatory events in hippocampal slice cultures prime neuronal susceptibility to excitotoxic injury: a crucial role of P2X7 receptormediated IL-1beta release. J Neurochem. 2008;106(1):271–80. doi: 10.1111/j.1471-4159.2008.05387.x. [DOI] [PubMed] [Google Scholar]
- 21.Sperlagh B, Vizi ES, Wirkner K, Illes P. P2X7 receptors in the nervous system. Prog Neurobiol. 2006;78(6):327–46. doi: 10.1016/j.pneurobio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Narcisse L, Scemes E, Zhao Y, Lee SC, Brosnan CF. The cytokine IL-1beta transiently enhances P2X7 receptor expression and function in human astrocytes. Glia. 2005;49(2):245–58. doi: 10.1002/glia.20110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagasawa K, Escartin C, Swanson RA. Astrocyte cultures exhibit P2X7 receptor channel opening in the absence of exogenous ligands. Glia. 2009;57(6):622–33. doi: 10.1002/glia.20791. [DOI] [PubMed] [Google Scholar]
- 24.Duan S, Anderson CM, Keung EC, Chen Y, Swanson RA. P2X7 receptor-mediated release of excitatory amino acids from astro-cytes. J Neurosci. 2003;23(4):1320–8. doi: 10.1523/JNEUROSCI.23-04-01320.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lo JC, Huang WC, Chou YC, Tseng CH, Lee WL, Sun SH. Activation of P2X(7) receptors decreases glutamate uptake and glutamine synthetase activity in RBA-2 astrocytes via distinct mechanisms. J Neurochem. 2008;105(1):151–64. doi: 10.1111/j.1471-4159.2007.05119.x. [DOI] [PubMed] [Google Scholar]
- 26.Jiang LH, Mackenzie AB, North RA, Surprenant A. Brilliant blue G selectively blocks ATP-gated rat P2X(7) receptors. Mol Pharmacol. 2000;58(1):82–8. [PubMed] [Google Scholar]
- 27.Arbeloa J, Perez-Samartin A, Gottlieb M, Matute C. P2X7 receptor blockade prevents ATP excitotoxicity in neurons and reduces brain damage after ischemia. Neurobiol Dis. 2012;45(3):954–61. doi: 10.1016/j.nbd.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Arcuino G, Takano T, Lin J, Peng WG, Wan P, et al. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med. 2004;10(8):821–7. doi: 10.1038/nm1082. [DOI] [PubMed] [Google Scholar]
- 29.Peng W, Cotrina ML, Han X, Yu H, Bekar L, Blum L, et al. Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proc Natl Acad Sci USA. 2009;106(30):12489–93. doi: 10.1073/pnas.0902531106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diaz-Hernandez M, Diez-Zaera M, Sanchez-Nogueiro J, Gomez-Villafuertes R, Canals JM, Alberch J, et al. Altered P2X7-receptor level and function in mouse models of Huntington's disease and therapeutic efficacy of antagonist administration. FASEB J. 2009;23(6):1893–906. doi: 10.1096/fj.08-122275. [DOI] [PubMed] [Google Scholar]
- 31.Le Feuvre RA, Brough D, Touzani O, Rothwell NJ. Role of P2X7 receptors in ischemic and excitotoxic brain injury in vivo. J Cereb Blood Flow Metab. 2003;23(3):381–4. doi: 10.1097/01.WCB.0000048519.34839.97. [DOI] [PubMed] [Google Scholar]
- 32.Anderson KD, Panayotatos N, Corcoran TL, Lindsay RM, Wiegand SJ. Ciliary neurotrophic factor protects striatal output neurons in an animal model of Huntington disease. Proc Natl Acad Sci USA. 1996;93(14):7346–51. doi: 10.1073/pnas.93.14.7346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Emerich DF, Winn SR, Hantraye PM, Peschanski M, Chen EY, Chu Y, et al. Protective effect of encapsulated cells producing neurotrophic factor CNTF in a monkey model of Huntington's disease. Nature. 1997;386(6623):395–9. doi: 10.1038/386395a0. [DOI] [PubMed] [Google Scholar]
