Abstract
Objective
Understanding dietary tracking may help to inform interventions to improve dietary intakes and health outcomes. We investigated how a dietary pattern (DP) associated with increased adiposity in childhood, tracked from 7 to 13 y of age in the Avon Longitudinal Study of Parents and Children (ALSPAC).
Design and Methods
Three-day food diaries were collected at 7, 10 and 13 y. Reduced rank regression was used to score respondents for an energy-dense, high fat, low fibre DP at each age. Tracking coefficients were estimated for the DP and its key foods using data from 7,027 children.
Results
The DP tracking coefficient was 0.48 (95% CI: 0.44-0.52) for boys and 0.38 (95% CI: 0.35-0.41) for girls. Of ten key food groups, fruit, vegetables, high fibre bread, high fibre breakfast cereals and full fat milk intakes exhibited the strongest tracking, particularly among low consumers. Lower maternal education and greater prepregnancy maternal BMI predicted higher DP z-scores and lower fruit and vegetable intakes.
Conclusions
A dietary pattern associated with increased adiposity tracks moderately from 7 to 13 y of age in this large UK cohort. Specific groups of families may require additional support to foster lifelong healthy dietary habits in their children.
Keywords: dietary pattern, tracking, obesity, children, adolescents
Introduction
An understanding of the stability or tracking of dietary intake is crucial for formulating policy and interventions to improve nutrition-related health outcomes such as obesity. Strong dietary tracking signifies maintenance of a similar level of dietary intake over time whereas, poor dietary tracking indicates a susceptibility to alter dietary intake over time. Knowledge of dietary tracking over the life course may assist in identifying which dietary intakes are maintained more (or less) consistently over time and therefore, their potential susceptibility to intervention, as well as identification of key times for intervention. This is especially relevant to dietary intakes linked to health outcomes.
Early childhood and adolescence are critical times for physical development as well as changes in psycho-social environments. Yet, relatively few studies have examined the tracking of dietary intake during the transition from childhood to adolescence (1, 2, 3, 4, 5). Dietary patterns may be more informative for investigating diet-disease relationships than individual foods or nutrients, as they consider total dietary intake and the colinearity between many foods and nutrients, as well as the potentially synergistic effects of foods and nutrients (6). Only two studies to date have examined how empirically derived dietary patterns track during childhood (1, 7).
We previously identified an energy-dense, high fat, low fibre dietary pattern prospectively associated with increased and excess adiposity during childhood and adolescence in the UK Avon Longitudinal Study of Parents and Children (ALSPAC) (8). The current paper examines how scores for this dietary pattern and intakes of its key foods tracked between 7 and 13 y of age in the ALSPAC cohort. As maternal education and maternal overweight have been linked with both offspring dietary intakes (9, 10) and weight status (11) we also hypothesised that low maternal education and greater maternal prepregnancy BMI would be positively associated with stronger tracking and higher scores for the energy-dense, high fat, low fibre dietary pattern, between 7 and 13 years of age.
Methods and Procedures
Data were from children and parents participating in the Avon Longitudinal Study of Parents and Children (ALSPAC). Full details of the study have been reported elsewhere (12, 13, 14). In brief, ALSPAC commenced with the recruitment of 14,541 pregnant women residing in Avon, England, with an expected delivery date between April 1991 and December 1992. The total sample of children consisted 14,610 live births and 14,536 were alive at one year. A range of health data have been collected from the women, their partners and children, at regular intervals thereafter, via mailed questionnaires and clinic visits at Bristol University (13). Parents provided written consent for their child to participate in the study. Ethical approval for the study was obtained from the ALSPAC Law and Ethics Committee and the Local Research Ethics Committees.
Dietary Assessment
The current analysis utilises dietary data collected at follow ups that took place when study children were approximately 7, 10 and 13 years of age. At each of these ages, the children were requested to complete a 3-d unweighed food diary over 2 week days and 1 weekend day. Parents completed the diary on behalf of the child at 7 y of age. At 10 and 13 y of age, the children completed the diary with input from an adult as required, and these diaries were later checked and queried with a nutritionist. All completed food diaries were coded and linked to food composition tables using DIDO, a program developed by MRC Human Nutrition Research, Cambridge. Nutrient intakes were calculated using McCance and Widdowson’s British food composition data. Individual food intakes were collapsed into predefined ‘Cambridge food groups’, used previously in this cohort (n=45) (15). As dietary misreporting, particularly under-reporting, contributes to measurement error in dietary assessment, an individualised method based on the ratio of energy intake (EI) to estimated energy requirement (EER) and its 95% confidence interval (16) was used to identify plausible, over- and under-reporting of dietary intake at 7, 10 and 13 y of age (8).
