Abstract
BACKGROUND & AIMS
Excellent single-center outcomes of neoadjuvant chemoradiation and liver transplantation (LT) for unresectable perihilar cholangiocarcinoma caused the United Network of Organ Sharing (UNOS) to offer a standardized model of end-stage liver disease (MELD) exception for this disease. We analyzed data from multiple centers to determine the effectiveness of this treatment and the appropriateness of the MELD exception.
METHODS
We collected and analyzed data from 12 large-volume transplant centers in the US who met the inclusion criteria of treating three or more patients with perihilar cholangiocarcinoma using neoadjuvant therapy followed by liver transplantation from 1993–2010 (n=287 total patients). Center-specific protocols and medical charts were reviewed on-site.
RESULTS
The patients completed external radiation (99%), brachytherapy (75%), radio-sensitizing (98%), and/or maintenance chemotherapy (65%). Seventy-one patients dropped out before liver transplantation (rate of 11.5% in 3 months). Intent-to-treat survival was 68% and 53%, 2 and 5 years after therapy, respectively; post-transplantation, recurrence-free survival rates were 78% and 65%, respectively. Patients outside the UNOS criteria (those with tumor mass >3 cm, trans-peritoneal tumor biopsy, or metastatic disease) or with a prior malignancy had significantly shorter survival times (P<.001). There were no differences in outcomes among patients based on differences in operative staging or brachytherapy. Although most patients came from 1 center (n=193), the other 11 centers had similar survival times after therapy.
CONCLUSION
Patients with perihilar cholangiocarcinoma who were treated with neoadjuvant therapy followed by liver transplantation at 12 US centers had a 65% rate of recurrence-free survival after 5 years, demonstrating this therapy to be highly effective. An 11.5% dropout rate after 3.5 months of therapy indicates the appropriateness of the MELD exception. Rigorous selection is important for the continued success of this treatment.
Keywords: liver cancer, biliary, hepatic, treatment efficacy
INTRODUCTION
Perihilar cholangiocarcinoma is a highly aggressive malignancy with features of biliary epithelial differentiation. Anatomically perihilar cholangiocarcinoma is defined by disease occurring above the junction of the cystic duct up to the secondary branches of the right and left hepatic ducts 1. It is the second most common primary liver cancer with an annual incidence of 1.2/100.000 in the United States 2. Historically, treatment options for this devastating disease have been limited. Resection is the standard of care, though many patients present with unresectable disease due to involvement of bilateral hilar structures, or underlying parenchymal liver disease (primary sclerosing cholangitis). Even when resection is possible, 5-year survival is only 20–40% 3–11. While initially endorsed as an indication for orthotopic liver transplantation (LT), the experience with LT alone was disappointing due to a high rate of tumor recurrence (53–84%) 12–16 and thus, perihilar cholangiocarcinoma became a contra-indication to LT.
However, inspired by small reports of long-term survival noted in patients who received radiotherapy alone 17,18, first the University of Nebraska 19 and later Mayo Clinic 20,21 developed a protocol using neoadjuvant chemoradiation followed by LT. This protocol includes selected patients with unresectable early stage (I – II) perihilar cholangiocarcinoma, who consecutively undergo external beam radiotherapy (EBRT) combined with radio-sensitizing chemotherapy (i.e. 5-Flouracil), brachytherapy with endoscopically placed Iridium-192 beads, maintenance chemotherapy (i.e. oral capecitabine), staging surgery to rule out metastases, and finally LT. Subsequent reports consistently showed 5-year recurrence-free survival of approximately 70% 5,20–24. Encouraged by these outcomes, in June 2009, United Network of Organ Sharing / Organ Procurement and Transplantation Network (UNOS/OPTN ) approved the allocation of a standard Model of End-stage Liver Disease (MELD) exception score for patients with perihilar cholangiocarcinoma who complete an approved neoadjuvant therapy protocol 25. Hampered by lack of data, the MELD score was set to equal the current standard assigned score for hepatocellular carcinoma, representing an expected 10% increase in waitlist mortality every 3 months.
Information on how many centers in the U.S. are actively transplanting these patients, what type of neoadjuvant therapy is being used and, most importantly, what outcomes are achieved, is currently unknown. Given the severe shortage of donor organs, these data are crucial to determine whether use of liver allografts for this indication is justified and if so, what the appropriate waitlist priority should be. Therefore, we examined the U.S. experience to: 1) evaluate the overall effectiveness of neoadjuvant therapy followed by LT for perihilar cholangiocarcinoma; 2) assess the impact of inter-center variance in selection and neoadjuvant therapies; and 3) determine whether the current MELD exception score is appropriate.
METHODS
Study design, center selection
In this multicenter retrospective study, we invited 50 large-volume adult U.S. liver transplant programs to participate if they had 1) an established protocol for transplantation for perihilar cholangiocarcinoma employing neoadjuvant therapy, and 2) transplanted three or more patients under this protocol from January 1993 to July 2010. In total 30 centers (60%) responded to the mailing; 8 (27%) did not have a protocol and had not treated anyone with neoadjuvant chemoradiotherapy and LT, 10 (33%) did have an approved protocol but had performed less than three transplants, and 12 centers (40%) fulfilled both criteria. All centers with approved protocols with the Liver-Intestine committee of UNOS responded to our invitation though not all were eligible to participate. These 12 centers formed the multicenter consortium of this study. Each center obtained approval from their Institutional Review Board (IRB). All patient identifiers were coded and could not be traced back to the patient.
Data collection
One Principle Investigator (PI) per center was responsible for identifying patients enrolled in their protocol. Subsequently, all but two (UNMC and UCD), centers were visited by one clinical investigator (SDM) for on-site data collection and eligibility verification to ensure homogenous and standardized data collection. In the other two centers, data was collected by the site-PI as required by their IRB. Clinical, radiographic, laboratory and pathology data as well as data on neoadjuvant therapy, staging surgery and LT, were systematically collected from patient charts.
Patient population
We adhered to the following inclusion criteria: 1) perihilar cholangiocarcinoma; 2) diagnosis by a malignant-appearing stricture on cholangiography with malignant endoluminal brushing/biopsy, CA 19-9 greater than 100 U/ml, mass on cross-sectional imaging and/or polysomy on Fluorescent In-Situ Hybridization (FISH)); 3) unresectable disease or arising in Primary Sclerosing Cholangitis; 4) completion of neoadjuvant therapy before LT; and 5) medical suitability for transplantation. Patients with intrahepatic or distal cholangiocarcinoma were excluded.
A mass was defined as a well-circumscribed solid lesion on cross-sectional imaging, excluding perihilar thickening or enhancement alone. Considering the limitations of radiographic detection of a small perihilar mass, those without a visible mass were considered as having a mass < 3 cm for statistical purposes. MELD was calculated as previously described 26. Staging surgery was defined as a surgical procedure performed either before or at time of transplantation to rule out intra- or extrahepatic metastases by routine excisional biopsy of hilar lymph nodes plus any suspicious lesion. Generally, patients with biopsies demonstrating metastasis were excluded from transplantation, but this was center-dependent. Neoadjuvant therapy was defined as any combination of chemotherapy, external beam radiotherapy and/or brachytherapy given prior to LT. Protocol details for each center can be found in supplementary table 1.
Transplant allocation and UNOS/OPTN policy for standard MELD exception
Transplant allocation was mainly driven by waiting time before MELD was implemented in February 2002. After February 2002, patients were granted MELD exception scores based on individual regional agreements or underwent LT using a living donor. Since January 2010, all patients within specified criteria who underwent transplant at a center with an approved neoadjuvant protocol have been granted a standardized MELD exception score equivalent to a 10% waitlist mortality at 3 month intervals 25. Excluded from the MELD exception are patients with metastases (lymph node, intrahepatic or extrahepatic) or a mass larger than 3 cm in radial diameter. Moreover, direct trans-peritoneal biopsies of the primary tumor are highly discouraged in the policy.
Outcome definition and statistical analysis
Dropout was defined as positive staging, tumor metastasis, death or withdrawal at any time before transplantation. Probability of dropout was calculated from end of chemoradiation with censoring at time of transplant or, if actively waiting, at last follow-up. Recurrence was defined as radiographic or pathologically confirmed evidence of cholangiocarcinoma post-transplantation. Recurrence-free survival was calculated from time of transplantation to recurrence, death or last follow-up. Continuous variables were expressed as median (range), and categorical variables as N (% of total). Survival was calculated by the Kaplan Meier method and compared by the log-rank test. Uni- and multivariate Cox regression analysis was used to assess the effect of several individual components of the protocol on recurrence-free survival. Statistical significance was set at P<.05.