- 34.Zala D, Bensadoun JC, PereiradeAlmeida L, Leavitt BR, Gutekunst CA, Aebischer P, et al. Long-term lentiviral-mediated expression of ciliary neurotrophic factor in the striatum of Huntington's disease transgenic mice. Exp Neurol. 2004;185(1):26–35. doi: 10.1016/j.expneurol.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 35.Nobbio L, Sturla L, Fiorese F, Usai C, Basile G, Moreschi I, et al. P2X7-mediated increased intracellular calcium causes functional derangement in Schwann cells from rats with CMT1A neuropathy. J Biol Chem. 2009;284(34):23146–58. doi: 10.1074/jbc.M109.027128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vigo T, Nobbio L, Hummelen PV, Abbruzzese M, Mancardi G, Verpoorten N, et al. Experimental Charcot–Marie–Tooth type 1A: a cDNA microarrays analysis. Mol Cell Neurosci. 2005;28(4):703–14. doi: 10.1016/j.mcn.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 37.Anderson CM, Nedergaard M. Emerging challenges of assigning P2X7 receptor function and immunoreactivity in neurons. Trends Neurosci. 2006;29(5):257–62. doi: 10.1016/j.tins.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Cotrina ML, Nedergaard M. Physiological and pathological functions of P2X7 receptor in the spinal cord. Purinergic Signal. 2009;5(2):223–32. doi: 10.1007/s11302-009-9138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Norenberg W, Schunk J, Fischer W, Sobottka H, Riedel T, Oliveira JF, et al. Electrophysiological classification of P2X7 receptors in rat cultured neocortical astroglia. Br J Pharmacol. 2010;160(8):1941–52. doi: 10.1111/j.1476-5381.2010.00736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oliveira JF, Riedel T, Leichsenring A, Heine C, Franke H, Krugel U, et al. Rodent cortical astroglia express in situ functional P2X7 receptors sensing pathologically high ATP concentrations. Cereb Cortex. 2011;21(4):806–20. doi: 10.1093/cercor/bhq154. [DOI] [PubMed] [Google Scholar]
- 41.Sim JA, Young MT, Sung HY, North RA, Surprenant A. Reanalysis of P2X7 receptor expression in rodent brain. J Neurosci. 2004;24(28):6307–14. doi: 10.1523/JNEUROSCI.1469-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Genzen JR, Platel JC, Rubio ME, Bordey A. Ependymal cells along the lateral ventricle express functional P2X(7) receptors. Purinergic Signal. 2009;5(3):299–307. doi: 10.1007/s11302-009-9143-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lim DA, Tramontin AD, Trevejo JM, Herrera DG, Garcia-Verdugo JM, Alvarez-Buylla A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28(3):713–26. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- 44.Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–84. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gajera CR, Emich H, Lioubinski O, Christ A, Beckervordersandforth-Bonk R, Yoshikawa K, et al. LRP2 in ependymal cells regulates BMP signaling in the adult neurogenic niche. J Cell Sci. 2010;123(Pt 11):1922–30. doi: 10.1242/jcs.065912. [DOI] [PubMed] [Google Scholar]
- 46.Del Bigio MR. Ependymal cells: biology and pathology. Acta Neuropathol. 2010;119(1):55–73. doi: 10.1007/s00401-009-0624-y. [DOI] [PubMed] [Google Scholar]
- 47.Naemsch LN, Dixon SJ, Sims SM. Activity-dependent development of P2X7 current and Ca2+ entry in rabbit osteoclasts. J Biol Chem. 2001;276(42):39107–14. doi: 10.1074/jbc.M105881200. [DOI] [PubMed] [Google Scholar]
- 48.Nakamoto T, Brown DA, Catalan MA, Gonzalez-Begne M, Romanenko VG, Melvin JE. Purinergic P2X7 receptors mediate ATP-induced saliva secretion by the mouse submandibular gland. J Biol Chem. 2009;284(8):4815–22. doi: 10.1074/jbc.M808597200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baker SA, Baker KA, Hagg T. Dopaminergic nigrostriatal projections regulate neural precursor proliferation in the adult mouse subventricular zone. Eur J Neurosci. 2004;20(2):575–9. doi: 10.1111/j.1460-9568.2004.03486.x. [DOI] [PubMed] [Google Scholar]
- 50.De Ryck M, Van Reempts J, Duytschaever H, Van Deuren B, Clincke G. Neocortical localization of tactile/proprioceptive limb placing reactions in the rat. Brain Res. 1992;573(1):44–60. doi: 10.1016/0006-8993(92)90112-m. [DOI] [PubMed] [Google Scholar]
- 51.Carroll P, Sendtner M, Meyer M, Thoenen H. Rat ciliary neurotrophic factor (CNTF): gene structure and regulation of mRNA levels in glial cell cultures. Glia. 1993;9(3):176–87. doi: 10.1002/glia.440090303. [DOI] [PubMed] [Google Scholar]