Dietary Patterns
We have previously described an ‘energy-dense, high fat, low fibre’ dietary pattern identified using reduced rank regression (RRR) in this cohort, in detail (8). This dietary pattern, or pattern in food intake, explained the maximum variation in three dietary response variables hypothesised to be related with obesity risk: 1) dietary energy density, 2) the proportion of total energy from fat (%EF), and 3) dietary fibre density. Separate exploratory RRR analyses were conducted at 7, 10 and 13 y of age (17), modelling 45 food group intakes as predictor variables and energy density, %EF and fibre density as response variables. This identified an almost identical dietary pattern at all ages positively correlated with dietary energy density and %EF and negatively correlated with dietary fibre density. This energy-dense, high fat, low fibre dietary pattern was characterised mostly by low intakes of fruit, vegetables and high fibre breakfast cereals and high intakes of confectionery, crisps, low fibre bread, cakes and biscuits (Table 1); full details are published elsewhere (8).
Table 1.
Five highest and lowest factor loadings for the energy-dense, high fat, low fibre dietary pattern.
| 7 y | 10 y | 13 y | |||
|---|---|---|---|---|---|
| Five Highest Factor Loadings | |||||
| Chocolate, confectionery | 0.23 | Chocolate, confectionery | 0.25 | Chocolate, confectionery | 0.24 |
| Bread, low fibre | 0.20 | Potato Crisps | 0.21 | Bread, low fibre | 0.23 |
| Cakes, biscuits | 0.19 | Bread, low fibre | 0.20 | Potato Crisps | 0.19 |
| Potato Crisps | 0.18 | Cakes, biscuits | 0.16 | Processed meats | 0.18 |
| Milk, full fat | 0.14 | Processed meats | 0.16 | Cakes, biscuits | 0.17 |
| Five Lowest Factor Loadings | |||||
| Fruit, fresh | −0.48 | Fruit, fresh | −0.43 | Fruit, fresh | −0.41 |
| Vegetables, raw or boiled | −0.41 | Vegetables, raw or boiled | −0.42 | Vegetables, raw or boiled | −0.41 |
| Breakfast cereal, high fibre | −0.25 | Legumes | −0.24 | Legumes | −0.23 |
| Potato, boiled | −0.21 | Potato, boiled | −0.22 | Potato, boiled | −0.21 |
| Bread, high fibre | −0.21 | Breakfast cereal, high fibre | −0.20 | Breakfast cereal, high fibre | −0.20 |
Each subject received a z-score quantifying the degree to which their reported dietary intake reflected the energy dense, high fat, low fibre dietary pattern at 7, 10 and 13 y of age (where data available). In order to track z-scores for exactly the same dietary pattern from 7 to 13 y of age, an applied dietary pattern z-score was calculated at 10 and 13 y using the scoring coefficients produced by the 7 y RRR analysis. This allowed a z-score for the dietary pattern identified at 7 y of age to be calculated at 10 and 13 y of age, and to more accurately track z-scores for the same dietary pattern.
Covariates
Information on maternal prepregnancy BMI and maternal education was available from questionnaires completed by the study child’s mother at 32 week’s gestation. Mothers were classified as overweight or obese if their BMI was ≥ 25 kg/m2. Maternal education level (reflecting the mother’s highest educational achievement) was coded into one of five ordinal categories (in decreasing order): University degree, A level (academic examination passed at 18 y), O level (academic examination passed at 16 y), vocational (non-academic training or apprenticeship) and CSE (Certificate of Secondary Education, non-academic study to 16 y).
Statistical analyses
Tracking was defined as how consistently individuals maintained their position or z-score in the study population distribution during follow up. Tracking (or stability) coefficients were calculated for dietary pattern z-scores using the method suggested by Twisk (18). Using a generalised estimating equation (GEE) model, the first dietary pattern score measurement (for the large majority this was measured at 7 y of age) was regressed on later dietary pattern scores (measured at either or both 10 y and 13 y of age) (Equation 1). This models the longitudinal association between the first z-score measurement and each subsequent measurement.
| Equation 1 |
Where DPit is the dietary pattern z-score for individual i at later time t, β0 is the intercept, DPit1 is the first measured dietary pattern z-score for individual i, β1 is the tracking coefficient for the dietary pattern , t is time, β2 is the beta coefficient for time, x is a fixed covariate and β3 is the beta coefficient for covariate x. The tracking coefficient ranges from 0 to 1, with 1 indicating perfect tracking and 0 indicating no tracking. There are no universally accepted cutoffs to classify good or poor tracking, as the magnitude of the tracking coefficient can depend on the length of follow up and measurement error in the variable being tracked (19). However, it is possible to contrast tracking coefficients observed within the same study, and we considered tracking coefficients ≥ 0.40 to indicate ‘moderate’ tracking. These GEE models were used as they can handle data from more than two time points, utilise all available data points, as well as account for repeated measurements on the same individuals being correlated and the different lengths of time between measurements. An exchangeable correlation structure was used and all models were adjusted for age at first dietary pattern measurement. The GEE models were run using PROC GENMOD in SAS v9.1.3.