RESULTS
Patient Population
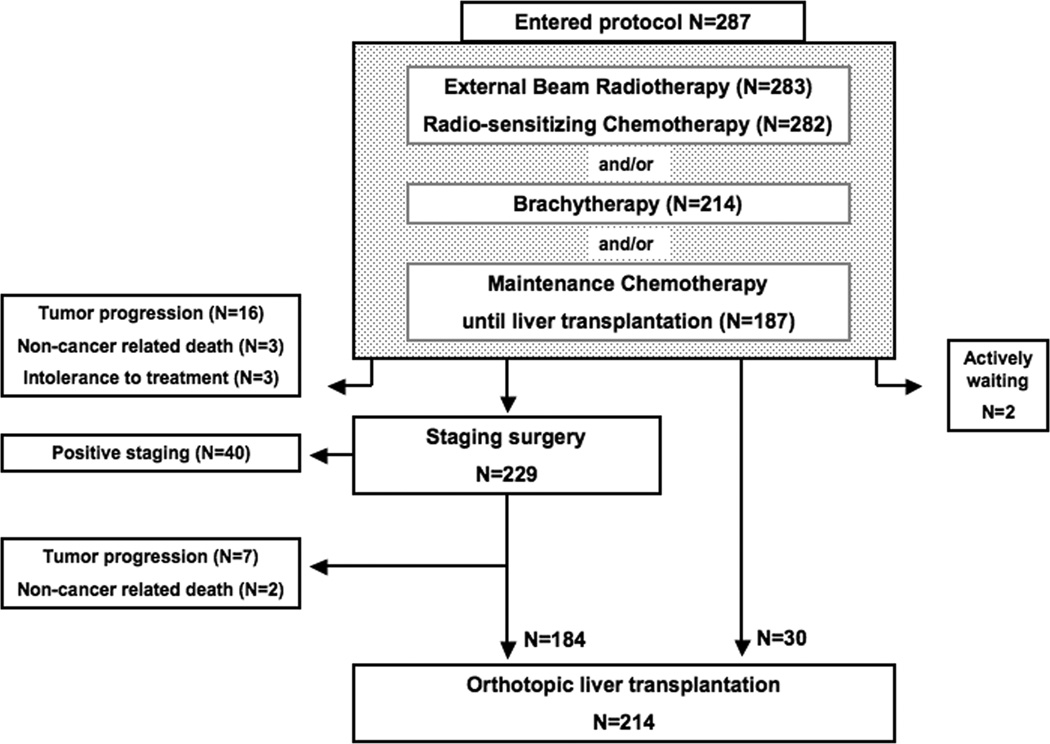
In total, 12 participating centers reported 319 patients. Upon review, 26 patients were excluded (intrahepatic cholangiocarcinoma (N=7), incidental explant diagnosis (N=11) and transplantation without neoadjuvant treatment (N=8)) and 6 patients were duplicates (received neoadjuvant therapy in one and transplantation in another participating center). Thus, 287 eligible patients were included in this study. Figure 1 summarizes the flow of patients through the protocol. Clinical characteristics at presentation and transplantation are presented in Table 1. Overall and center-specific treatment data can be found in supplementary Table 2.
Figure 1.
Flow chart of 287 patients with perihilar cholangiocarcinoma who underwent neoadjuvant therapy, staging surgery, and, finally, LT. The outer left boxes represent patients who dropped out from the protocol (total, 71 patients).
Table 1.
Clinical characteristics at time of presentation and at time of transplantation of patients with perihilar cholangiocarcinoma treated with neoadjuvant therapy in anticipation for liver transplantation. Results are shown for the total population first, and are subsequently compared between center 1 and centers 2–12. Results are expressed in N (%) or median (range).
| Characteristics at presentation | Total population (N=287) |
Center 1 (N=193) |
Centers 2–12 (N=94) |
|---|---|---|---|
| Age | 51 (17–70) | 51 (22–70) | 49 (17–69) |
| Gender (male) | 207 (72%) | 138 (72%) | 69 (73%) |
| Underlying Primary Sclerosing Cholangitis | 181 (63%) | 122 (63%) | 59 (63%) |
| Type of cholangiocarcinoma | |||
| - perihilar | 255 (89%) | 177 (92%) | 78 (83%) |
| - combined perihilar + IH | 13 (5%) | 6 (3%) | 7 (7%) |
| - combined perihilar + distal | 19 (7%) | 10 (5%) | 9 (10%) |
| Mass on cross-sectional imaging | 158 (55%) | 109 (56%) | 49 (52%) |
| Mass size (cm) | 2.7 (0.6–6.2) | 2.5 (0.6–5.0) | 2.9 (1.0–6.2) |
| Mass size | |||
| 0–3 cm | 119 (75%) | 84 (77%) | 35 (71%) |
| > 3 cm | 39 (25%) | 25 (23%) | 14 (29%) |
| Intraluminal brushing result | |||
| - positive | 96 (33%) | 64 (33%) | 32 (34%) |
| - suspicious | 57 (20%) | 37 (19%) | 20 (21%) |
| - negative | 110 (38%) | 78 (40%) | 32 (34%) |
| - could not be obtained | 24 (8%) | 14 (7%) | 10 (11%) |
| Intraluminal biopsy result * | |||
| - positive | 65 (23%) | 49 (25%) | 16 (17%) |
| - suspicious | 24 (8%) | 19 (10%) | 5 (5%) |
| - negative | 85 (30%) | 70 (36%) | 15 (16%) |
| - could not be obtained | 113 (39%) | 55 (28%) | 58 (62%) |
| Tissue diagnosis of malignancy from either brushing or biopsy | |||
| - positive / suspicious | 200 (70%) | 138 (73%) | 62 (72%) |
| - negative | 75 (26%) | 51 (26%) | 24 (26%) |
| - could not be obtained | 12 (4%) | 4 (2%) | 8 (9%) |
| FISH result * | |||
| - polysomy | 70 (24%) | 52 (27%) | 18 (19%) |
| - trisomy / negative | 74 (26%) | 60 (31%) | 14 (15%) |
| - not obtained | 143 (50%) | 81 (42%) | 62 (66%) |
| CA 19-9 (U/ml) | 76 (0–28750) | 79 (0–28750) | 73 (0–25856) |
| CA 19-9 > 100 U/ml | 126 (45%) | 89 (47%) | 37 (42%) |
| Bilirubin (mg/dl) | 2.2 (0.2–33.6) | 2.4 (0.3–33.6) | 1.9 (0.2–29.2) |
| INR * | 1.0 (0.8–5.6) | 1.0 (0.8–5.6) | 1.1 (0.8–1.9) |
| Creatinine (mg/dl) * | 0.9 (0.5–2.6) | 0.9 (0.5–2.6) | 0.8 (0.5–2.6) |
| Platelet count (x10E9/l) | 284 (47–897) | 282 (56–867) | 296 (47–662) |
| MELD (calculated) | 10 (6–34) | 10 (6–34) | 10 (6–27) |
| Tumor grade * | |||
| - well differentiated (G1) | 4 (1%) | 1 (1%) | 3 (3%) |
| - moderately differentiated (G2) | 20 (7%) | 16 (8%) | 4 (4%) |
| - poorly differentiated (G3) | 34 (12%) | 26 (13%) | 8 (9%) |
| - undifferentiated (G4) | 5 (2%) | 4 (2%) | 1 (1%) |
| - low-grade dysplasia | 3 (1%) | 1 (1%) | 2 (2%) |
| - high-grade dysplasia | 18 (6%) | 13 (7%) | 5 (5%) |
| - no tissue available or grade unobtainable | 203 (71%) | 132 (68%) | 71 (72%) |
| Characteristics at Liver Transplantation |
Total population (N=214) |
Center 1 (N=131) |
Centers 2–12 (N=83) |
| CA 19-9 (U/ml) | 48 (0–3300) | 55 (0–2370) | 35 (0–3300) |
| MELD (calculated) | 10 (6–40) | 11 (6–40) | 9 (6–26) |
| Type of allograft | |||
| - deceased donor | 152 (71%) | 88 (67%) | 64 (77%) |
| - living donor | 52 (29%) | 43 (33%) | 19 (23%) |
| Quality of allograft | |||
| - standard criteria donor | 173 (81%) | 106 (81%) | 67 (81%) |
| - extended criteria donor | 41 (19%) | 25 (19%) | 16 (19%) |
| Additional pancreaticoduodenectomy for distal bile duct involvement | 22 (10%) | 12 (9%) | 10 (12%) |
| Residual tumor tissue in explant * | 112 (52%) | 61 (47%) | 51 (61%) |
| Final tumor grade in explant * | |||
| - well differentiated (G1) | 10 (5%) | 1 (8%) | 9 (11%) |
| - moderately differentiated (G2) | 42 (20%) | 22 (17%) | 20 (24%) |
| - poorly differentiated (G3) | 40 (19%) | 29 (22%) | 11 (13%) |
| - undifferentiated (G4) | 5 (2%) | 4 (3%) | 1 (1%) |
| - no tumor tissue seen or grade not obtainable due to complete radiation-induced necrosis | 117 (54%) | 75 (57%) | 42 (51%) |
Comparison is based on Chi-square tests for categorical and Mann-Whitney-U tests for continuous variables.
P<.05.