- 52.Adriouch S, Dox C, Welge V, Seman M, Koch-Nolte F, Haag F. Cutting edge: a natural P451L mutation in the cytoplasmic domain impairs the function of the mouse P2X7 receptor. J Immunol. 2002;169(8):4108–12. doi: 10.4049/jimmunol.169.8.4108. [DOI] [PubMed] [Google Scholar]
- 53.Young MT, Pelegrin P, Surprenant A. Identification of Thr283 as a key determinant of P2X7 receptor function. Br J Pharmacol. 2006;149(3):261–8. doi: 10.1038/sj.bjp.0706880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suadicani SO, Iglesias R, Spray DC, Scemes E. Point mutation in the mouse P2X7 receptor affects intercellular calcium waves in astrocytes. ASN Neuro. 2009;1(1) doi: 10.1042/AN20090001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kempermann G, Kuhn HG, Gage FH. Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc Natl Acad Sci USA. 1997;94(19):10409–14. doi: 10.1073/pnas.94.19.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17(15):5820–9. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bauer S, Patterson PH. Leukemia inhibitory factor promotes neural stem cell self-renewal in the adult brain. J Neurosci. 2006;26(46):12089–99. doi: 10.1523/JNEUROSCI.3047-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vallieres L, Campbell IL, Gage FH, Sawchenko PE. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J Neurosci. 2002;22(2):486–92. doi: 10.1523/JNEUROSCI.22-02-00486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iosif RE, Ahlenius H, Ekdahl CT, Darsalia V, Thored P, Jovinge S, et al. Suppression of stroke-induced progenitor proliferation in adult subventricular zone by tumor necrosis factor receptor 1. J Cereb Blood Flow Metab. 2008;28(9):1574–87. doi: 10.1038/jcbfm.2008.47. [DOI] [PubMed] [Google Scholar]
- 60.Iosif RE, Ekdahl CT, Ahlenius H, Pronk CJ, Bonde S, Kokaia Z, et al. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J Neurosci. 2006;26(38):9703–12. doi: 10.1523/JNEUROSCI.2723-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suzuki S, Yamashita T, Tanaka K, Hattori H, Sawamoto K, Okano H, et al. Activation of cytokine signaling through leukemia inhibitory factor receptor (LIFR)/gp130 attenuates ischemic brain injury in rats. J Cereb Blood Flow Metab. 2005;25(6):685–93. doi: 10.1038/sj.jcbfm.9600061. [DOI] [PubMed] [Google Scholar]
- 62.Zafra F, Castren E, Thoenen H, Lindholm D. Interplay between glutamate and gamma-aminobutyric acid transmitter systems in the physiological regulation of brain-derived neurotrophic factor and nerve growth factor synthesis in hippocampal neurons. Proc Natl Acad Sci USA. 1991;88(22):10037–41. doi: 10.1073/pnas.88.22.10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zafra F, Lindholm D, Castren E, Hartikka J, Thoenen H. Regulation of brain-derived neurotrophic factor and nerve growth factor mRNA in primary cultures of hippocampal neurons and astrocytes. J Neurosci. 1992;12(12):4793–9. doi: 10.1523/JNEUROSCI.12-12-04793.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu H, Friedman WJ, Dreyfus CF. Differential regulation of neurotrophin expression in basal forebrain astrocytes by neuronal signals. J Neurosci Res. 2004;76(1):76–85. doi: 10.1002/jnr.20060. [DOI] [PubMed] [Google Scholar]
- 65.Muller A, Hauk TG, Leibinger M, Marienfeld R, Fischer D. Exogenous CNTF stimulates axon regeneration of retinal ganglion cells partially via endogenous CNTF. Mol Cell Neurosci. 2009;41(2):233–46. doi: 10.1016/j.mcn.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 66.Zigmond RE. gp130 cytokines are positive signals triggering changes in gene expression and axon outgrowth in peripheral neurons following injury. Front Mol Neurosci. 2011;4:62. doi: 10.3389/fnmol.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Keasey MP, Kang SS, Lovins C, Hagg T. Inhibition of a novel specific neuroglial integrin signaling pathway increases STAT3-mediated CNTF expression. Cell Commun Signal. 2013;11(1):35. doi: 10.1186/1478-811X-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rudge JS, Morrissey D, Lindsay RM, Pasnikowski EM. Regulation of ciliary neurotrophic factor in cultured rat hippocampal astrocytes. Eur J Neurosci. 1994;6(2):218–29. doi: 10.1111/j.1460-9568.1994.tb00264.x. [DOI] [PubMed] [Google Scholar]
