Abstract
Little is known about how alcohol causes liver disease and cirrhosis. The strongest evidence of the causality between alcohol and liver disease stems from epidemiological observations. Factors contributing to alcohol-induced fibrosis and cirrhosis include cytokines, oxidative stress, and toxic metabolites of ethanol. Patients with alcoholic cirrhosis generally have complications at diagnosis, and cirrhotic complications should be actively assessed because they are closely associated with subsequent morbidity as well as mortality. Abstinence is strictly required to prevent disease progression and is critical for eventual liver transplantation. In addition, nutritional therapy remains the mainstay of managing alcoholic cirrhosis.
Keywords: alcohol, cirrhosis, complication, treatment
Introduction
Alcoholism is a global health problem. Liver metabolizes most of the ingested alcohol. Among individuals who consume more than 70 drinks (1 drink = one 12 oz. beer at 4% alcohol or one 1.5 oz glass of wine at 11% alcohol) per week for over 20 years, 19% developed alcoholic liver disease and 7% developed cirrhosis.1 Thresholds of ethanol consumption per week for the development of alcoholic liver disease were 7 to 13 drinks for women and 14 to 27 drinks for men.1 Alcoholic liver disease can be divided on histology into steatosis, hepatitis, hepatitis superimposed on early cirrhosis, and cirrhosis.2 When symptoms occur in individuals with alcohol abuse, many of them already have progressed to cirrhosis. The risk of cirrhosis correlates strongly with past and current alcohol drinking,3 and many patients with alcoholic cirrhosis have complications at diagnosis.
Pathogenesis of alcoholic fibrosis and cirrhosis
It is incompletely understood how alcohol causes liver disease and cirrhosis. The strongest evidence of the causality is deduced from epidemiological studies, showing a strong correlation between ethanol consumption and alcoholic liver disease as well as cirrhosis.1,3 Prohibition of alcohol drinking can result in a marked reduction of liver-related deaths in patients with alcoholic liver disease.4 Data from genetic polymorphisms in alcohol metabolism further support this association. Ethanol is metabolized in hepatocyte cytosol by alcohol dehydrogenase (ADH) to acetaldehyde, which is subsequently metabolized in the mitochondria by acetaldehyde dehydrogenase (ALDH) to acetate. Polymorphisms of ADH and ALDH genes correlate with occurrence of alcoholic liver disease in Japanese5,6 and with alcoholic cirrhosis in Taiwanese.7,8
Most patients with alcoholic steatosis progress to steatohepatitis and subsequent fibrosis or even cirrhosis. Reactive oxygen species (ROS) are produced in hepatocytes through induction of cytochrome P450 2E1.9,10 Oxidative stress, hepatocytes injury by ROS, is a major determinant in alcoholic liver injury and fibrosis.11,12 Production of ROS results in reduced antioxidant glutathione/glutathione disulfide ratio.13,14 On liver histology, hepatocyte injury is most significant in pericentral regions, where pericentral fibrosis occurs. The latter is also known as sclerosing hyaline necrosis or perivenular fibrosis. In addition, Mallory bodies are an important marker of alcoholic liver injury,15 but they do not have a pathogenic role in the liver damage.
Hepatic stellate cells, the major source of extracellular matrix in hepatic fibrosis, are also stimulated by ROS. Activation of stellate cells is observed during liver injury, resulting in their proliferation and resulting fibrogenesis.16 Studies in experimental alcoholic injury and human alcoholic fibrosis support the central pathway of stellate cell activation in their pathogenesis.17,18 Initiation of the activation results mostly from paracrine stimulation by Kupffer cells, hepatocytes, sinusoidal endothelium, and platelets. Recent identification of the receptor for bacterial lipopolysaccharide, Toll-like receptor 4 (TLR4), in stellate cells and Kupffer cells reveals its role in fibrogenesis.19 TLR4 enhances TGF-beta-1 signaling, which is the major fibrogenic cytokine in the liver.20
Other mediators may also play a role in alcoholic fibrogenesis. Acetaldehyde, but not alcohol itself, has adjunctive fibrogenic activity in cultured stellate cells.21 In experimental rodents fed with alcohol, there is an increased in lipid aldehydes, which are unstable intermediates during interaction of ROS and cellular proteins.22,23 In addition, several reports have addressed the involvement of pro-inflammatory cytokines such as tumor necrosis factor and interleukin-6 in hemodynamic changes of patients with cirrhosis.24,25
Clinical manifestations and diagnosis of alcoholic cirrhosis
Clinical presentations of alcoholic cirrhosis vary from asymptomatic to hepatic decompensation with complications.26 Specific diagnosis for alcoholic cirrhosis is summarized in Table 1. Detail history taking is mandatory for diagnosis. Alcoholic cirrhosis is diagnosed in many individuals with alcohol abuse when they have symptoms. These patients may have malnutrition, parotid enlargement, vascular spiders, palmar erythema, hepatosplenomegaly, portal hypertension, fluid and electrolyte redistribution, feminization, neuropathy, and encephalopathy.27 Laboratory findings show disproportionate elevation of serum aspartate transaminase compared to alanine transaminase, hypoalbuminemia, hyperbilirubinemia, anemia, leukopenia, thrombocytopenia, prolonged prothrombin time, and partial thromboplastin time. Reduced platelet count and function reflect hypersplenism.
Table 1.
Specific diagnosis for alcoholic cirrhosis
| Detail history taking |
| Clinical: hepatosplenomegaly and/or malnutrition |
| Biochemical: disproportionately high aspartate aminotransferase compared to alanine aminotransferase |
| Imaging studies: |
| Ultrasound: convenient and repeatable |
| Magnetic resonance imaging: etiologically specific |
| Hepatic phosphorus-31 magnetic resonance spectroscopy: etiologically specific |
| Liver biopsy: pericentral fibrosis and/or micronodular cirrhosis |
Ultrasound, computed tomography scan, and magnetic resonance imaging (MRI) are well-known imaging tools to diagnose cirrhosis or concomitant neoplastic diseases. Typical imaging studies of alcoholic cirrhosis show hepatomegaly, bluntness of liver edge, irregular liver surface, and coarse liver texture.28,29 MRI may also be used to differentiate alcoholic cirrhosis from cirrhosis of viral hepatitis by the detection of caudate lobe enlargement and the presence of the right posterior hepatic notch.29 Patients with alcoholic cirrhosis with lower phosphodiesterase to adenosine triphosphate ratios may be differentiated from cirrhosis of other etiologies by using hepatic phosphorus-31 magnetic resonance spectroscopy (HP31 MRS).30 In addition, HP31 MRS has the ability to calculate hepatic phospholipid metabolism, which can be used to distinguish alcoholic cirrhosis from noncirrhosis.31
On liver histology, cirrhosis is characterized by hepatic architecture distortion and the formation of regenerative nodules. For patients with advanced alcoholic cirrhosis, the diagnosis is usually made on the basis of clinical, biochemical, imaging, and hemodynamic findings. However, for those without typical manifestations, a liver biopsy is required to establish the diagnosis.2,32,33 Liver biopsy is also beneficial to exclude coexisting liver diseases and to evaluate the severity of concurrent alcoholic hepatitis.34 The reversibility of alcoholic fibrosis may be greater than other causes of fibrosis if there is a prominent inflammatory component due to recent ethanol abuse. Histological factors predicting the irreversibility and advanced cirrhosis include micronodular cirrhosis and thickened septae.35,36 Both alcoholic hepatitis and fibrosis first appear in the pericentral zone, and progress to panlobular fibrosis in continuous drinkers.37 Therefore, the pericentral fibrosis can serve as an early marker of progression to cirrhosis.32,38
Transient elastography (TE) has been introduced as a noninvasive assessment of hepatic fibrosis in patients with chronic viral hepatitis.39 However, TE may not be reliable in patients with concurrent hepatitis and cirrhosis.40,41 The clinical application of TE in alcoholic cirrhosis, especially in those with concurrent alcoholic hepatitis, thus requires further examinations.