A similar GEE model was applied to estimate tracking coefficients for key food group intakes. Only those food groups in the top five and bottom five factor loadings for the energy-dense, high fat, low fibre dietary pattern were examined (Table 1) as these explained over 80% of the variation in DP scores (8). To account for varying ages at each follow up, food group intakes were firstly adjusted for total energy intake (food group intake (g)/total energy intake (kJ)). Energy-adjusted food intakes were then standardised to z-scores to estimate standardised tracking coefficients. Maternal education and maternal prepregnancy BMI were included as covariates in the GEE models to examine their influence on the tracking coefficients and to quantify their prospective associations with later dietary pattern z-scores and food group intake z-scores. Dietary misreporting was included in the models (as a categorical variable) as a potential predictor of dietary tracking.
A secondary analysis was conducted to enable comparisons with other studies that have not reported tracking coefficients. Dietary pattern and food group z-scores were firstly categorised into quartiles. Tracking was then examined simply as the proportion of individuals in the highest or lowest quartiles at 7 y of age who maintained their quartile position at 13 y of age (maintenance of extreme quartiles). This enabled differences in tracking between high and low consumers to be identified. As there were fewer consumers of full fat milk, high fibre breakfast cereal, high fibre bread and legumes, these food intakes were categorised into tertiles rather than quartiles.
Tracking analyses were conducted separately for boys and girls. Only those respondents who completed a food diary on at least two follow ups were included in the analyses.
Results
A total of 7,078 participants (3,506 boys and 3,572 girls) provided dietary data from at least two follow ups and were considered for these analyses. Of the 6,202 in this sample who provided dietary data at 7 years of age, 5,949 had dietary data at 10 y of age and 4,986 had dietary data at 13 y of age. A total of 4,733 had dietary data at all three ages and 876 had dietary data at 10 and 13 y of age only. Participants included in this analysis were slightly more likely to be white and have higher levels of maternal education compared with the remainder of the cohort, who either did not complete or completed only one food diary; maternal prepregnancy BMI status did not differ (Table S1). Mean intakes of the ten key food groups at each follow up are shown in Table 2.
Table 2.
Mean dietary intakes at 7, 10 and 13 years of age
| 7 y | 10 y | 13 y | |
|---|---|---|---|
|
| |||
| n | 7285 | 7471 | 6106 |
| Z-score for energy-dense, high fat, low fibre dietary pattern |
0 (1.03) (−6.84, 3.91) |
−0.07 (1.19) (−6.92, 4.27) |
0.02 (1.48) (−7.08, 8.78) |
| Total energy intake (MJ) | 7.16 (1.32) (2.52, 14.33) |
7.79 (1.62) (2.14, 15.5) |
8.21 (2.19) (2.22, 23.9) |
| Dietary energy density (kJ/g) | 8.8 (1.5) (4, 17.8) |
8.8 (1.6) (4.3, 16.9) |
8.5 (1.7) (2.8, 18.7) |
| Dietary fibre density (g/MJ) | 1.5 (0.4) (0, 3.9) |
1.5 (0.4) (0.4, 4.3) |
1.6 (0.5) (0.2, 5.5) |
| Energy from fat (%) | 35.5 (4.4) (15.4, 56.3) |
35.6 (4.8) (10.3, 55.6) |
34.8 (5.8) (8.9, 60.1) |
| Chocolate, confectionery (g/d) | 26 (21) (0, 170) |
31 (28) (0, 284) |
26 (33) (0, 954) |
| Bread, low fibre (g/d) | 59 (40) (0, 375) |
63 (48) (0, 410) |
61 (56) (0, 453) |
| Cakes, biscuits (g/d) | 49 (34) (0, 324) |
46 (37) (0, 280) |
44 (45) (0, 455) |
| Potato crisps (g/d) | 18 (14) (0, 100) |
20 (17) (0, 164) |
17 (19) (0, 429) |
| Milk, full fat (g/d) | 139 (183) (0, 1389) |
79 (146) (0, 1343) |
65 (151) (0, 2387) |
| Processed meats (g/d) | 24 (25) (0, 261) |
29 (31) (0, 358) |
31 (37) (0, 424) |
| Fresh fruit (g/d) | 78 (77) (0, 828) |
66 (75) (0, 645) |
74 (92) (0, 1150) |
| Vegetables, raw or boiled (g/d) | 49 (41) (0, 432) |
59 (54) (0, 535) |
70 (65) (0, 520) |
| Breakfast cereals, high fibre (g/d) | 16 (24) (0, 255) |
14 (24) (0, 382) |
19 (32) (0, 400) |
| Potato, boiled (g/d) | 30 (35) (0, 275) |
33 (45) (0, 353) |
38 (55) (0, 400) |
| Bread, high fibre (g/d) | 12 (24) (0, 239) |
13 (27) (0, 316) |
24 (38) (0, 352) |
| Legumes (g/d) | 17 (29) (0, 400) |
21 (42) (0, 523) |
20 (44) (0, 450) |
Figures shown are mean (SD) and (ranges).