Dropout rate
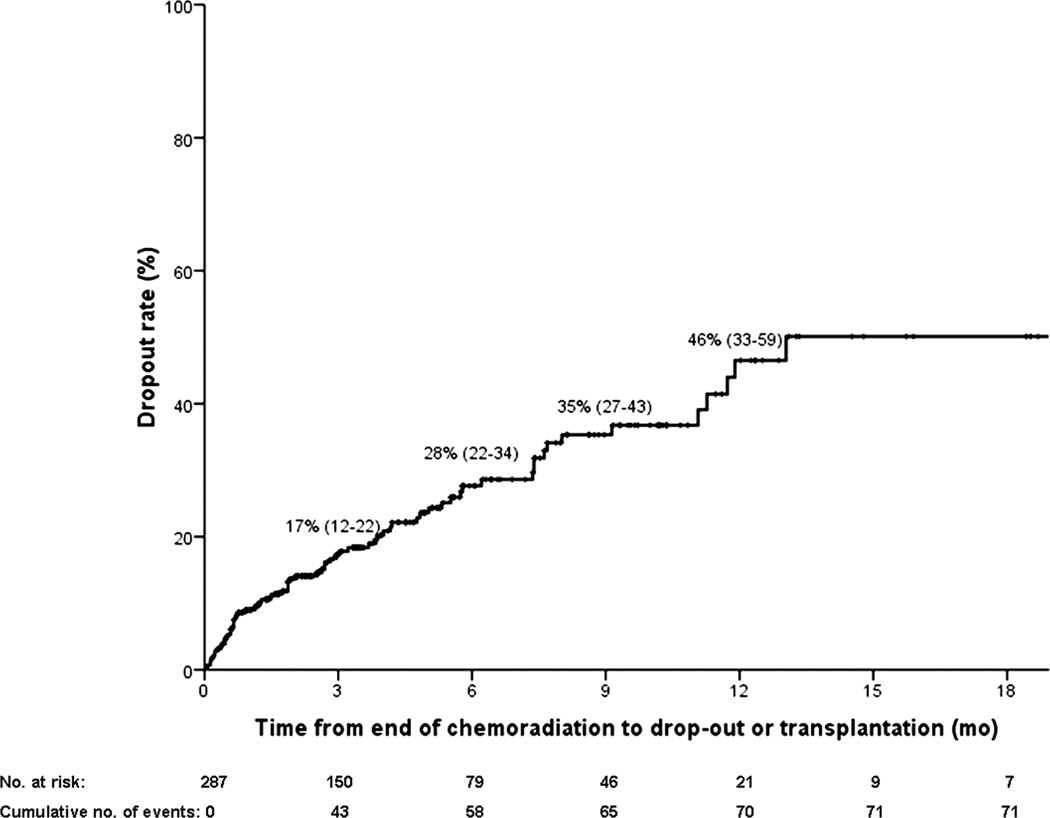
In total, 71 patients (25%) dropped out after a median of 4.6 months (1.1–17.1) from presentation. Table 2 compares characteristics between those who dropped out versus those who remained eligible for transplantation. Figure 2 shows the cumulative dropout rate starting from end of chemoradiation (i.e. time of listing). Nearly all dropouts occurred in the first year. On average, dropout rate increased every 3 months by 11.5% (range 7%–17%).
Table 2.
Clinical characteristics at time of presentation of patients who dropped out (N=71) versus those who remained eligible for liver transplantation (N=216). Results are expressed in N (%) or median (range).
| Clinical characteristics at presentation | Dropout (N=71) |
Eligible (N=216) |
|---|---|---|
| Age | 52 (27–70) | 50 (17–69) |
| Gender (male) | 47 (66%) | 160 (74%) |
| Underlying Primary Sclerosing Cholangitis * | 37 (52%) | 144 (66.7) |
| Type of cholangiocarcinoma | ||
| - perihilar | 62 (87%) | 193 (89%) |
| - combined perihilar + IH | 3 (4%) | 10 (5%) |
| - combined perihilar + distal | 6 (9%) | 13 (6%) |
| Mass on cross-sectional imaging * | 50 (70%) | 108 (50%) |
| Mass size (cm) | 3.0 (0.9–6.2) | 2.5 (0.6–5.6) |
| Intraluminal brushing result | ||
| - positive | 28 (39%) | 68 (32%) |
| - suspicious | 12 (17%) | 45 (21%) |
| - negative | 22 (31%) | 88 (41%) |
| - could not be obtained | 9 (13%) | 15 (7%) |
| Intraluminal biopsy result | ||
| - positive | 20 (28%) | 45 (21%) |
| - suspicious | 4 (6%) | 20 (9%) |
| - negative | 19 (27%) | 66 (31%) |
| - could not be obtained | 28 (39%) | 85 (39%) |
| FISH result | ||
| - polysomy | 16 (23%) | 54 (25%) |
| - trisomy / negative | 14 (20%) | 60 (28%) |
| - not obtained | 41 (58%) | 102 (47%) |
| CA 19-9 (U/ml) * | 218 (0–13200) | 58 (0–28750) |
| Bilirubin (mg/dl) * | 3.7 (0.4–33.6) | 1.85 (0.2–29.4) |
| INR | 1.0 (0.8–2.0) | 1.0 (0.8–5.6) |
| Creatinine (mg/dl) | 0.9 (0.5–2.0) | 0.9 (0.5–2.6) |
| Platelet count (x10E9/l) * | 305 (80–897) | 281 (47–870) |
| MELD (calculated) * | 11 (6–26) | 9 (6–34) |
| Tumor grade | ||
| - well differentiated (G1) | 1 (1%) | 3 (1%) |
| - moderately differentiated (G2) | 8 (11%) | 12 (6%) |
| - poorly differentiated (G3) | 9 (13%) | 25 (12%) |
| - undifferentiated (G4) | 3 (4%) | 2 (1%) |
| - low-grade dysplasia | 0 | 3 (1%) |
| - high-grade dysplasia | 3 (4%) | 15 (7%) |
| - no tissue available or grade could not be obtained | 47 (66%) | 156 (72%) |
Comparison is based on the Chi-square test (categorical variables) and Mann-Whitney-U test (continuous variables).
P<.05
Figure 2.
Cumulative drop-out rate in 3-month intervals from the end of chemoradiation (ie, time of listing).
Overall effectiveness
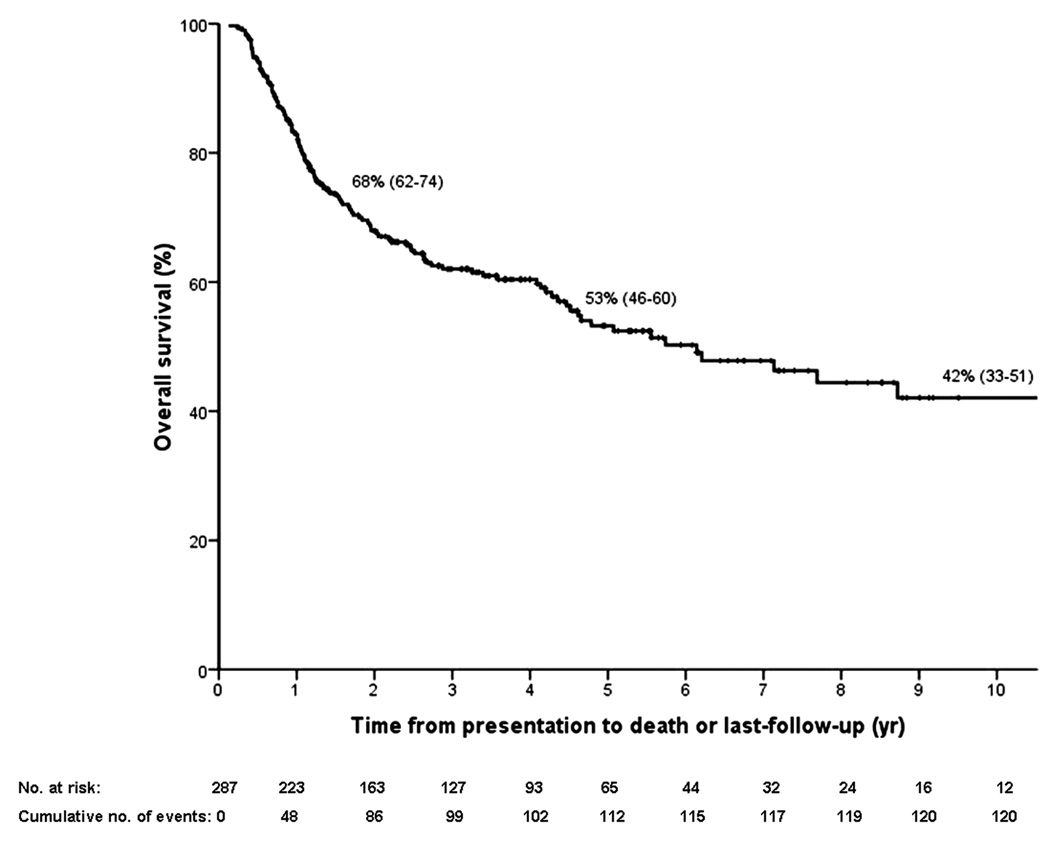
Median follow-up time was 2.5 years (range 0.1–17.8) from time of listing for transplantation. In total, 122 patients died (43%) after a median of 1.2 years from presentation (0.1–17.5), of whom 60 (49%) pre-transplant. Causes of pre-transplant death were tumor progression (N=52), liver failure (N=3), cardiovascular (N=2), multi-organ failure (N=2) and sepsis (N=1). Intent-to-treat survival is shown in Figure 3a.