Complications and prognosis of alcoholic cirrhosis
Patients with alcoholic cirrhosis are vulnerable to various complications which threaten their life expectancy (Figure 1). Studies have shown the high prevalence of complications at the time of initial diagnosis of alcoholic cirrhosis.42,43 Complications include occurrence of ascites, varices with their related hemorrhage, hepatic encephalopathy, spontaneous bacterial peritonitis, hepatorenal syndrome, hepatopulmonary syndrome, and hepatocellular carcinoma. These complications lead to high mortality in patients with alcoholic cirrhosis. In contrast, prevention and treatment of complications may lead to the prolonged survival.
Figure 1.
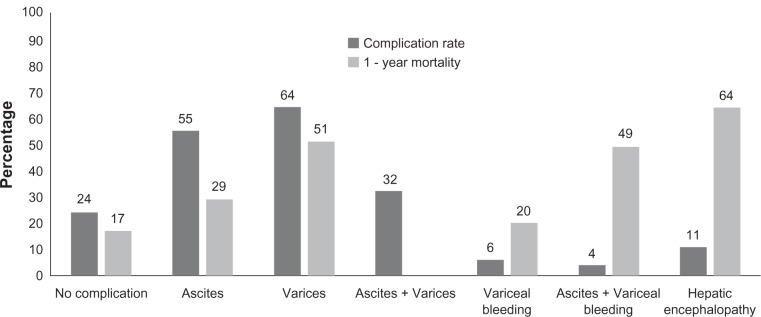
Rates of complications at diagnosis of alcoholic cirrhosis and 1-year mortality following complications. From data of Jepsen et al,42 Huang et al,43 and Lin et al.52
Patients are considered to have decompensated liver disease when complications of cirrhosis develop. Factors predicting poor prognosis include continued alcohol use and the presence of hepatic inflammation. For example, patients with decompensated alcoholic cirrhosis who continue drinking have a 5-year transplant-free survival of 30% versus 60% for those who quit drinking.26,44–46
Ascites
Portal hypertension leads to development of ascites, ie, fluid retention in the peritoneal cavity. Ascites is the most common complication of cirrhosis. The mechanisms of sodium and water retention include activation of the renin–angiotensin–aldosterone system and sympathetic nervous system. The impairment in urinary sodium excretion in cirrhosis correlates with liver function.47 In addition, nonosmotic hypersecretion of antidiuretic hormone is observed. Ascites develop in 58% of patients within 10 years of compensated cirrhosis.48 In a population-based cohort study on the clinical course of alcoholic cirrhosis, the presence and type of 3 complications (ie, ascites, variceal hemorrhage, and encephalopathy) could predict mortality.42 However, this study may have underestimated the rate of complications, due to the detection of ascites mainly by physical findings and nonassessment of varices per se.43 Ultrasound is useful to detect minimal amount of ascites, as well as the presence of portal hypertension.28,49 Early detection of ascites in patients with cirrhosis is important to predict long-term outcomes.50 Complications of cirrhosis should be assessed actively to allow early management of complications and reduce subsequent mortality.
Varices and related hemorrhage
The detection of varices in patients with cirrhosis is important.50 Endoscopy is well known to detect varices in patients with cirrhosis. The newly developed narrow band imaging system may assist the detection of varices.51 The occurrence of varices per se has been reported to predict mortality in patients with alcoholic cirrhosis.52 Variceal hemorrhage occurs in 25% to 40% of patients with cirrhosis,53 and it is a devastating complication, with 1-year mortality of 20%.42
Hepatic encephalopathy
Hepatic encephalopathy is a potentially reversible alteration of brain function in patients with liver decompensation. The diagnosis of hepatic encephalopathy should be made after exclusion of unrelated neurologic or metabolic abnormalities. The definition of hepatic encephalopathy is difficult in patients with alcoholic liver disease who have initial neurologic manifestations as part of diffuse central and peripheral neuropathy. Patients with alcoholic cirrhosis have delayed nerve conduction and evoked potentials.54,55 Neuropsychiatric testing to detect minimal hepatic encephalopathy may be considered for patients with alcoholic liver disease who are at increased risk. A study reported that alcoholic cirrhotic patients with minimal hepatic encephalopathy have driving impairment and should be avoided.56
Spontaneous bacterial peritonitis
Spontaneous bacterial peritonitis (SBP) refers to an ascitic fluid infection without evidence for an intra-abdominal surgically treatable source. SBP mostly occurs in patients with advanced cirrhosis.57 Diagnostic criteria for SBP are a positive ascitic fluid bacterial culture and elevated ascitic fluid absolute polymorphonuclear leukocyte (PMN) count (≥250 cells/mm3). The latter is adequate to start empirical antibiotic treatment.
Hepatorenal syndrome
The hepatorenal syndrome (HRS) is an acute renal failure usually found in patients with cirrhosis or severe alcoholic hepatitis.58–60 HRS represents the end-stage of a sequence of reductions in renal perfusion with the scenario of deteriorating liver injury. The diagnosis of HRS is made by excluding other causes of renal impairment. The prognosis is poor unless underlying liver disease can be improved or liver transplantation can be performed.
Hepatopulmonary syndrome
Hepatopulmonary syndrome (HPS) is diagnosed in patients with the triad of liver disease, increased alveolar–arterial gradient while breathing room air, and evidence for intrapulmonary vascular dilatations.61,62 Prevalence of HPS ranges from 4% to 47%.63,64 Patients with cirrhosis commonly have mild hypoxemia which may have resulted from ascites or pleural fluid compression to the lung parenchyma.65 HPS should be suspected if severe hypoxemia (PaO2 < 60 mmHg) is detected in the absence of cardiopulmonary disease.66
Hepatocellular carcinoma
Although advanced fibrosis is known to be a strong risk factor for hepatocellular carcinoma (HCC) development, patients with alcoholic liver disease are not at an increased risk until cirrhosis develops. Patients with small HCC (<3 cm) are often asymptomatic. The development of HCC should be suspected in a patient with cirrhosis who has serum alpha-fetoprotein (AFP) elevation. However, serum AFP level is normal in up to 40% of small HCCs.67 Elevated AFP may be more likely in viral hepatitis-related than in alcoholic liver disease-related HCC.68
Patients with alcoholic cirrhosis should undergo surveillance for HCC by using ultrasound and AFP. AFP alone should not be used for the screening purpose. Regenerative nodules, as one of histological features of patients with alcoholic cirrhosis, are commonly detected on ultrasound or other imaging studies. Sometimes it may be difficult to distinguish regenerative nodules from HCC, even by contrast-enhanced imaging modalities. In such cases, liver biopsy to the target lesion should be performed.
Prognosis and predictive models
The prognosis of liver cirrhosis is influenced by the following factors: etiology, severity, presence of complications, and comorbid illness. Child–Pugh classification based on 5 parameters (ascites, bilirubin, albumin, prothrombin time, and encephalopathy) is useful to predict the overall prognosis, development of complications, and surgical risk. One-year survival rates for patients with Child–Pugh A, B, and C cirrhosis are 100%, 80%, and 45%, respectively.69,70 Model for End Stage Liver Disease (MELD) using bilirubin, creatinine, INR, and etiology of cirrhosis can also be used to predict outcomes in cirrhosis.