Tracking Coefficients
The tracking coefficient for the energy-dense, high fat, low fibre dietary pattern was 0.48 (95% CI, 0.44-0.52) for boys and 0.38 (95% CI, 0.35-0.41) for girls (Table 3). Tracking coefficients for the food group intakes were generally lower, ranging from 0.14 to 0.40 (Table 3). Tracking was consistently higher among boys, particularly for the dietary pattern and intakes of full fat milk, cakes and biscuits, and high fibre breakfast cereal. After full fat milk, the strongest tracking for both boys and girls was for fresh fruit (0.35 and 0.31, respectively) and vegetables (0.31 and 0.28, respectively).
Table 3.
Tracking coefficients for the dietary pattern and food group z-scores from 7 to 13 years of age.
| BOYS |
GIRLS |
|||
|---|---|---|---|---|
| Tracking Coeff a |
95% CI | Tracking Coeff a |
95% CI | |
|
Energy-dense, high fat, low fibre dietary pattern |
0.48 | 0.44 - 0.52 | 0.38 | 0.35 - 0.41 |
| Food groups positively loaded onto the dietary pattern | ||||
| Confectionery, chocolate | 0.18 | 0.14 - 0.21 | 0.14 | 0.11 - 0.17 |
| Bread, low fibre | 0.22 | 0.19 - 0.25 | 0.17 | 0.14 - 0.20 |
| Cakes, biscuits | 0.22 | 0.19 - 0.25 | 0.15 | 0.12 - 0.18 |
| Potato Crisps | 0.23 | 0.20 - 0.26 | 0.23 | 0.20 - 0.25 |
| Milk, full fat | 0.40 | 0.36 - 0.45 | 0.31 | 0.27 - 0.34 |
| Processed meat | 0.15 | 0.12 - 0.18 | 0.14 | 0.11 - 0.18 |
| Food groups negatively loaded onto the dietary pattern | ||||
| Fresh fruit | 0.35 | 0.32 - 0.39 | 0.31 | 0.28 - 0.35 |
| Vegetables, raw or boiled | 0.31 | 0.28 - 0.35 | 0.28 | 0.25 - 0.31 |
| Breakfast cereal, high fibre | 0.21 | 0.17 - 0.26 | 0.14 | 0.10 - 0.17 |
| Potato, boiled | 0.15 | 0.11 - 0.19 | 0.14 | 0.10 - 0.18 |
| Bread, high fibre | 0.23 | 0.20 - 0.27 | 0.22 | 0.19 - 0.26 |
| Legumes | 0.21 | 0.16 - 0.26 | 0.15 | 0.11 - 0.18 |
GEE adjusted for age at first measurement and time between measurements, all p-value <.0001 (Ho: r = 0)
Maintenance of Extreme Quartile Position
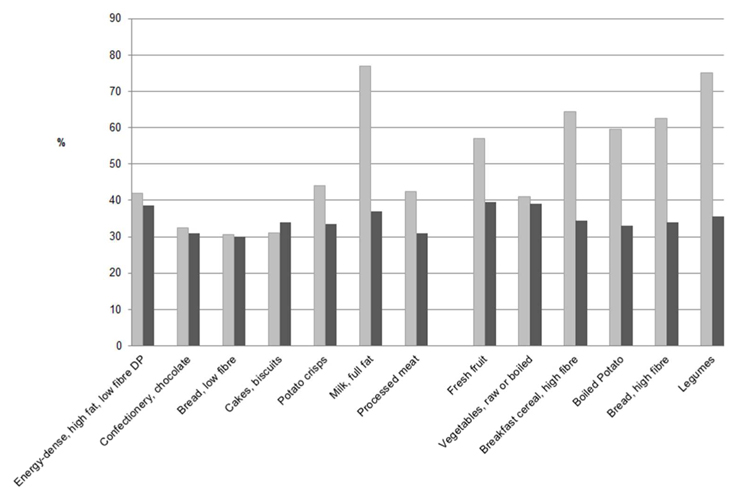
A total of 4,986 individuals (2,460 boys, 2,526 girls) provided dietary data at 7 and 13 y of age. Figure 1 shows the proportion of respondents who maintained their ranking in either the highest or lowest quartile of dietary pattern z-scores and food group intakes between 7 and 13 y of age. For simplicity, Figure 1 shows results for boys and girls combined, as there were few gender differences, with the exception of the dietary pattern. Among girls, tracking for the dietary pattern was stronger among low scorers (43%) compared with high scorers (32%), whereas among boys, there was little difference between high (45%) and low scorers (41%).
Figure 1.