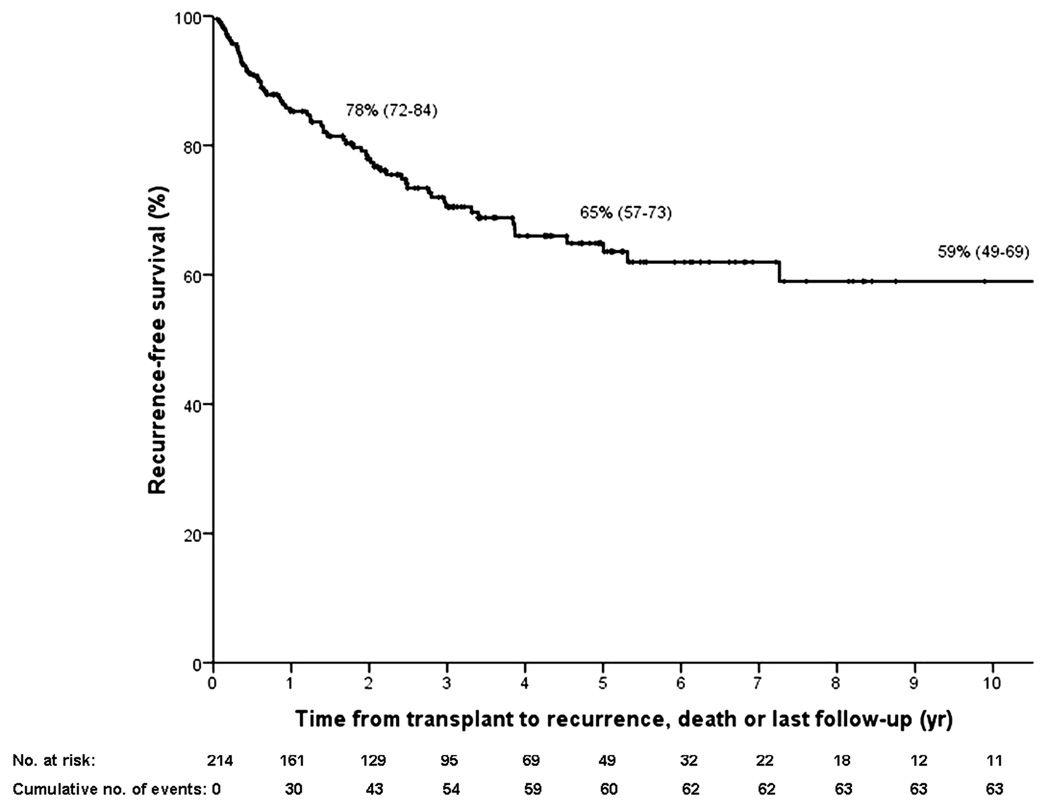
Figure 3.
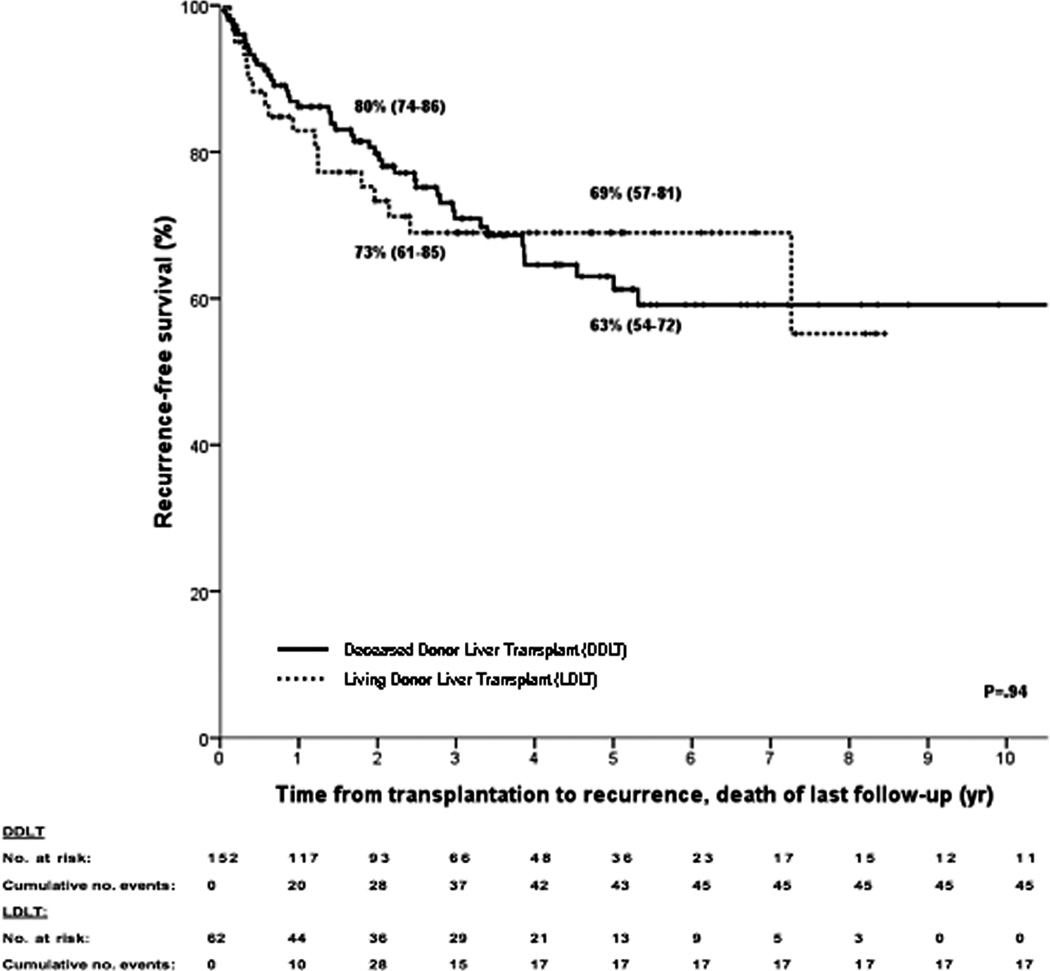
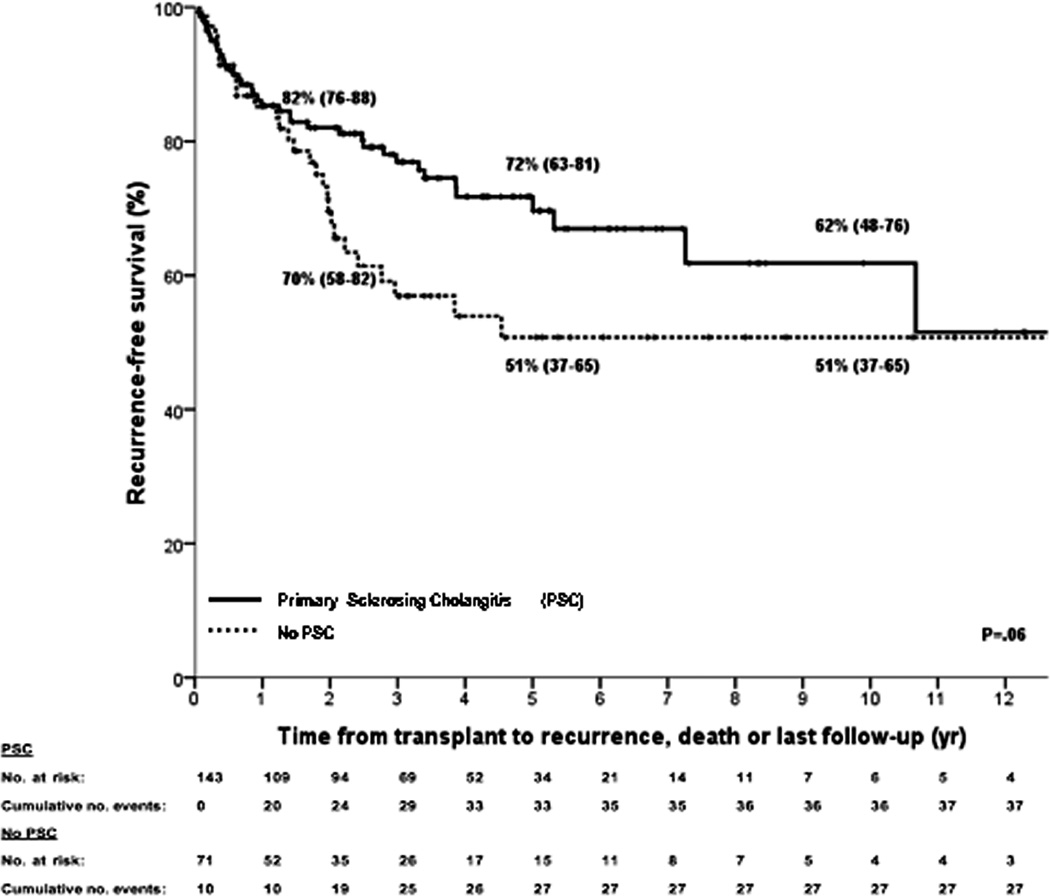
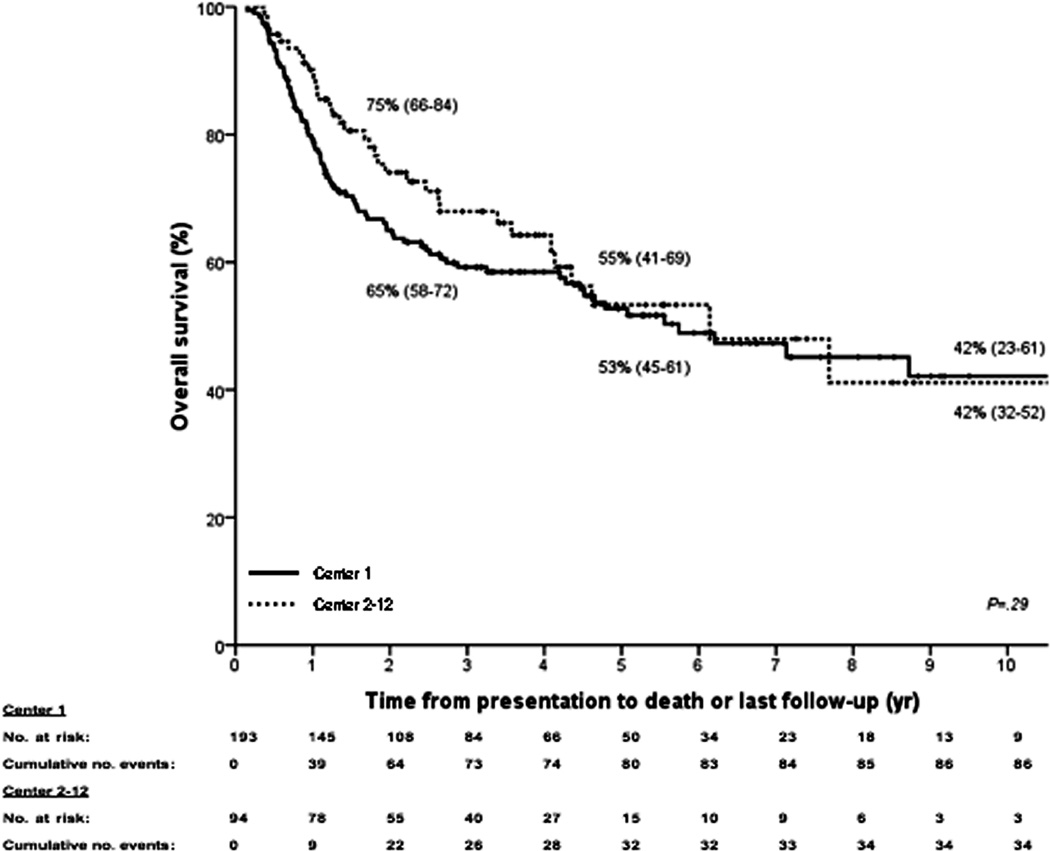
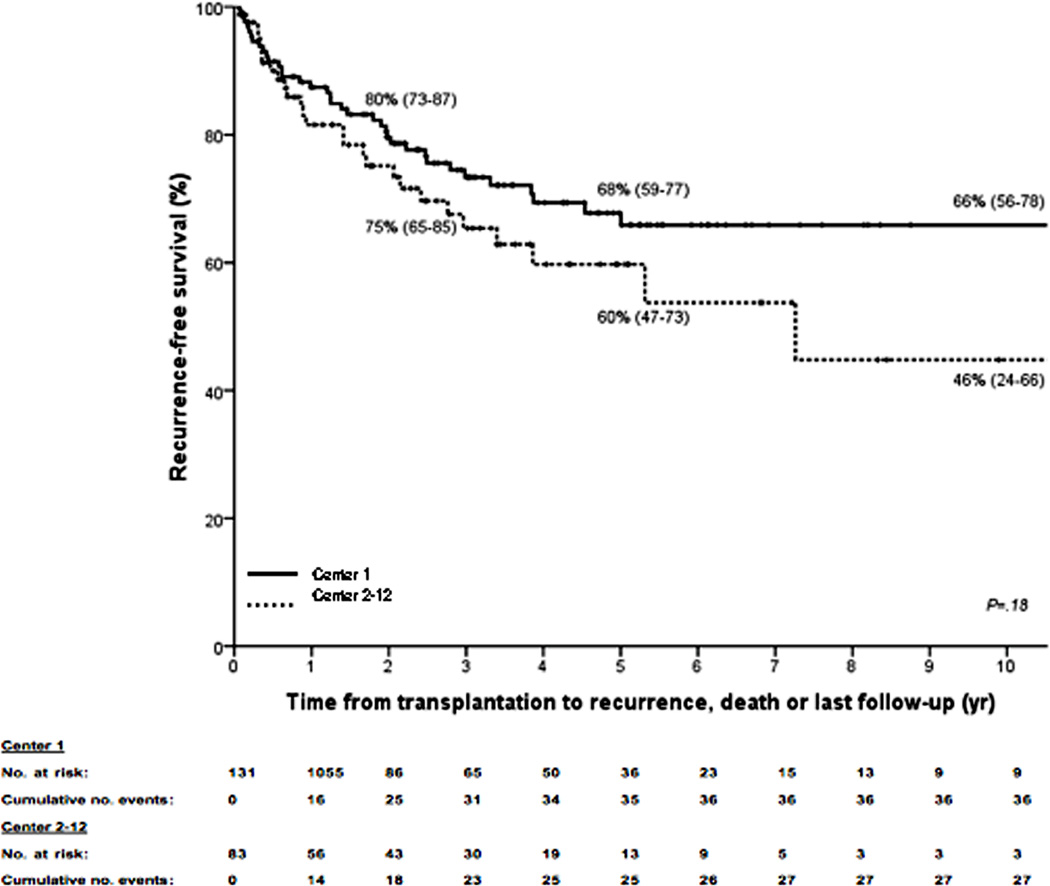
Kaplan–Meier curves for (A) intent-to-treat survival for the total population (N = 287); (B) recurrence-free survival for all transplanted patients (N = 214); (C) recurrence-free survival for deceased donor (N = 152) vs living donor (N = 52) liver transplantation; (D) recurrence-free survival for primary sclerosing cholangitis (N = 143) vs those without (N = 71); (E) intent-to-treat survival comparing center 1 (N = 193) vs all other centers (N = 94); and (F) recurrence-free survival comparing center 1 (N = 131) vs all other centers (N = 83). The 2-, 5-, and 10-year survival rates are shown as percentages (95% CI).
Post-transplant, 43 patients (20%) developed recurrence, and 62 patients died (22%) from either recurrence (N=40), sepsis (N=8), multiorgan failure (N=3), liver failure (N=3), post-transplant lymphoproliferative disease (N=2), or other causes (N=6). Table 3 compares transplant characteristics between those who recurred versus those who remained free of recurrence. Recurrence-free survival is depicted in figure 3b and was 78% (95% CI 72–84), 65% (95% CI 57–73) and 59% (95% 49–69) at 2, 5 and 10 years respectively. There were no significant differences in recurrence-free survival in patients who underwent deceased versus living donor liver transplantation (figure 3c) or in patients with underlying primary sclerosing cholangitis compared to those without (figure 3d).