Histologic findings can also predict prognosis in alcoholic cirrhosis, ie, patients with alcoholic hepatitis on cirrhosis had higher mortality.71 In addition, patients with alcoholic hepatitis on cirrhosis usually have elevated sum of asymmetric dimethylarginine and its stereoisomer.72 To predict disease severity and mortality risk in alcoholic hepatitis, Maddrey’s discriminant function, Glasgow alcoholic hepatitis score, and the Lille model are commonly used.73–76 Finally, obese patients with alcohol abuse may have higher risk of advanced liver disease.77
Prevention and treatment of alcoholic cirrhosis
Current prevention and treatment of alcoholic cirrhosis are summarized in Table 2. Among these measures, abstinence of alcohol drinking is fundamental and still beneficial even in cirrhotic patients to prevent disease progression and is critical for eventual liver transplantation. Patients who stop drinking may reduce or normalize portal pressure,78 reduction of ascites,79 and improve fibrosis.80,81
Table 2.
Prevention and treatment of alcoholic cirrhosis
| Alcohol abstinence |
| Nutritional support: short- and long-term benefit |
| Frequent small quantity feeding |
| Micronutrients and vitamin replacement |
| High protein and kilocalorie |
| Liver transplantation |
| Psychosocial support |
| Quit smoking |
| Avoid hepatotoxic agents |
| Vaccination (hepatitis A, B, pneumococcus, and influenza) |
Serum albumin level has been used as an indicator for nutritional status in patients with cirrhosis,82 and nutritional therapy is a mainstay of good practice in alcoholic cirrhosis. In order to improve nutritional balance, frequent small quantity feeding with a morning meal and snack at night time are recommended.83 Micronutrients and vitamin replacement should also be provided as part of the treatment.83 Nutritional deficiencies are common in alcoholism.84 However, few data have confirmed that aggressive nutritional supplementation can reverse the catabolism and inflammation of alcoholic liver disease. Nevertheless, short-term survival outcomes are improved in enteral feeding during hospitalization for severely malnourished alcoholic patients with hepatic decompensation.85,86 Long-term nutritional supports are also beneficial in patients with alcoholic cirrhosis or chronic hepatic decompensation.87,88
A higher-than-usual dietary intake with protein amount of 1.2 to 1.5 g/kg and kilocalorie intake of 35 to 40 kcal/kg is recommended.83 This recommendation by the American Association for the Study of Liver Diseases and the American College of Gastroenterology is based on a study in patients with cirrhosis,89 and such approaches were found to improve the efficiency of nitrogen metabolism and then reduce hospitalization as well as complications.27 It is also recommended that patients could have protein amount of 1.5 g/kg and kilocalorie intake of 40 kcal/kg during acute illness or intermittent exacerbation of chronic disease.83 Branched-chain amino acids may be considered in patients who develop encephalopathy during protein feeding.87
Patients with decompensated cirrhosis should be referred for the possibility of liver transplantation. Transplantation for alcoholic liver disease provides survival benefit, better quality of life, and is thus cost-effective.90–92 Delayed referral for evaluation of transplantation leads to mortality during the waiting period.93 However, delayed referral may be due to active alcoholism.94 Psychosocial support and abstinence are important before transplantation. A 6-month period of abstinence has been widely used as a minimal listing criterion.91,95,96 Living donor liver transplantation (LDLT) in alcoholic liver disease has ethical issues on the indications and timing.97,98 LDLT should be considered only when there is a low risk of recidivism and a likelihood of good outcome.
The rate of recidivism after orthotopic liver transplantation (OLT) ranges from 7% to 31% in patients with alcoholic cirrhosis.99,100 A higher percentage of cirrhotic patients resuming alcohol use after transplantation has been reported, but only 5% to 7% have excessive drinking.100,101 The latter has similar overall compliance to immunosuppression and survival with transplant recipients for other diseases. Nevertheless, a study described the success of post-OLT alcoholism treatment programs to reduce the relapse rate of any drinking.102 Post-transplantation alcoholic liver disease patients in the United Kingdom have regular appointments with a psychiatrist in addictions treatment training.103,104
Alcoholic liver disease patients who receive liver transplantation have increased risk of lung, liver, and oropharyngeal cancer.105,106 It has been reported that up to 40% of post-transplant patients with alcoholic liver disease resume smoking.107 The high rate of post-transplant cancer may be due to prior smoking in combination with the impact of immunosuppression on tumor surveillance.
Two meta-analyses reported the benefit of corticosteroids in severe alcoholic hepatitis.108,109 A pooled analysis of data from randomized controlled trials also revealed their efficacy in improving short-term survival.110 However, the benefit of corticosteroids in severe alcoholic hepatitis superimposed on alcoholic cirrhosis remains unknown. Pentoxifylline of 400 mg 3 times daily for 6 months can reduce bacterial infection, renal failure, and hepatic encephalopathy in patients with advanced cirrhosis but not the short-term mortality.111
Potential hepatotoxic agents should be avoided in patients with cirrhosis, which include herbal remedies, acetaminophen, and drugs with hepatotoxic side effects. Hepatitis A and B vaccination should be considered to prevent additional insult to the liver with limited functional reserve. Pneumococcal and yearly influenza vaccination may also be beneficial.
Prevention and treatment of complications of alcoholic cirrhosis
Ascites
Nonsteroidal anti-inflammatory drugs may induce water retention and should be avoided.112 Dietary sodium restriction to 88 meq (2000 mg) per day is recommended, which includes sodium in all foods, liquids, and medications.113,114 When liver function deteriorates, urinary sodium excretion declines.79 A combination of diuretics with sodium restriction is required. Successful treatment refers to reducing ascitic fluid volume without intravascular volume depletion. Treatment of ascites improves quality of life and protects against SBP.
Varices and related hemorrhage
Patients with alcoholic cirrhosis should undergo evaluation to assess the presence of varices and to determine the risk of variceal hemorrhage.43,50,52 To prevent varices and a first variceal bleeding, nonselective beta blockers are recommended for small varices which have a high risk of bleeding or varices in Child–Pugh B or C cirrhosis. Furthermore, nonselective beta blockers or endoscopic band ligation are suggested for medium or large varices.115 For treatment of acute variceal bleeding, combination of vasoconstrictor and endoscopic band ligation are recommended for Child–Pugh A or B patients or patients with an hepatic venous pressure gradient (HVPG) of less than 20 mmHg,116,117 together with short-term prophylactic norfloxacin or ceftriaxone.118,119 In patients with Child–Pugh C or an HVPG of more than 20 mmHg, more aggressive treatment should be considered. Transjugular intrahepatic portosystemic shunt is a salvage therapy for patients who responded poorly to previous treatment, and early placement is suggested. Endoscopic variceal obturation with butyl cyanoacrylate is recommended for acute bleeding of gastric varices.120,121 To prevent recurrent variceal bleeding, a combination of endoscopic band ligation and nonselective beta-blockers is recommended.122
Hepatic encephalopathy
A precipitating factor can usually be identified when hepatic encephalopathy occurs. These factors include constipation, infection, gastrointestinal bleeding, increased protein intake, hypokalemic alkalosis, hypoxia, and sedatives.123 Treatment of these precipitating factors leads to a continuous and rapid improvement of encephalopathy. Nevertheless, protein restriction in acute encephalopathy is not recommended.124
Elevation of serum ammonia is detected in 60% to 80% of patients with hepatic encephalopathy. Lowering of the ammonia levels improves encephalopathy. Hypokalemia should be corrected because hypokalemia increases renal ammonia production. Lactulose is widely used to reduce ammonia and to inhibit its production. Although there is limited evidence from controlled trials, lactulose is the mainstay of treatment to improve encephalopathy,125 and to prevent its recurrence.126 Lactulose enemas have also been reported to be more effective than tap water enemas.127
Antibiotics can be also used to inhibit intestinal ammonia production and absorption. Rifaximin prevents recurrent hepatic encephalopathy over a 6-month period128 and is better tolerated than nonabsorbable disaccharides.129 Neomycin has been found to be similar in efficacy to lactulose,130 but also to placebo.131 However, the alteration in gut flora due to antibiotics use is a concern.