Proportions remaining in the same extreme quartile of consumption levels at 7 and 13 y of age
Plotted values are proportions of respondents who maintained their ranking in either the lowest (light) or highest (dark) quartile at 7 years and 13 years of age. Tertiles replace quartiles for full fat milk, high fibre breakfast cereal, high fibre bread and legumes. Results are for boys and girls combined.
Overall, there was fair to strong tracking of food group intakes; 30% to 77% of subjects maintained their position in an extreme quartile during follow up, more than the 25% expected to remain in the same quartile by chance (Figure 1). Almost 80% of children who were low consumers of full fat milk by 7 y of age remained low consumers at 13 y. The proportions of children maintaining a low consumption of fresh fruit, high fibre breakfast cereals, high fibre bread, potato (boiled) and legumes was considerably greater than those who maintained a high consumption of these foods. The majority of children (57% to 75%) who were low consumers of these foods at 7 years, remained low consumers at 13 y. Among those foods positively loaded onto the dietary pattern, there was some evidence that low consumers of processed meats and crisps at 7 y of age were less likely to alter their intake level than high consumers.
Maternal Education and Prepregnancy BMI
Adjustment for maternal education, pre-pregnancy BMI and dietary misreporting made little difference to the GEE tracking coefficients (not shown).
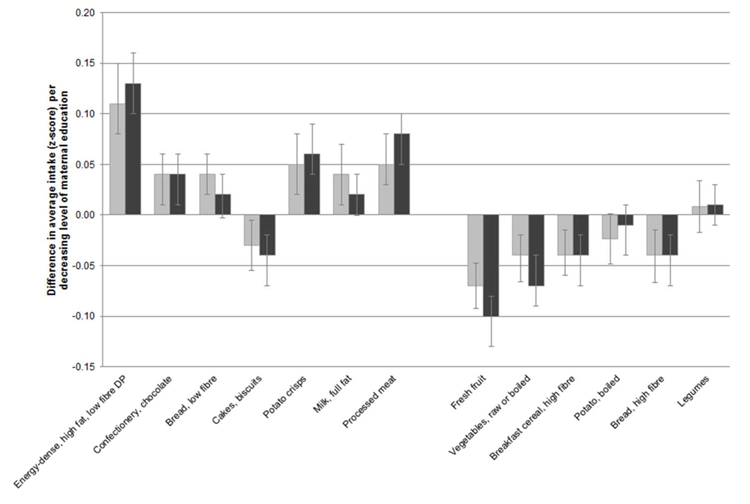
However, maternal education level was a predictor of later dietary pattern z-scores. A lower maternal education level was associated with higher scores for the energy-dense, high fat, low fibre dietary pattern in boys and girls (p-value for trend <0.0001). For example among boys, each decreasing level of maternal education was associated with a dietary pattern score in later years that was on average, 0.11 SD units greater (95% CI, 0.08-0.15, p<0.0001) than those with the highest level of maternal education (Figure 2). As such, boys in the lowest maternal education group had a dietary pattern z-score that was 0.45 SD units (95% CI, 0.30-0.61) greater on average, than those in the highest maternal education group.
Figure 2.
Difference in average intake (z-score) per decreasing level of maternal education (relative to the highest level).
Plotted values are beta coefficients and 95% confidence intervals for maternal education level included as an ordinal covariate (5 levels) in GEE models (Equation 1) to calculate tracking coefficients for dietary pattern z-scores and food intakes (energy-adjusted z-scores).

A lower level of maternal education predicted greater z-scores for later intakes of nearly all foods positively loaded onto the energy-dense, high fat, low fibre dietary pattern; confectionery and chocolate, low fibre bread, crisps, full fat milk and processed meat (Figure 2). Conversely, lower intakes of foods negatively loaded onto the dietary pattern, including fresh fruit, vegetables, high fibre breakfast cereals and high fibre bread, were observed with each decreasing level of maternal education (Figure 2). For example among girls, for each decreasing level of maternal education, fresh fruit intake (energy-adjusted z-score) was lower on average by 0.10 SD units (95% CI, 0.08-0.13, p<0.001) (Figure 2). As such, girls in the lowest maternal education group would be expected to have an average z-score for fruit intake that was 0.37 SD units (95% CI, 0.26-0.47) lower than those in the highest maternal education group.