Table 3.
Clinical characteristics at time of transplantation of patients who developed recurrence (N=43) versus those who remained free of recurrence (N=171) post transplantation. Results are expressed in N (%) or median (range).
| Clinical characteristics at transplantation | Recurrence (N=43) |
No recurrence (N=171) |
|---|---|---|
| Age | 51 (24–68) | 51 (18–70) |
| Gender (male) | 30 (70%) | 128 (75%) |
| Underlying Primary Sclerosing Cholangitis * | 20 (47%) | 123 (72%) |
| CA 19-9 (U/ml) | 78 (0–2370) | 45 (0–3300) |
| MELD (calculated) | 9 (6–23) | 9 (6–34) |
| Type of allograft | ||
| - deceased donor | 32 (74%) | 120 (70%) |
| - living donor | 11 (26%) | 51 (30%) |
| Quality of allograft | ||
| - standard criteria donor | 36 (84%) | 137 (80%) |
| - extended criteria donor | 7 (16%) | 34 (20%) |
| Additional pancreaticoduodenectomy for distal bile duct involvement * | 8 (19%) | 14 (8%) |
| Residual tumor tissue in explant * | 40 (93%) | 72 (42%) |
| Lymph node invastion on explant * | 6 (14%) | 6 (4%) |
| Vascular invasion on explant * | 5 (12%) | 3 (2%) |
| Perineural invasion on explant * | 23 (54%) | 27 (16%) |
| Tumor grade on explant * | ||
| - well differentiated (G1) | 3 (7%) | 7 (4%) |
| - moderately differentiated (G2) | 15 (35%) | 27 (16%) |
| - poorly differentiated (G3) | 16 (37%) | 24 (14%) |
| - undifferentiated (G4) | 3 (7%) | 2 (1%) |
| - no tissue tisse seen or grade could not obtainable due to complete radiation-induced necrosis | 6 (14%) | 111 (65%) |
Comparison is based on the Chi-square test (categorical variables) and Mann-Whitney-U test (continuous variables).