Intestinal ammonia production may also be inhibited by acarbose, an alpha glycosidase inhibitor. Acarbose has been shown in a randomized trial to reduce blood ammonia and improve encephalopathy in diabetes patients.132 Its efficacy and possible side effects in nondiabetic patients need further study.
Another approach to reduce ammonia is by stimulating glutamine synthesis which leads to enhancement of ammonia metabolism and removal. This can be achieved by ornithine and aspartate. Ornithine–aspartate has been described to be effective in mild hepatic encephalopathy,133,134 but not in acute liver failure.135
An increase in the plasma aromatic amino acids to branched-chain amino acids (BCAA) ratio has been proposed as another hypothesis of hepatic encephalopathy, besides the ammonia hypothesis. Parenteral BCAA supplementation has contradictory results in mortality.136,137 Several trials have found beneficial effects of oral BCAA in cirrhotic patients intolerant to protein or under a low protein diet.87,138
A third hypothesis of hepatic encephalopathy is the neuronal inhibition by GABA-receptor complex. This principal inhibitory network in the central nervous system includes the benzodiazepine receptor site. An increase in benzodiazepine receptor ligands has been proven in patients with hepatic encephalopathy.139 A meta-analysis showed that treatment with flumazenil, a benzodiazepine receptor antagonist, improves encephalopathy in some patients.140
Zinc and melatonin have been suggested in the treatment of hepatic encephalopathy. Cirrhotic patients with encephalopathy have zinc deficiency.141 Zinc supplement to improve encephalopathy has been described in case reports,142,143 but has not been proven in a double-blind trial.144 Cirrhotic patients have elevated daytime melatonin levels which may contribute to the disturbances of the sleep–wake cycle.145 Melatonin supplement may be an option to alter the sleep–wake cycle in these patients.146
Spontaneous bacterial peritonitis
Judicious use of diuresis can increase ascitic fluid opsonic activity and may prevent SBP.147 Aggressive treatment of infections at other sites can also prevent SBP. Antibiotics should be initiated early to maximize survival of the patients.114 Empirical antibiotics should be started when there is an unexplained presence of fever, abdominal pain or tenderness, altered mental status, or ascitic polymorphonuclear count of ≥250 cells/mm3. Most of the ascitic cultures in SBP reveal gut bacteria such as Escherichia coli and Klebsiella. A third-generation cephalosporin is a reasonable empirical antibiotic.57,148
A follow-up ascitic fluid after antibiotics treatment is not necessary in most patients with SBP who have dramatic clinical response. A 5-day treatment duration has been proven to be as effective as a 10-day.149
Hepatorenal syndrome
The onset of renal impairment is insidious and may be precipated by gastrointestinal bleeding, infection, or overly rapid diuresis. The combination of a systemic vasoconstrictor (midodrine) and an inhibitor of endogenous vasodilator release (octreotide) but not octreotide alone improves renal and systemic hemodynamics.150,151 Vasopressin analogs plus plasma volume expansion may also be beneficial for hepatorenal syndrome.152–154
Hepatopulmonary syndrome
Currently, no medical therapy has been shown to significantly improve oxygenation. Liver transplantation is indicated for patients with severe and refractory hypoxemia.
Hepatocellular carcinoma
Surveillance for hepatocellular carcinoma in patients with alcoholic cirrhosis is recommended to allow earlier detection of hepatocellular carcinoma to achieve better treatment response.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Becker U, Deis A, Sorensen TI, et al. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology. 1996;23:1025–1029. doi: 10.1002/hep.510230513. [DOI] [PubMed] [Google Scholar]
- 2.Review by an international group Alcoholic liver disease: morphological manifestations. Lancet. 1981;317:707–711. [PubMed] [Google Scholar]
- 3.Klatsky AL, Armstrong MA. Alcohol, smoking, coffee, and cirrhosis. Am J Epidemiol. 1992;136:1248–1257. doi: 10.1093/oxfordjournals.aje.a116433. [DOI] [PubMed] [Google Scholar]
- 4.Zakim D, Boyer TD, Montgomery C. Hepatology: Textbook of Liver Disease. Philadelphia: Saunders; 1990. Alcoholic liver disease; p. 821. [Google Scholar]
- 5.Enomoto N, Takase S, Takada N, Takada A. Alcoholic liver disease in heterozygotes of mutant and normal aldehyde dehydrogenase-2 genes. Hepatology. 1991;13:1071–1075. [PubMed] [Google Scholar]
- 6.Shibuya A, Yoshida A. Genotypes of alcohol-metabolizing enzymes in Japanese with alcohol liver diseases: a strong association of the usual Caucasian-type aldehyde dehydrogenase gene (ALDH12) with the disease. Am J Hum Genet. 1988;43:744–748. [PMC free article] [PubMed] [Google Scholar]
- 7.Chao YC, Liou SR, Chung YY, et al. Polymorphism of alcohol and aldehyde dehydrogenase genes and alcoholic cirrhosis in Chinese patients. Hepatology. 1994;19:360–366. [PubMed] [Google Scholar]
- 8.Chao YC, Young TH, Tang HS, Hsu CT. Alcoholism and alcoholic organ damage and genetic polymorphisms of alcohol metabolizing enzymes in Chinese patients. Hepatology. 1997;25:112–117. doi: 10.1002/hep.510250121. [DOI] [PubMed] [Google Scholar]
- 9.Nieto N, Friedman SL, Cederbaum AI. Cytochrome P450 2E1-derived reactive oxygen species mediate paracrine stimulation of collagen I protein synthesis by hepatic stellate cells. J Biol Chem. 2002;277:9853–9864. doi: 10.1074/jbc.M110506200. [DOI] [PubMed] [Google Scholar]
- 10.Castillo T, Koop DR, Kamimura S, Triadafilopoulos G, Tsukamoto H. Role of cytochrome P-450 2E1 in ethanol-, carbon tetrachloride- and iron-dependent microsomal lipid peroxidation. Hepatology. 1992;16:992–996. doi: 10.1002/hep.1840160423. [DOI] [PubMed] [Google Scholar]
- 11.Parola M, Robino G. Oxidative stress-related molecules and liver fibrosis. J Hepatology. 2001;35:297–306. doi: 10.1016/s0168-8278(01)00142-8. [DOI] [PubMed] [Google Scholar]
- 12.Sastre J, Serviddio G, Pereda J, et al. Mitochondrial function in liver disease. Front Biosci. 2007;12:1200–1209. doi: 10.2741/2138. [DOI] [PubMed] [Google Scholar]
- 13.Lieber CS. Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol. 2004;34:9–19. doi: 10.1016/j.alcohol.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Yang SS, Huang CC, Chen JR, et al. Effects of ethanol on antioxidant capacity in isolated rat hepatocytes. World J Gastroenterol. 2005;11:7272–7276. doi: 10.3748/wjg.v11.i46.7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.French SW, Nash J, Shitabata P, et al. Pathology of alcoholic liver disease. Semin Liver Dis. 1993;13:154–169. doi: 10.1055/s-2007-1007346. [DOI] [PubMed] [Google Scholar]