- 69.Yu Y, Ugawa S, Ueda T, Ishida Y, Inoue K, Kyaw Nyunt A, et al. Cellular localization of P2X7 receptor mRNA in the rat brain. Brain Res. 2008;1194:45–55. doi: 10.1016/j.brainres.2007.11.064. [DOI] [PubMed] [Google Scholar]
- 70.Deuchars SA, Atkinson L, Brooke RE, Musa H, Milligan CJ, Batten TF, et al. Neuronal P2X7 receptors are targeted to presynaptic terminals in the central and peripheral nervous systems. J Neurosci. 2001;21(18):7143–52. doi: 10.1523/JNEUROSCI.21-18-07143.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu YP, Yang CS, Chen MC, Sun SH, Tzeng SF. Ca(2+)-dependent reduction of glutamate aspartate transporter GLAST expression in astrocytes by P2X(7) receptor-mediated phosphoinositide 3-kinase signaling. J Neurochem. 2010;113(1):213–27. doi: 10.1111/j.1471-4159.2010.06589.x. [DOI] [PubMed] [Google Scholar]
- 72.Skaper SD, Debetto P, Giusti P. The P2X7 purinergic receptor: from physiology to neurological disorders. FASEB J. 2010;24(2):337–45. doi: 10.1096/fj.09-138883. [DOI] [PubMed] [Google Scholar]
- 73.Sperlagh B, Kofalvi A, Deuchars J, Atkinson L, Milligan CJ, Buckley NJ, et al. Involvement of P2X7 receptors in the regulation of neurotransmitter release in the rat hippocampus. J Neurochem. 2002;81(6):1196–211. doi: 10.1046/j.1471-4159.2002.00920.x. [DOI] [PubMed] [Google Scholar]
- 74.Franke H, Gunther A, Grosche J, Schmidt R, Rossner S, Reinhardt R, et al. P2X7 receptor expression after ischemia in the cerebral cortex of rats. J Neuropathol Exp Neurol. 2004;63(7):686–99. doi: 10.1093/jnen/63.7.686. [DOI] [PubMed] [Google Scholar]
- 75.Melani A, Amadio S, Gianfriddo M, Vannucchi MG, Volonte C, Bernardi G, et al. P2X7 receptor modulation on microglial cells and reduction of brain infarct caused by middle cerebral artery occlusion in rat. J Cereb Blood Flow Metab. 2006;26(7):974–82. doi: 10.1038/sj.jcbfm.9600250. [DOI] [PubMed] [Google Scholar]
- 76.Zimmermann H. Purinergic signaling in neural development. Semin Cell Dev Biol. 2011;22(2):194–204. doi: 10.1016/j.semcdb.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 77.Neary JT, Shi YF, Kang Y, Tran MD. Opposing effects of P2X(7) and P2Y purine/pyrimidine-preferring receptors on proliferation of astrocytes induced by fibroblast growth factor-2: implications for CNS development, injury, and repair. J Neurosci Res. 2008;86(14):3096–105. doi: 10.1002/jnr.21765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Monif M, Reid CA, Powell KL, Smart ML, Williams DA. The P2X7 receptor drives microglial activation and proliferation: a trophic role for P2X7R pore. J Neurosci. 2009;29(12):3781–91. doi: 10.1523/JNEUROSCI.5512-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Walton NM, Sutter BM, Laywell ED, Levkoff LH, Kearns SM, Marshall GP, 2nd, et al. Microglia instruct subventricular zone neurogenesis. Glia. 2006;54(8):815–25. doi: 10.1002/glia.20419. [DOI] [PubMed] [Google Scholar]
- 80.Ekdahl CT, Kokaia Z, Lindvall O. Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience. 2009;158(3):1021–9. doi: 10.1016/j.neuroscience.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 81.Hayakawa K, Mishima K, Nozako M, Hazekawa M, Mishima S, Fujioka M, et al. Delayed treatment with minocycline ameliorates neurologic impairment through activated microglia expressing a high-mobility group box1-inhibiting mechanism. Stroke. 2008;39(3):951–8. doi: 10.1161/STROKEAHA.107.495820. [DOI] [PubMed] [Google Scholar]
- 82.Yanagisawa D, Kitamura Y, Takata K, Hide I, Nakata Y, Taniguchi T. Possible involvement of P2X7 receptor activation in microglial neuroprotection against focal cerebral ischemia in rats. Biol Pharm Bull. 2008;31(6):1121–30. doi: 10.1248/bpb.31.1121. [DOI] [PubMed] [Google Scholar]