P<.05
Twenty-two patients (10%) underwent re-transplantation after a median of 4.6 months (0.03–163.5) for primary non-function (N=5), biliary complication (N=4), arterial compromise (N=11), recurrence of PSC (N=1) and donor-derived neuro-endocrine tumor (N=1). Graft survival was 77% (95% CI 71–83), 60% (95% CI 68–52) and 51% (95% CI 41–61) at 2, 5 and 10 years, respectively.
Protocol effects
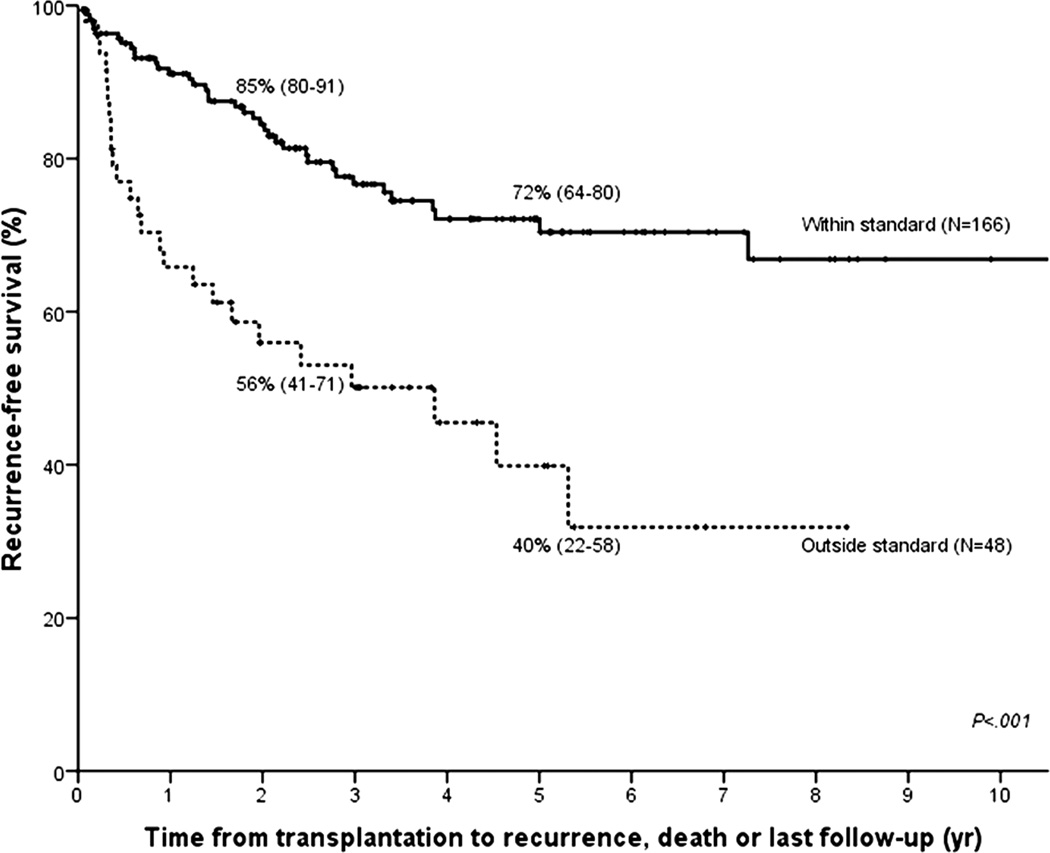
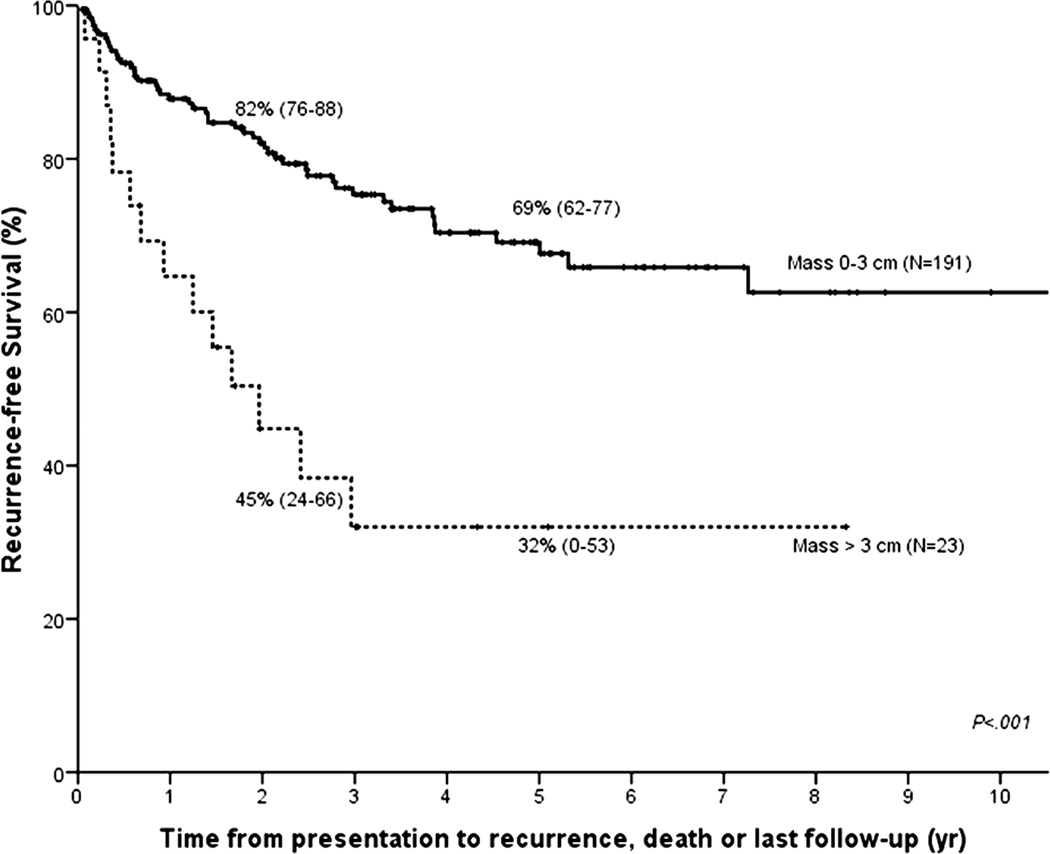
We studied the impact of differences in selection, staging surgery and type of neoadjuvant therapy on recurrence-free survival post-transplant. Patients who were transplanted outside of current UNOS/OPTN criteria for MELD exception (i.e. mass > 3 cm (N=21), metastatic disease at transplantation (N=4), or direct tumor biopsy (N=16)), or who had a history of previous malignancy within 5 years (N=7) had significantly worse recurrence-free survival as compared to those within criteria (HR 2.98 (95% CI 1.79–4.95); Figure 4a). Mass size caused the greatest disparity with 5-year recurrence-free survival of 32% for those larger than 3 cm compared to 69% for smaller tumors (P<.001; Figure 4b).
Figure 4.
Kaplan–Meier recurrence-free survival curves for (A) patients who are within UNOS/OPTN criteria for standard MELD exception (N = 166) vs those who are not (N = 48); and (B) patients with a mass larger than 3 cm (N = 23) vs 3 cm or smaller (N = 191).
We did not find a significant difference in recurrence-free survival (HR 0.73; 95% CI 0.37–1.43) among patients who underwent operative staging (recurrence in 36/184 (20%)) compared to those who did not (recurrence in 7/30 (23%)). Likewise, timing of staging (separate procedure (N=142) versus at transplantation (N=42)) was not significantly associated with survival (HR 0.79; 95% CI 0.43–1.43).
While nearly all patients received EBRT (99%) with radio-sensitizing chemotherapy (98%), 25% did not receive additional brachytherapy. Recurrence-free survival for patients who had received brachytherapy was similar to those who did not (HR 1.05; 95% CI 0.60–1.85).
Center effects
One center has transplanted patients with perihilar cholangiocarcinoma since 1993, and hence contributed the largest number of patients (center 1; N=193). Baseline characteristics are compared in table 1. However, we found no significant difference in median follow-up (2.49 vs. 2.54; P=.91), intent-to-treat (P=.29; Figure 3e) or recurrence-free survival (P=.18; Figure 3f) between this center and all other centers (N=94). This remained true (HR 0.82; 95% CI 0.43–1.54) even after correcting for differences in staging (HR 0.95; 95% 0.44–2.07), brachytherapy (HR 1.31; 95% CI 0.70–2.46) and selection criteria (HR 2.89; 95% CI 1.70–4.97) in a multivariate Cox regression model. Note that selection remained the only significant determinant of recurrence-free survival (P<.001).