- 16.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsukamoto H, Gaal K, French SW. Insights into the pathogenesis of alcoholic liver necrosis and fibrosis: status report. Hepatology. 1990;12:599–608. doi: 10.1002/hep.1840120325. [DOI] [PubMed] [Google Scholar]
- 18.Okanoue T, Burbige EJ, French SW. The role of the Ito cell in perivenular and intralobular fibrosis in alcoholic hepatitis. Arch Pathol Lab Med. 1983;107:459–463. [PubMed] [Google Scholar]
- 19.Paik YH, Schwabe RF, Bataller R, Russo MP, Jobin C, Brenner DA. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology. 2003;37:1043–1055. doi: 10.1053/jhep.2003.50182. [DOI] [PubMed] [Google Scholar]
- 20.Seki E, de Minicis S, Osterreicher CH, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 21.Casini A, Cunningham M, Rojkind M, Lieber CS. Acetaldehyde increases procollagen type I and fibronectin gene transcription in cultured rat fat-storing cells through a protein synthesis-dependent mechanism. Hepatology. 1991;13:758–765. [PubMed] [Google Scholar]
- 22.Kamimura S, Gaal K, Britton RS, Bacon BR, Triadafilopoulos G, Tsukamoto H. Increased 4-hydroxynonenal levels in experimental alcoholic liver disease: association of lipid peroxidation with liver fibrogenesis. Hepatology. 1992;16:448–453. doi: 10.1002/hep.1840160225. [DOI] [PubMed] [Google Scholar]
- 23.Kawase T, Kato S, Lieber CS. Lipid peroxidation and antioxidant defense systems in rat liver after chronic ethanol feeding. Hepatology. 1989;10:815–821. doi: 10.1002/hep.1840100511. [DOI] [PubMed] [Google Scholar]
- 24.Sheron N, Bird G, Koskinas J, et al. Circulating and tissue levels of the neutrophil chemotaxin interleukin-8 are elevated in severe acute alcoholic hepatitis, and tissue levels correlate with neutrophil infiltration. Hepatology. 1993;18:41–46. [PubMed] [Google Scholar]
- 25.McClain CS, Hill D, Schmidt J, Diehl AM. Cytokines and alcoholic liver disease. Semin Liver Dis. 1993;13:170–182. doi: 10.1055/s-2007-1007347. [DOI] [PubMed] [Google Scholar]
- 26.Adachi M, Brenner DA. Clinical syndromes of alcoholic liver disease. Dig Dis. 2005;23:255–263. doi: 10.1159/000090173. [DOI] [PubMed] [Google Scholar]
- 27.McCullough AJ, O’Connor JF. Alcoholic liver disease: proposed recommendations for the American College of Gastroenterology. Am J Gastroenterol. 1998;93:2022–2036. doi: 10.1111/j.1572-0241.1998.00587.x. [DOI] [PubMed] [Google Scholar]
- 28.Yang SS. Alcoholic liver disease: clinical and sonographic features. J Med Ultrasound. 2008;16:140–149. [Google Scholar]
- 29.Okazaki H, Ito K, Fujita T, Koike S, Takano K, Matsunaga N. Discrimination of alcoholic from virus-induced cirrhosis on MR imaging. AJR Am J Roentgenol. 2000;175:1677–1681. doi: 10.2214/ajr.175.6.1751677. [DOI] [PubMed] [Google Scholar]
- 30.Menon DK, Sargentoni J, Taylor-Robinson SD, et al. Effect of functional grade and etiology on in vivo hepatic phosphorus-31 magnetic resonance spectroscopy in cirrhosis: biochemical basis of spectral appearances. Hepatology. 1995;21:417–427. [PubMed] [Google Scholar]
- 31.Schlemmer HP, Sawatzki T, Sammet S, et al. Hepatic phospholipids in alcoholic liver disease assessed by proton-decoupled 31P magnetic resonance spectroscopy. J Hepatol. 2005;42:752–759. doi: 10.1016/j.jhep.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 32.Worner TM, Lieber CS. Perivenular fibrosis as precursor lesion of cirrhosis. JAMA. 1985;254:627–630. [PubMed] [Google Scholar]
- 33.Chedid A, Mendenhall CL, Tosch T, et al. Significance of megamitochondria in alcoholic liver disease. Gastroenterology. 1986;90:1858–1864. doi: 10.1016/0016-5085(86)90253-2. [DOI] [PubMed] [Google Scholar]
- 34.Talley NJ, Roth A, Woods J, Hench V. Diagnostic value of liver biopsy in alcoholic liver disease. J Clin Gastroenterol. 1988;10:647–650. doi: 10.1097/00004836-198812000-00015. [DOI] [PubMed] [Google Scholar]
- 35.Nagula S, Jain D, Groszmann RJ, Garcia-Tsao G. Histological-hemodynamic correlation in cirrhosis – a histological classification of the severity of cirrhosis. J Hepatol. 2006;44:111–117. doi: 10.1016/j.jhep.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 36.Issa R, Zhou X, Constandinou CM, et al. Spontaneous recovery from micronodular cirrhosis: evidence for incomplete resolution associated with matrix cross-linking. Gastroenterology. 2004;126:1795–1808. doi: 10.1053/j.gastro.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Teli MR, Day CP, Burt AD, Bennett MK, James OF. Determinants of progression to cirrhosis or fibrosis in pure alcoholic fatty liver. Lancet. 1995;346:987–990. doi: 10.1016/s0140-6736(95)91685-7. [DOI] [PubMed] [Google Scholar]
- 38.Nakano M, Worner TM, Lieber CS. Perivenular fibrosis in alcoholic liver injury: ultrastructure and histologic progression. Gastroenterology. 1982;83:777–785. [PubMed] [Google Scholar]
- 39.Castera L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343–350. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 40.Arena U, Vizzutti F, Corti G, et al. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology. 2008;47:380–384. doi: 10.1002/hep.22007. [DOI] [PubMed] [Google Scholar]
- 41.Sagir A, Erhardt A, Schmitt M, Haussinger D. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology. 2008;47:592–595. doi: 10.1002/hep.22056. [DOI] [PubMed] [Google Scholar]
- 42.Jepsen P, Ott P, Andersen PK, Sorensen HT, Vilstrup H. Clinical course of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology. 2010;51:1675–1682. doi: 10.1002/hep.23500. [DOI] [PubMed] [Google Scholar]
- 43.Huang YW, Hu JT, Yang SS. Complications of alcoholic liver cirrhosis: active assessment by endoscopy and sonography. Hepatology. 2010;52:1864–1865. doi: 10.1002/hep.23799. [DOI] [PubMed] [Google Scholar]
- 44.Galambos JT. Alcoholic hepatitis: its therapy and prognosis. Prog Liver Dis. 1972;4:567–588. [PubMed] [Google Scholar]
- 45.Pares A, Caballeria J, Bruguera M, Torres M, Rodes J. Histological course of alcoholic hepatitis: influence of abstinence, sex and extent of hepatic damage. J Hepatol. 1986;2:33–42. doi: 10.1016/s0168-8278(86)80006-x. [DOI] [PubMed] [Google Scholar]
- 46.Morgan MY. The prognosis and outcome of alcoholic liver disease. Alcohol Alcohol Suppl. 1994;2:335–343. [PubMed] [Google Scholar]