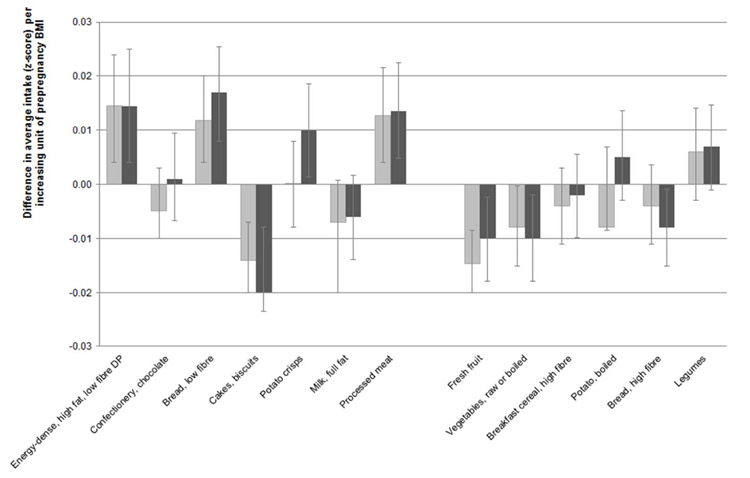
Approximately 17% of study childrens’ mothers were overweight or obese according to their pre-pregnancy BMI. Maternal prepregnancy BMI was a predictor of later dietary pattern z-score and food group intakes, albeit to a lesser extent than maternal education (Figure 3). A one unit higher prepregnancy BMI was associated with later dietary pattern z-scores that were greater on average by 0.015 SD units (95% CI, 0.004-0.025, p=0.005) in girls and 0.010 SD units (95% CI 0.004-0.024, p=0.006) in boys (Figure 3). More markedly, maternal prepregnancy overweight or obesity was associated with later dietary pattern z-scores that were on average, 0.11 SD units (95% CI, 0.02-0.20, p=0.02) higher in boys and 0.08 SD units (95% CI,-0.01-0.18, p=0.08) higher in girls (not shown). A greater prepregnancy BMI predicted higher intakes of low fibre bread, crisps (girls only), processed meat and lower intakes of fresh fruit, vegetables, high fibre bread (girls only) and unexpectedly, cakes and biscuits (Figure 3). Dietary misreporting was not an important predictor of dietary pattern scores or food group intakes.
Figure 3.
Difference in average intake (z-score) per increasing unit of maternal prepregnancy BMI
Plotted values are beta coefficients and 95% confidence intervals for maternal prepregnancy BMI included as a continuous covariate in GEE models (Equation 1) to calculate tracking coefficients for dietary pattern z-scores and food intakes (energy-adjusted z-scores).

Discussion
This analysis of a large pregnancy cohort demonstrates that a dietary pattern longitudinally associated with increased adiposity in childhood and adolescence tracks moderately between 7 and 13 y of age. The individual food groups important to this dietary pattern showed varying but generally lower degrees of tracking. This suggests that, although intakes of individual food groups may be less stable, children and adolescents may maintain a similar profile of dietary energy density, fibre density and energy from fat that can contribute to obesity risk (8).
The differences in tracking among food groups indicates that some food intakes are less stable than others. The stronger tracking coefficients for fruit, vegetables and high fibre bread suggest that early interventions to increase intakes of these foods would be anticipated to have a lasting effect on dietary habits into adolescence. In this study with no specific dietary interventions, it is also notable that children who were low consumers of fruit, high fibre breakfast cereals and high fibre bread at 7 years of age were likely to remain low consumers in adolescence. This suggests that unhealthy dietary habits may be established by the age of 7 years. Conversely, the weaker tracking seen for low fibre bread, processed meat, cakes and biscuits, confectionery and chocolate, suggests that intakes of these foods may be more amenable to intervention throughout childhood and adolescence,.
Maternal education was an important predictor of dietary intakes between 7 and 13 y of age. This corresponds with studies of maternal determinants of offspring diet (9) and suggests that mothers with low levels of education may need extra support to encourage healthy intakes in their children.
The positive relationship between maternal prepregnancy BMI and the energy-dense, high fat, low fibre dietary pattern among children in this study supports the ‘environment hypothesis’ for obesity development. Parents are important role models for children’s dietary intake, and there is considerable evidence that children’s eating behaviours, including food preferences, and levels of intake, are closely aligned with that of their parents (9).
To our knowledge, only two studies have examined how empirically derived dietary patterns track in young people and both focussed on the maintenance of extreme quartiles. The Cardiovascular Risk in Young Finns Study reported how two dietary patterns tracked 6 y and 21 y after baseline in 1,768 children aged 3 to 18 years. The dietary patterns were identified using principal components analysis (PCA); one pattern containing ‘traditional Finnish’, the other ‘health conscious’ foods. Both patterns exhibited similar degrees of tracking however, fewer subjects (approximately 25-42%) maintained the same extreme quartiles than the present study (1). The HEALTH-KIDS study tracked three dietary patterns derived using factor analysis and selected food intakes over 1 year in 181 low-income African-American adolescents (7). A ‘Western’ dietary pattern showed stronger tracking than an ‘Eastern’ or ‘Dairy’ dietary pattern. Similar to our study, tracking was greater among low consumers of fruits and vegetables. However, both studies compared scores for dietary patterns identified using exploratory pattern analyses at each follow up, rather than calculating scores for the same pattern at each follow up. Although these patterns appeared qualitatively similar at different time points, there were some important differences. As such, these studies have not tracked exactly the same dietary pattern over time.