Effect of histological confirmation
In 87 patients (30%), biopsy or brushings at diagnosis had either led to negative results or were unsuccessful in obtaining sufficient tissue. However, of these, 55 patients did have pathological proof at explant (i.e. residual tumor cells were found despite chemoradiation) and/or developed recurrence of cholangiocarcinoma after liver transplantation. Of the 32 patients without a tissue diagnosis, 17 had clear evidence of malignancy, by either a visible tumor mass on imaging and/or CA 19-9 above 100 U/ml in the absence of biliary obstruction. Hence, only 15 patients (5%) were enrolled and transplanted in whom there was a strong clinical suspicion of malignancy (malignant appearing stricture, FISH polysomy, weight loss, etc.) but in whom despite multiple attempts no histological proof of malignancy was found. However, excluding these patients, 5-year intent-to-treat survival (N=272) was 50% (43–57) and recurrence-free survival (N=199) was 62% (54–70), similar to results seen in the entire cohort.
Toxicity neoadjuvant therapy
Recurrent (sub)clinical cholangitis occurred in all patients in more and less severe forms during treatment. Toxicity from radiation included fatigue (41%), gastroduodenal ulcers (34%), and gastrointestinal dysmotility (18%). In addition, radiation caused friability of the portal vein in 23% and hepatic artery in 12% leading to (sub)total stenosis and/or thrombosis. Short-term biliary complications included post-surgical bile leak (11%) and biloma (N=2), though given the radiated native bile duct is completely resected, and reconstruction was with choledochojejunostomy the biliary complications are unlikely to be related to radiation. Long-term biliary complications included anastomotic strictures at the choledochojejunostomy or duct-to-duct site in 17% and ischemic cholangiopathy related to arterial compromise in 7%. The strictures were either managed conservatively of surgically by anastomotic revision. Toxicity of chemotherapy included nausea/vomiting (81%), cytopenia (21%), mucositis (18%) and hand/foot syndrome (17%). Besides a few patients for whom the toxicity was fatal or too severe to continue with transplantation (Figure 1), most toxicities were medically or surgically managed.
DISCUSSION
In this first multi-center study, we examined the effectiveness of a novel modality combining neoadjuvant chemoradiotherapy followed by LT for unresectable perihilar cholangiocarcinoma. Combining data from 12 transplant programs from different regions within the U.S. allowed us to address previous concerns regarding the broader applicability of this resource-intense strategy and the generalizability of results previously only achieved by a few single-center series. In addition to these 12, we identified at least 10 other centers with UNOS/OPTN approved protocols who are actively enrolling and treating patients. Our data serves to justify the use of scarce liver allografts for this otherwise lethal disease, as the unadjusted 5-year disease-free survival of 65% is not only similar to results from earlier single-center series 5,20–24 but also similar to outcomes of liver transplantation for other malignant and non-malignant indications 27. Finally, the observed 3-month dropout rate provides justification for the use of MELD exception to expedite transplantation for this indication.
The selection criteria, upon which the UNOS/OPTN policy for MELD exception is based, were derived from earlier single-center series showing that mass size 24,28 and direct biopsy of tumors 29 were associated with poorer outcome. Transplantation of metastatic patients would by reasoning alone lead to higher recurrence rates and a recent history of malignancy is a concern for any transplantation, regardless of the presence of cholangiocarcinoma. In the current multicenter analysis, these criteria were re-evaluated and found to be highly predictive of successful outcome. As a matter of fact, selection represents the only variable that acts as an independent predictor of outcome and is modifiable at the same time. By adjusting selection alone, 5-year recurrence-free survival can be maximized to 72%.
The standard MELD score exception that was designated for patients with perihilar cholangiocarcinoma was set to mirror that of hepatocellular carcinoma, as the actual waitlist dropout risk was unknown. We were able to demonstrate that the drop-out rate per three months averaged 11.5%, which approximates the expected 10%, and hence justifies its use. Interestingly, this rate is higher than what was most recently reported for patients with hepatocellular carcinoma, who have been served by the MELD exception for many years 30. The majority of patients drop out due to tumor progression. Because the tumor biology remains incompletely understood, the effect of more rapid access to transplant is unknown. While more patients may get to transplant, it may pose an increased risk for recurrence as some patients have aggressive tumor biology resistant to neoadjuvant therapy. A small number of patients also died from complications of therapy such as cholangitis and liver failure and for them earlier access to transplantation would have been of benefit.
Pre-transplant operative staging failed to show a statistical advantage in recurrence-free survival post-transplant. However, this was mostly due to lack of power given the small number of patients without staging (N=30). Staging however did lead to detection of metastases in 17% (40/229), and hence prevented ineffective use of allografts. Applying this rate to the non-staged patients, metastatic disease would have been detected in approximately 5 patients should they have been staged. Thus, while a firm conclusion cannot be reached, the question remains whether some of the recurrences in the non-staged patients could have been prevented by operative staging. The actual timing of the staging surgery also seemed of less statistical importance. Although combining staging and LT in one surgery has the obvious advantage over two separate procedures, other factors such as the ethics of bringing double bad news, and practical aspects related to re-allocation of the allograft to the regional pool need to be taken into consideration.
The variability in neoadjuvant protocols was largely due to the variable administration of brachytherapy and maintenance chemotherapy, whereas almost everybody (98–99%) received EBRT and radio-sensitizing chemotherapy. Brachytherapy is technically challenging and resource-intensive while exact positioning of the Iridium beads may be tricky. In our analysis, brachytherapy was not shown to have added benefit compared to EBRT alone, suggesting that the method of delivery of radiation may be less important. Most patients who did not receive brachytherapy did receive a higher dose of EBRT so that the total radiation exposure was fairly similar. Stereotactic radiotherapy, as utilized by 2 centers, may provide another alternative. Going forward, newer methods of radiotherapy, such as proton beam therapy, may eventually prove to be beneficial as well.
The main limitation of our study is that it is not a randomized controlled trial. However, a trial to compare outcomes for those who undergo LT for perihilar cholangiocarcinoma with or without neoadjuvant therapy, would be extremely difficult to conduct given the rarity of this disease and ethical considerations associated with unacceptably high recurrence and mortality rates historically reported with LT alone, even for incidentally discovered, and therefore presumably very early, perihilar cholangiocarcinoma 12–16. Another limitation was that a large proportion of patients were from one center, creating a potential for bias. To address this concern, outcomes were compared between this center and all others, and there were no statistical differences (figures 3c and 3d), even after correcting for variability in selection, staging and brachytherapy. The fact that we did not find a difference underlines the broader applicability of the protocol. Finally, due to heterogeneity in duration, type, and dose of maintenance chemotherapy administered at different centers, we were unable to determine the independent impact of maintenance chemotherapy.
In conclusion, this study confirms excellent outcomes of neoadjuvant chemoradiotherapy followed by LT for patients with perihilar cholangiocarcinoma across 12 U.S. institutions with variable neoadjuvant protocols. The assigned MELD score adjustment set by current UNOS/OPTN policy appears to be appropriate based on an observed dropout rate of 11.5% per 3-month increment. Patient selection clearly impacts outcome as a 3-fold increased risk of recurrence and death post-transplant was seen in patients with larger tumors, metastatic disease at transplantation, direct tumor biopsy and prior history of malignancy. While we could not find an independent benefit from the addition of brachytherapy, it is clear that at a minimum, EBRT with concomitant chemotherapy should be provided. The central challenge for the future will be to gain a greater understanding of the tumor biology in order to reduce waitlist dropout and post-transplant recurrence either by further refinements in patient selection or, ideally, by more effective chemoradiotherapy.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to acknowledge the following research staff for their invaluable logistical support: Patrice Al-Saden (Northwestern University, Chicago, IL), Rita Lerner (University of Colorado, Denver, CO), L. Susan Draeger (University of Nebraska Medical Center, Omaha, NE), Diane Bock (University of Wisconsin School of Medicine and Public Health, Maddison, WI), Helen Kaemmerer (Washington University School of Medicine, St. Louis, MO), Heather F. Thiesset (University of Utah, Salt Lake City, UT), Janet Mooney (University of California, Los Angeles, CA), Grace Bayona and Theresa Lukose, Pharm.D. (Colombia University Medical Center, New York, NY), and Melissa Satterlee (University of Illinois, Chicago, IL). We would also like to thank Joseph J. Larson (Mayo Clinic, Rochester, MN) for his assistance with some of the statistical analyses.