- 47.Wensing G, Lotterer E, Link I, Hahn EG, Fleig WE. Urinary sodium balance in patients with cirrhosis: relationship to quantitative parameters of liver function. Hepatology. 1997;26:1149–1155. doi: 10.1002/hep.510260510. [DOI] [PubMed] [Google Scholar]
- 48.Gines P, Quintero E, Arroyo V, et al. Compensated cirrhosis: natural history and prognostic factors. Hepatology. 1987;7:122–128. doi: 10.1002/hep.1840070124. [DOI] [PubMed] [Google Scholar]
- 49.Yang SS, Ralls PW, Korula J. The effect of oral nitroglycerin on portal blood velocity as measured by ultrasonic Doppler: a double blind, placebo controlled study. J Clin Gastroenterol. 1991;13:173–177. doi: 10.1097/00004836-199104000-00011. [DOI] [PubMed] [Google Scholar]
- 50.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 51.Yang SS, Hu JT, Huang YW, Hsu MY, Chang HY. The study of narrow band imaging system in the evaluation of esophageal varices. Gastroenterol J Taiwan. 2009;26:164–169. [Google Scholar]
- 52.Lin CW, Chen YS, Lai CH, et al. Esophagogastric varices predict mortality in hospitalized patients with alcoholic liver disease in Taiwan. Hepatogastroenterol. 2010;57:285–289. [PubMed] [Google Scholar]
- 53.Grace ND. Prevention of initial variceal hemorrhage. Gastroenterol Clin North Am. 1992;21:149–161. [PubMed] [Google Scholar]
- 54.Chu NS, Yang SS. Somatosensory and brainstem auditory evoked potentials in alcoholic liver disease with and without encephalopathy. Alcohol. 1987;4:225–230. doi: 10.1016/0741-8329(87)90016-4. [DOI] [PubMed] [Google Scholar]
- 55.Yang SS, Chu NS, Wu CH. The role of somatosensory evoked potentials on hepatic encephalopathy. Biomed Eng Appl Basis Comm. 1997;9:154–157. [Google Scholar]
- 56.Wein C, Koch H, Popp B, Oehler G, Schauder P. Minimal hepatic encephalopathy impairs fitness to drive. Hepatology. 2004;39:739–745. doi: 10.1002/hep.20095. [DOI] [PubMed] [Google Scholar]
- 57.Such J, Runyon BA. Spontaneous bacterial peritonitis. Clin Infect Dis. 1998;27:669–674. doi: 10.1086/514940. [DOI] [PubMed] [Google Scholar]
- 58.Gines P, Schrier RW. Renal failure in cirrhosis. N Engl J Med. 2009;361:1279–1290. doi: 10.1056/NEJMra0809139. [DOI] [PubMed] [Google Scholar]
- 59.Gines P, Guevara M, Arroyo V, Rodes J. Hepatorenal syndrome. Lancet. 2003;362:1819–1827. doi: 10.1016/S0140-6736(03)14903-3. [DOI] [PubMed] [Google Scholar]
- 60.Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:1637–1648. doi: 10.1053/gast.2000.20189. [DOI] [PubMed] [Google Scholar]
- 61.Hoeper MM, Krowka MJ, Strassburg CP. Portopulmonary hypertension and hepatopulmonary syndrome. Lancet. 2004;363:1461–1468. doi: 10.1016/S0140-6736(04)16107-2. [DOI] [PubMed] [Google Scholar]
- 62.Rodriguez-Roisin R, Krowka MJ. Hepatopulmonary syndrome – a liver-induced lung vascular disorder. N Engl J Med. 2008;358:2378–2387. doi: 10.1056/NEJMra0707185. [DOI] [PubMed] [Google Scholar]
- 63.Stoller JK, Lange PA, Westveer MK, Carey WD, Vogt D, Henderson JM. Prevalence and reversibility of the hepatopulmonary syndrome after liver transplantation. The Cleveland Clinic experience. West J Med. 1995;163:133–138. [PMC free article] [PubMed] [Google Scholar]
- 64.Hopkins WE, Waggoner AD, Barzilai B. Frequency and significance of intrapulmonary right-to-left shunting in end-stage hepatic disease. Am J Cardiol. 1992;70:516–519. doi: 10.1016/0002-9149(92)91200-n. [DOI] [PubMed] [Google Scholar]
- 65.Naeije R, Melot C, Hallemans R, Mols P, Lejeune P. Pulmonary hemodynamics in liver cirrhosis. Semin Respir Med. 1985;7:164–170. [Google Scholar]
- 66.Lange PA, Stoller JK. The hepatopulmonary syndrome. Ann Intern Med. 1995;122:521–529. doi: 10.7326/0003-4819-122-7-199504010-00008. [DOI] [PubMed] [Google Scholar]
- 67.Chen DS, Sung JL, Sheu JC, et al. Serum alpha-fetoprotein in the early stage of human hepatocellular carcinoma. Gastroenterology. 1984;86:1404–1409. [PubMed] [Google Scholar]
- 68.Fasani P, Sangiovanni A, de Fazio C, et al. High prevalence of multinodular hepatocellular carcinoma in patients with cirrhosis attributable to multiple risk factors. Hepatology. 1999;29:1704–1707. doi: 10.1002/hep.510290604. [DOI] [PubMed] [Google Scholar]
- 69.Infante-Rivard C, Esnaola S, Villeneuve JP. Clinical and statistical validity of conventional prognostic factors in predicting short-term survival among cirrhotics. Hepatology. 1987;7:660–664. doi: 10.1002/hep.1840070408. [DOI] [PubMed] [Google Scholar]
- 70.Albers I, Hartmann H, Bircher J, Creutzfeldt W. Superiority of the Child-Pugh classification to quantitative liver function tests for assessing prognosis of liver cirrhosis. Scand J Gastroenterol. 1989;24:269–276. doi: 10.3109/00365528909093045. [DOI] [PubMed] [Google Scholar]
- 71.Orrego H, Blake JR, Blendis LM, Medline A. Prognosis of alcoholic cirrhosis in the presence and absence of alcoholic hepatitis. Gastroenterology. 1987;92:208–214. doi: 10.1016/0016-5085(87)90861-4. [DOI] [PubMed] [Google Scholar]
- 72.Mookerjee RP, Malaki M, Davies NA, et al. Increasing dimethylarginine levels are associated with adverse clinical outcome in severe alcoholic hepatitis. Hepatology. 2007;45:62–71. doi: 10.1002/hep.21491. [DOI] [PubMed] [Google Scholar]
- 73.Imperiale TF, McCullough AJ. Do corticosteroids reduce mortality from alcoholic hepatitis? A meta-analysis of the randomized trials. Ann Intern Med. 1990;113:299–307. doi: 10.7326/0003-4819-113-4-299. [DOI] [PubMed] [Google Scholar]
- 74.Maddrey WC, Boitnott JK, Bedine MS, Weber FL, Jr, Mezey E, White RI., Jr Corticosteroid therapy of alcoholic hepatitis. Gastroenterology. 1978;75:193–199. [PubMed] [Google Scholar]
- 75.Forrest EH, Evans CD, Stewart S, et al. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut. 2005;54:1174–1179. doi: 10.1136/gut.2004.050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Louvet A, Naveau S, Abdelnour M, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology. 2007;45:1348–1354. doi: 10.1002/hep.21607. [DOI] [PubMed] [Google Scholar]
- 77.Naveau S, Cassard-Doulcier AM, Njike-Nakseu M, et al. Harmful effect of adipose tissue on liver lesions in patients with alcoholic liver disease. J Hepatol. 2010;52:895–902. doi: 10.1016/j.jhep.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 78.Reynolds TB, Geller HM, Kuzma OT, Redeker AG. Spontaneous decrease in portal pressure with clinical improvement in cirrhosis. N Engl J Med. 1960;263:734–739. doi: 10.1056/NEJM196010132631505. [DOI] [PubMed] [Google Scholar]
- 79.Runyon BA. Historical aspects of treatment of patients with cirrhosis and ascites. Semin Liver Dis. 1997;17:163–173. doi: 10.1055/s-2007-1007195. [DOI] [PubMed] [Google Scholar]