Two other studies have reported tracking coefficients for food intakes in young people using GEE. An Australian study of children aged 5-6 y and 10-12 y (296 in total) reported strong tracking coefficients for frequency of fruit consumption (0.73 to 0.89) and frequency of vegetable consumption (0.52 to 0.86), based on three dietary assessments over five years (2). These tracking coefficients are higher than those reported in the current study, however they are based on the frequency of consumption, not the amount of food consumed, reported by parents. The Amsterdam Growth and Health Study tracked dietary intakes measured six times between 12 and 36 y of age in 168 subjects. The tracking coefficients were 0.33 for fruit (95% CI, 0.25-0.41) and 0.27 for vegetable (95% CI, 0.19-0.36) intakes (20), which are comparable to those observed in the present study. However differences in the length of follow up need to be taken into consideration when making comparisons.
A number of other studies have attempted to track food and nutrient intakes from childhood to adolescence (3, 4, 5, 21, 22, 23). Direct comparison of these studies is challenging, owing to widely varying sample sizes, number and length of follow ups, statistical methods, and dietary assessment methods. However, these studies suggest considerable potential for dietary change between childhood and adolescence (3, 4, 5, 21, 22, 23).
The current study has several strengths. We tracked a dietary pattern known to be longitudinally associated with obesity, or an ‘obesogenic’ dietary pattern (8). This is one of the first studies to track a dietary pattern identified using reduced rank regression. The availability of up to three dietary assessments over the course of childhood, starting from early school age up until mid-adolescence, covers more than one key developmental phase. The application of longitudinal models makes use of all available data. The use of 3-d food diaries provides detail on the types of foods consumed and depends less on dietary recall than other commonly used dietary assessment methods. However, residual error cannot be ruled out, and while we attempted to adjust for dietary misreporting, the degree of error may have increased with age, as the 7 y diaries were completed by the parents, while later diaries were mainly completed by the children. This tracking analysis included over 7,000 children, far more than in previous studies. Although approximately half of the original cohort could not be included in the analysis because of missing data, we adjusted for some of the differences between respondents included and not included in this analysis.
In conclusion, an energy-dense, high fat and low fibre dietary pattern that is longitudinally associated with increased adiposity (8), tracks moderately between 7 and 13 y of age in this cohort. Early interventions (before 7 y of age) to increase intakes of fruit, vegetables, high fibre bread and high fibre breakfast cereals, key components of this dietary pattern, would be anticipated to influence dietary habits into adolescence. Interventions to reduce low fibre bread, processed meat, cakes, biscuits, chocolate and confectionery are also warranted. While policies are needed to support healthy eating among all children, children whose mothers have a history of overweight or low levels of education may need the most support to make these changes.
Supplementary Material
Table S1. Tracking study population and remainder of cohort
What is already known on this subject ?
Dietary intake is an important determinant of obesity in childhood.
Information on how dietary intakes relevant to obesity track during childhood and adolescence is limited, yet this may help to inform interventions to prevent obesity.
What does this study add ?
An energy-dense, high fat, low fibre dietary pattern that is longitudinally associated with increased adiposity is likely to be maintained from mid childhood to adolescence.
Early interventions (before 7 y of age) to increase intakes of fruit, vegetables, high fibre bread and high fibre breakfast cereals, key components of this dietary pattern, would be anticipated to influence dietary habits into adolescence.
Children whose mothers have a history of overweight or low levels of education may need the most support to make these changes.
Acknowledgements
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. SAJ designed research, PME and KN conducted research, GLA analyzed data, GLA and SAJ wrote paper, SAJ and GLA had primary responsibility for final content. All authors read and approved the final manuscript. The authors declare no conflicts of interest.
Financial Disclosure The current analysis was funded by a project grant from the World Cancer Research Fund (2008/31) and a Medical Research Council (MRC) programme grant (U105960389). Core support for ALSPAC is provided by the MRC (74882), the Wellcome Trust (092731) and the University of Bristol.
Footnotes
Supporting Information Table S1: Comparison of tracking study population to rest of cohort
Conflicts of Interest All authors have no conflicts to declare.