Grant Support
Sarwa Darwish Murad is a recipient of the 2010/2011 AASLD/LIFER Clinical and Translational Research Fellowship in Liver Diseases Award
Disclosures
| Sarwa Darwish Murad | nothing to disclose, no conflict of interest |
| W. Ray Kim | nothing to disclose, no conflict of interest |
| Denise Harnois | nothing to disclose, no conflict of interest |
| David Douglas | nothing to disclose, no conflict of interest |
| James Burton | nothing to disclose, no conflict of interest |
| Laura Kulik | nothing to disclose, no conflict of interest |
| Jean Botha | nothing to disclose, no conflict of interest |
| Joshua Mezrich | nothing to disclose, no conflict of interest |
| William Chapman | nothing to disclose, no conflict of interest |
| Jason Schwartz | nothing to disclose, no conflict of interest |
| Jonny Hong | nothing to disclose, no conflict of interest |
| Jean Emond | nothing to disclose, no conflict of interest |
| Hoonbae Jeon | nothing to disclose, no conflict of interest |
| Charles B. Rosen | nothing to disclose, no conflict of interest |
| Gregory J. Gores | nothing to disclose, no conflict of interest |
| Julie K. Heimbach | nothing to disclose, no conflict of interest |
Author Contributions
| Sarwa Darwish Murad | study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; statistical analyses; obtained funding |
| W. Ray Kim | study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; |
| Denise Harnois | |
| David Douglas | |
| James Burton | |
| Laura Kulik | |
| Jean Botha | |
| Joshua Mezrich | |
| William Chapman | |
| Jason Schwartz | |
| Jonny Hong | |
| Jean Emond | |
| Hoonbae Jeon | Study concept and design, acquisition of data, critical revision of the manuscript for important intellectual content; |
| Charles Rosen | |
| Gregory Gores | Study concept and design, critical revision of the manuscript for important intellectual content; |
| Julie K. Heimbach | study concept and design; analysis and interpretation of data; drafting and critical revision of the manuscript; study supervision; obtained funding |
REFERENCES
- 1.Deoliveira ML, Schulick RD, Nimura Y, et al. New staging system and a registry for perihilar cholangiocarcinoma. Hepatology (Baltimore, Md. 2011;53:1363–1371. doi: 10.1002/hep.24227. [DOI] [PubMed] [Google Scholar]
- 2.Gores GJ. Cholangiocarcinoma: current concepts and insights. Hepatology (Baltimore, Md. 2003;37:961–969. doi: 10.1053/jhep.2003.50200. [DOI] [PubMed] [Google Scholar]
- 3.Washburn WK, Lewis WD, Jenkins RL. Aggressive surgical resection for cholangiocarcinoma. Arch Surg. 1995;130:270–276. doi: 10.1001/archsurg.1995.01430030040006. [DOI] [PubMed] [Google Scholar]
- 4.Su CH, Tsay SH, Wu CC, et al. Factors influencing postoperative morbidity, mortality, and survival after resection for hilar cholangiocarcinoma. Annals of surgery. 1996;223:384–394. doi: 10.1097/00000658-199604000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rea DJ, Munoz-Juarez M, Farnell MB, et al. Major hepatic resection for hilar cholangiocarcinoma: analysis of 46 patients. Arch Surg. 2004;139:514–523. doi: 10.1001/archsurg.139.5.514. discussion 23-5. [DOI] [PubMed] [Google Scholar]
- 6.Rea DJ, Heimbach JK, Rosen CB, et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Annals of surgery. 2005;242:451–458. doi: 10.1097/01.sla.0000179678.13285.fa. discussion 8-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kosuge T, Yamamoto J, Shimada K, Yamasaki S, Makuuchi M. Improved surgical results for hilar cholangiocarcinoma with procedures including major hepatic resection. Annals of surgery. 1999;230:663–671. doi: 10.1097/00000658-199911000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobayashi A, Miwa S, Nakata T, Miyagawa S. Disease recurrence patterns after R0 resection of hilar cholangiocarcinoma. The British journal of surgery. 2010;97:56–64. doi: 10.1002/bjs.6788. [DOI] [PubMed] [Google Scholar]
- 9.Jarnagin WR, Ruo L, Little SA, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer. 2003;98:1689–1700. doi: 10.1002/cncr.11699. [DOI] [PubMed] [Google Scholar]
- 10.Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Annals of surgery. 2001;234:507–517. doi: 10.1097/00000658-200110000-00010. discussion 17-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeOliveira ML, Cunningham SC, Cameron JL, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Annals of surgery. 2007;245:755–762. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer CG, Penn I, James L. Liver transplantation for cholangiocarcinoma: results in 207 patients. Transplantation. 2000;69:1633–1637. doi: 10.1097/00007890-200004270-00019. [DOI] [PubMed] [Google Scholar]
- 13.Iwatsuki S, Todo S, Marsh JW, et al. Treatment of hilar cholangiocarcinoma (Klatskin tumors) with hepatic resection or transplantation. Journal of the American College of Surgeons. 1998;187:358–364. doi: 10.1016/s1072-7515(98)00207-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robles R, Figueras J, Turrion VS, et al. Spanish experience in liver transplantation for hilar and peripheral cholangiocarcinoma. Annals of surgery. 2004;239:265–271. doi: 10.1097/01.sla.0000108702.45715.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghali P, Marotta PJ, Yoshida EM, et al. Liver transplantation for incidental cholangiocarcinoma: analysis of the Canadian experience. Liver Transpl. 2005;11:1412–1416. doi: 10.1002/lt.20512. [DOI] [PubMed] [Google Scholar]
- 16.Brandsaeter B, Isoniemi H, Broome U, et al. Liver transplantation for primary sclerosing cholangitis; predictors and consequences of hepatobiliary malignancy. Journal of hepatology. 2004;40:815–822. doi: 10.1016/j.jhep.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Alden ME, Mohiuddin M. The impact of radiation dose in combined external beam and intraluminal Ir-192 brachytherapy for bile duct cancer. Int J Radiat Oncol Biol Phys. 1994;28:945–951. doi: 10.1016/0360-3016(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 18.Foo ML, Gunderson LL, Bender CE, Buskirk SJ. External radiation therapy and transcatheter iridium in the treatment of extrahepatic bile duct carcinoma. Int J Radiat Oncol Biol Phys. 1997;39:929–935. doi: 10.1016/s0360-3016(97)00299-x. [DOI] [PubMed] [Google Scholar]
- 19.Sudan D, DeRoover A, Chinnakotla S, et al. Radiochemotherapy and transplantation allow long-term survival for nonresectable hilar cholangiocarcinoma. Am J Transplant. 2002;2:774–779. doi: 10.1034/j.1600-6143.2002.20812.x. [DOI] [PubMed] [Google Scholar]
- 20.De Vreede I, Steers JL, Burch PA, et al. Prolonged disease-free survival after orthotopic liver transplantation plus adjuvant chemoirradiation for cholangiocarcinoma. Liver Transpl. 2000;6:309–316. doi: 10.1053/lv.2000.6143. [DOI] [PubMed] [Google Scholar]
- 21.Heimbach JK, Gores GJ, Haddock MG, et al. Liver transplantation for unresectable perihilar cholangiocarcinoma. Seminars in liver disease. 2004;24:201–207. doi: 10.1055/s-2004-828896. [DOI] [PubMed] [Google Scholar]
- 22.Rea DJ, Rosen CB, Nagorney DM, Heimbach JK, Gores GJ. Transplantation for cholangiocarcinoma: when and for whom? Surgical oncology clinics of North America. 2009;18:325–337. ix. doi: 10.1016/j.soc.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Heimbach JK, Gores GJ, Nagorney DM, Rosen CB. Liver transplantation for perihilar cholangiocarcinoma after aggressive neoadjuvant therapy: a new paradigm for liver and biliary malignancies? Surgery. 2006;140:331–334. doi: 10.1016/j.surg.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Heimbach JK, Gores GJ, Haddock MG, et al. Predictors of disease recurrence following neoadjuvant chemoradiotherapy and liver transplantation for unresectable perihilar cholangiocarcinoma. Transplantation. 2006;82:1703–1707. doi: 10.1097/01.tp.0000253551.43583.d1. [DOI] [PubMed] [Google Scholar]
- 25.Gores GJ, Gish RG, Sudan D, Rosen CB. Model for end-stage liver disease (MELD) exception for cholangiocarcinoma or biliary dysplasia. Liver Transpl. 2006;12:S95–S97. doi: 10.1002/lt.20965. [DOI] [PubMed] [Google Scholar]
- 26.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology (Baltimore, Md. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 27.Scientific Registry of Transplant Recipients. 2009 (Accessed at http://optn.transplant.hrsa.gov/ar2009/909a_li.htm.)
- 28.Darwish Murad S, Kim W, Gores G, Masuoka H, Rosen C, Heimbach J. Predictors of metastasis at operative staging in patients with hilar cholangiocarcinoma who undergo neoadjuvant chemoradiation in anticipation of liver transplantation. Am J of Transplantation. 2011;11:87. [Google Scholar]
- 29.Heimbach JK, Sanchez W, Rosen CB, Gores GJ. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB (Oxford) 2011;13:356–360. doi: 10.1111/j.1477-2574.2011.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Washburn K, Edwards E, Harper A, Freeman R. Hepatocellular carcinoma patients are advantaged in the current liver transplant allocation system. Am J Transplant. 2010;10:1643–1648. doi: 10.1111/j.1600-6143.2010.03127.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.