- 80.Niemela O, Risteli J, Blake JE, Risteli L, Compton KV, Orrego H. Markers of fibrogenesis and basement membrane formation in alcoholic liver disease. Relation to severity, presence of hepatitis, and alcohol intake. Gastroenterology. 1990;98:1612–1619. doi: 10.1016/0016-5085(90)91098-q. [DOI] [PubMed] [Google Scholar]
- 81.Alexander JF, Lischner MW, Galambos JT. Natural history of alcoholic hepatitis. II. The long-term prognosis. Am J Gastroenterol. 1971;56:515–525. [PubMed] [Google Scholar]
- 82.Yang SS, Wu CH, Chen LL, Mo SC, Chen DF. Nutritional status in non-alcoholic sub-clinical porto-systemic encephalopathy. World J Gastroenterol. 1998;4:380–384. doi: 10.3748/wjg.v4.i5.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology. 2010;51:307–328. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- 84.Mendenhall CL, Anderson S, Weesner RE, Goldberg SJ, Crolic KA. Protein-calorie malnutrition associated with alcoholic hepatitis. Veterans Administration Cooperative Study Group on Alcoholic Hepatitis. Am J Med. 1984;76:211–222. doi: 10.1016/0002-9343(84)90776-9. [DOI] [PubMed] [Google Scholar]
- 85.Kearns PJ, Young H, Garcia G, et al. Accelerated improvement of alcoholic liver disease with enteral nutrition. Gastroenterology. 1992;102:200–205. doi: 10.1016/0016-5085(92)91801-a. [DOI] [PubMed] [Google Scholar]
- 86.Cabre E, Gonzalez-Huix F, Abad-Lacruz A, et al. Effect of total enteral nutrition on the short-term outcome of severely malnourished cirrhotics. A randomized controlled trial. Gastroenterology. 1990;98:715–720. doi: 10.1016/0016-5085(90)90293-a. [DOI] [PubMed] [Google Scholar]
- 87.Marchesini G, Dioguardi FS, Bianchi GP, et al. Long-term oral branched-chain amino acid treatment in chronic hepatic encephalopathy. A randomized double-blind casein-controlled trial. The Italian Multicenter Study Group. J Hepatol. 1990;11:92–101. doi: 10.1016/0168-8278(90)90278-y. [DOI] [PubMed] [Google Scholar]
- 88.Hirsch S, Bunout D, de la Maza P, et al. Controlled trial on nutrition supplementation in outpatients with symptomatic alcoholic cirrhosis. JPEN J Parenter Enteral Nutr. 1993;17:119–124. doi: 10.1177/0148607193017002119. [DOI] [PubMed] [Google Scholar]
- 89.Swart GR, Zillikens MC, van Vuure JK, van den Berg JW. Effect of a late evening meal on nitrogen balance in patients with cirrhosis of the liver. BMJ. 1989;299:1202–1203. doi: 10.1136/bmj.299.6709.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.De Gottardi A, Dumortier J. Transplantation for alcoholic liver disease. Gut. 2007;56:735–736. doi: 10.1136/gut.2006.115022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kumar S, Stauber RE, Gavaler JS. Orthotopic liver transplantation for alcoholic liver disease. Hepatology. 1990;11:159–164. doi: 10.1002/hep.1840110202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Longworth L, Young T, Buxton MJ, et al. Midterm cost-effectiveness of the liver transplantation program of England and Wales for three disease groups. Liver Transpl. 2003;9:1295–1307. doi: 10.1016/j.lts.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 93.Everhart JE, Lombardero M, Detre KM, et al. Increased waiting time for liver transplantation results in higher mortality. Transplantation. 1997;64:1300–1306. doi: 10.1097/00007890-199711150-00012. [DOI] [PubMed] [Google Scholar]
- 94.Julapalli VR, Kramer JR, El-Serag HB. Evaluation for liver transplantation: adherence to AASLD referral guidelines in a large Veterans Affairs center. Liver Transpl. 2005;11:1370–1378. doi: 10.1002/lt.20434. [DOI] [PubMed] [Google Scholar]
- 95.Bird GL, O’Grady JG, Harvey FA, Calne RY, Williams R. Liver transplantation in patients with alcoholic cirrhosis: selection criteria and rates of survival and relapse. BMJ. 1990;301:15–17. doi: 10.1136/bmj.301.6742.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Osorio RW, Ascher NL, Avery M, Bacchetti P, Roberts JP, Lake JR. Predicting recidivism after orthotopic liver transplantation for alcoholic liver disease. Hepatology. 1994;20:105–110. doi: 10.1016/0270-9139(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 97.Bramstedt KA, Jabbour N. When alcohol abstinence criteria create ethical dilemmas for the liver transplant team. J Med Ethics. 2006;32:263–265. doi: 10.1136/jme.2005.012856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schiano TD, Kim-Schluger L, Gondolesi G, Miller CM. Adult living donor liver transplantation: the hepatologist’s perspective. Hepatology. 2001;33:3–9. doi: 10.1053/jhep.2001.21489. [DOI] [PubMed] [Google Scholar]
- 99.Berlakovich GA, Steininger R, Herbst F, Barlan M, Mittlbock M, Muhlbacher F. Efficacy of liver transplantation for alcoholic cirrhosis with respect to recidivism and compliance. Transplantation. 1994;58:560–565. doi: 10.1097/00007890-199409150-00006. [DOI] [PubMed] [Google Scholar]
- 100.Fabrega E, Crespo J, Casafont F, de las Heras G, de la Pena J, Pons-Romero F. Alcoholic recidivism after liver transplantation for alcoholic cirrhosis. J Clin Gastroenterol. 1998;26:204–206. doi: 10.1097/00004836-199804000-00011. [DOI] [PubMed] [Google Scholar]
- 101.Gerhardt TC, Goldstein RM, Urschel HC, et al. Alcohol use following liver transplantation for alcoholic cirrhosis. Transplantation. 1996;62:1060–1063. doi: 10.1097/00007890-199610270-00005. [DOI] [PubMed] [Google Scholar]
- 102.Bjornsson E, Olsson J, Rydell A, et al. Long-term follow-up of patients with alcoholic liver disease after liver transplantation in Sweden: impact of structured management on recidivism. Scand J Gastroenterol. 2005;40:206–216. doi: 10.1080/00365520410009591. [DOI] [PubMed] [Google Scholar]
- 103.Kotlyar DS, Burke A, Campbell MS, Weinrieb RM. A critical review of candidacy for orthotopic liver transplantation in alcoholic liver disease. Am J Gastroenterol. 2008;103:734–743. doi: 10.1111/j.1572-0241.2007.01691.x. [DOI] [PubMed] [Google Scholar]
- 104.Bathgate AJ. Recommendations for alcoholic liver disease. Lancet. 2006;367:2045–2046. doi: 10.1016/S0140-6736(06)68904-6. [DOI] [PubMed] [Google Scholar]
- 105.Jain A, DiMartini A, Kashyap R, Youk A, Rohal S, Fung J. Long-term follow-up after liver transplantation for alcoholic liver disease under tacrolimus. Transplantation. 2000;70:1335–1342. doi: 10.1097/00007890-200011150-00012. [DOI] [PubMed] [Google Scholar]
- 106.Boffetta P, Hashibe M. Alcohol and cancer. Lancet Oncol. 2006;7:149–156. doi: 10.1016/S1470-2045(06)70577-0. [DOI] [PubMed] [Google Scholar]