References
- 1.Mikkilä V, Räsänen L, Raitakari OT, Pietinen P, Viikari J. Consistent dietary patterns identified from childhood to adulthood: the Cardiovascular Risk in Young Finns Study. Br J Nutr. 2005;93:923–931. doi: 10.1079/bjn20051418. [DOI] [PubMed] [Google Scholar]
- 2.Pearson N, Salmon J, Campbell K, Crawford D, Timperio A. Tracking of children’s body-mass index, television viewing and dietary intake over five-years. Prev Med. 2011;53:268–270. doi: 10.1016/j.ypmed.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 3.Patterson E, Warnberg J, Kearney J, Sjostrom M. The tracking of dietary intakes of children and adolescents in Sweden over six years: the European Youth Heart Study. Int J Behav Nutr Phys Act. 2009;6:91. doi: 10.1186/1479-5868-6-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zive MM, Berry CC, Sallis JF, Frank GC, Nader PR. Tracking dietary intake in white and Mexican-American children from age 4 to 12 years. J Am Diet Assoc. 2002;102:683–689. doi: 10.1016/s0002-8223(02)90155-0. [DOI] [PubMed] [Google Scholar]
- 5.Robson PJ, Gallagher AM, Livingstone MBE, Cran GW, Strain JJ, Savage JM, et al. Tracking of nutrient intakes in adolescence: the experiences of the Young Hearts Project, Northern Ireland. Br J Nutr. 2000;84:541–548. [PubMed] [Google Scholar]
- 6.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13:3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Wang Y. Tracking of dietary intake patterns is associated with baseline characteristics of urban low-income African-American adolescents. J Nutr. 2008;138:94–100. doi: 10.1093/jn/138.1.94. [DOI] [PubMed] [Google Scholar]
- 8.Ambrosini GL, Emmett PM, Northstone K, Howe L, Tilling K, Jebb SA. Identification of a dietary pattern prospectively associated with increased adiposity in childhood and adolescence. Int J Obes. 2012;36:1299–1305. doi: 10.1038/ijo.2012.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patrick H, Nicklas TA. A review of family and social determinants of children’s eating patterns and diet quality. J Am Coll Nutr. 2005;24:83–92. doi: 10.1080/07315724.2005.10719448. [DOI] [PubMed] [Google Scholar]
- 10.Hendrie GA, Coveney J, Cox DN. Defining the complexity of childhood obesity and related behaviours within the family environment using structural equation modelling. Public Health Nutr. 2012;15:48–57. doi: 10.1017/S1368980011001832. [DOI] [PubMed] [Google Scholar]
- 11.Reilly JJ, Armstrong J, Dorosty AR, Emmett PM, Ness AR, Rogers I, et al. Early life risk factors for obesity in childhood: cohort study. BMJ. 2005;330:1357. doi: 10.1136/bmj.38470.670903.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golding J, Pembrey M, Jones J, Team TAS. ALSPAC - The Avon Longitudinal Study of Parents and Children. Paediatr Perinat Epidemiol. 2001;15:74–87. doi: 10.1046/j.1365-3016.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- 13.Boyd A, Golding J, Macleod J, Lawlor DA, Fraser A, Henderson J, et al. Cohort Profile: The ‘Children of the 90’s’ - the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol. 2013;42:111–127. doi: 10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraser A, Macdonald-Wallis C, Tilling K, Boyd A, Golding J, Davey Smith G, et al. Cohort Profile: The Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol. 2013;42:97–110. doi: 10.1093/ije/dys066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson L, Mander AP, Jones LR, Emmett PM, Jebb SA. Energy-dense, low-fiber, high-fat dietary pattern is associated with increased fatness in childhood. Am J Clin Nutr. 2008;87:846–854. doi: 10.1093/ajcn/87.4.846. [DOI] [PubMed] [Google Scholar]
- 16.Rennie KL, Coward A, Jebb SA. Estimating under-reporting of energy intake in dietary surveys using an individualised method. Br J Nutr. 2007;97:1169–1176. doi: 10.1017/S0007114507433086. [DOI] [PubMed] [Google Scholar]
- 17.SAS Institute Incorporated . SAS for Windows. SAS Institute Incorporated; Cary, NC, USA: 2002-2003. [Google Scholar]
- 18.Twisk JWR. Applied longitudinal data analysis for epidemiology. A practical guide. Cambridge University Press; Cambridge: 2003. [Google Scholar]
- 19.Twisk JW. The problem of evaluating the magnitude of tracking coefficients. Eur J Epidemiol. 2003;18:1025–1026. doi: 10.1023/a:1026161919170. [DOI] [PubMed] [Google Scholar]
- 20.te Velde SJ, Twisk JWR, Brug J. Tracking of fruit and vegetable consumption from adolescence into adulthood and its longitudinal association with overweight. Br J Nutr. 2007;98:431–438. doi: 10.1017/S0007114507721451. [DOI] [PubMed] [Google Scholar]
- 21.Singer MR, Moore LL, Garrahie EJ, Ellison RC. The tracking of nutrient intake in young children: the Framingham Children’s Study. Am J Public Health. 1995;85:1673–1677. doi: 10.2105/ajph.85.12.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Bentley ME, Zhai F, Popkin BM. Tracking of dietary intake patterns of Chinese from childhood to adolescence over a six-year follow-up period. The Journal of Nutrition. 2002;132:430–438. doi: 10.1093/jn/132.3.430. [DOI] [PubMed] [Google Scholar]
- 23.Resnicow K, Smith M, Baranowski T, Baranowski J, Vaughan R, Davis M. 2- Year tracking of children’s fruit and vegetable intake. J Am Diet Assoc. 1998;98:785–789. doi: 10.1016/S0002-8223(98)00177-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Tracking study population and remainder of cohort