- 107.Dimartini A, Javed L, Russell S, et al. Tobacco use following liver transplantation for alcoholic liver disease: an underestimated problem. Liver Transpl. 2005;11:679–683. doi: 10.1002/lt.20385. [DOI] [PubMed] [Google Scholar]
- 108.Imperiale TF, McCullough AJ. Do corticosteroids reduce mortality from alcoholic hepatitis? A meta-analysis of the randomized trials. Ann Intern Med. 1990;113:299–307. doi: 10.7326/0003-4819-113-4-299. [DOI] [PubMed] [Google Scholar]
- 109.Reynolds TB, Benhamou JP, Blake J, et al. Treatment of acute alcoholic hepatitis. Gastroenterol Int. 1989;2:308. [Google Scholar]
- 110.Mathurin P, Mendenhall CL, Carithers RL, Jr, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis (AH): individual data analysis of the last three randomized placebo controlled double blind trials of corticosteroids in severe AH. J Hepatol. 2002;36:480–487. doi: 10.1016/s0168-8278(01)00289-6. [DOI] [PubMed] [Google Scholar]
- 111.Lebrec D, Thabut D, Oberti F, et al. Pentoxifylline does not decrease short-term mortality but does reduce complications in patients with advanced cirrhosis. Gastroenterology. 2010;138:1755–1762. doi: 10.1053/j.gastro.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 112.Arroyo V, Ginés P, Rimola A, Gaya L. Renal function abnormalities, prostaglandins, and effects of nonsteroidal anti-inflammatory drugs in cirrhosis with ascites. An overview with emphasis on pathogenesis. Am J Med. 1986;81:104–122. doi: 10.1016/0002-9343(86)90912-5. [DOI] [PubMed] [Google Scholar]
- 113.Runyon BA, Montano AA, Akriviadis EA, Antillon MR, Irving MA, McHutchison JG. The serum-ascites albumin gradient is superior to the exudates-transudate concept in the differential diagnosis of ascites. Ann Intern Med. 1992;117:215–220. doi: 10.7326/0003-4819-117-3-215. [DOI] [PubMed] [Google Scholar]
- 114.Runyon BA. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087–2107. doi: 10.1002/hep.22853. [DOI] [PubMed] [Google Scholar]
- 115.Gluud LL, Klingenberg S, Nikolova D, Gluud C. Banding ligation versus beta-blockers as primary prophylaxis in esophageal varices: systemic review of randomized trials. Am J Gastroenterol. 2007;102:2842–2848. doi: 10.1111/j.1572-0241.2007.01564.x. [DOI] [PubMed] [Google Scholar]
- 116.Villanueva C, Piqueras M, Aracil C, et al. A randomized controlled trial comparing ligation and sclerotherapy as emergency endoscopic treatment added to somatostatin in acute variceal bleeding. J Hepatol. 2006;45:560–567. doi: 10.1016/j.jhep.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 117.Banares R, Albillos A, Rincon D, et al. Endoscopic treatment versus endoscopic plus pharmacologic treatment for acute variceal bleeding: a meta-analysis. Hepatology. 2002;35:609–615. doi: 10.1053/jhep.2002.31354. [DOI] [PubMed] [Google Scholar]
- 118.Bernard B, Grange JD, Khac EN, Amiot X, Opolon P, Poynard T. Antibiotic prophylaxis for the prevention of bacterial infections in cirrhotic patients with gastrointestinal bleeding: a meta-analysis. Hepatology. 1999;29:1655–1661. doi: 10.1002/hep.510290608. [DOI] [PubMed] [Google Scholar]
- 119.Fernandez J, Ruiz del Arbol L, Gomez C, et al. Norfloxacin vs ceftriaxone in the prophylaxis of infections in patients with advanced cirrhosis and hemorrhage. Gastroenterology. 2006;131:1049–1056. doi: 10.1053/j.gastro.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 120.Lo GH, Lai KH, Cheng JS, Chen MH, Chiang HT. A prospective, randomized trial of butyl cyanoacrylate injection versus band ligation in the management of bleeding gastric varices. Hepatology. 2001;33:1060–1064. doi: 10.1053/jhep.2001.24116. [DOI] [PubMed] [Google Scholar]
- 121.Tan PC, Hou MC, Lin HC, et al. A randomized trial of endoscopic treatment of acute gastric variceal hemorrhage: N-butyl-2-cyanoacrylate injection versus band ligation. Hepatology. 2006;43:690–697. doi: 10.1002/hep.21145. [DOI] [PubMed] [Google Scholar]; Hepatology. 2006;43:1410. Erratum. [Google Scholar]
- 122.Gonzalez R, Zamora J, Gomez-Camarero J, Molinero LM, Banares R, Albillos A. Combination endoscopic and drug therapy to prevent variceal rebleeding in cirrhosis. Ann Intern Med. 2008;149:109–122. doi: 10.7326/0003-4819-149-2-200807150-00007. [DOI] [PubMed] [Google Scholar]
- 123.Conn HO. A rational program for the management of hepatic coma. Gastroenterology. 1969;57:715–723. [PubMed] [Google Scholar]
- 124.Cordoba J, Lopez-Hellin J, Planas M, et al. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol. 2004;41:38–43. doi: 10.1016/j.jhep.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 125.Prasad S, Dhiman RK, Duseja A, Chawla YK, Sharma A, Agarwal R. Lactulose improves cognitive functions and health-related quality of life in patients with cirrhosis who have minimal hepatic encephalopathy. Hepatology. 2007;45:549–559. doi: 10.1002/hep.21533. [DOI] [PubMed] [Google Scholar]
- 126.Sharma BC, Sharma P, Agrawal A, Sarin SK. Secondary prophylaxis of hepatic encephalopathy: an open-label randomized controlled trial of lactulose versus placebo. Gastroenterology. 2009;137:885–891. doi: 10.1053/j.gastro.2009.05.056. [DOI] [PubMed] [Google Scholar]
- 127.Uribe M, Campollo O, Vargas F, et al. Acidifying enemas (lactitol and lactose) vs nonacidifying enemas (tap water) to treat acute portal-systemic encephalopathy: a double-blind, randomized clinical trial. Hepatology. 1987;7:639–643. doi: 10.1002/hep.1840070404. [DOI] [PubMed] [Google Scholar]
- 128.Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071–1081. doi: 10.1056/NEJMoa0907893. [DOI] [PubMed] [Google Scholar]
- 129.Jiang Q, Jiang XH, Zheng MH, Jiang LM, Chen YP, Wang L. Rifaximin versus nonabsorbable disaccharides in the management of hepatic encephalopathy: a meta-analysis. Eur J Gastroenterol Hepatol. 2008;20:1064–1070. doi: 10.1097/MEG.0b013e328302f470. [DOI] [PubMed] [Google Scholar]
- 130.Conn HO, Leevy CM, Vlahcevic ZR, et al. Comparison of lactulose and neomycin in the treatment of chronic portal-systemic encephalopathy. A double blind controlled trial. Gastroenterology. 1977;72:573–583. [PubMed] [Google Scholar]
- 131.Strauss E, Tramote R, Silva EP, et al. Double-blind randomized clinical trial comparing neomycin and placebo in the treatment of exogenous hepatic encephalopathy. Hepatogastroenterology. 1992;39:542–545. [PubMed] [Google Scholar]
- 132.Gentile S, Guarino G, Romano M, et al. A randomized controlled trial of acarbose in hepatic encephalopathy. Clin Gastroenterol Hepatol. 2005;3:184–191. doi: 10.1016/s1542-3565(04)00667-6. [DOI] [PubMed] [Google Scholar]
- 133.Stauch S, Kircheis G, Adler G, et al. Oral L-ornithine-L-aspartate therapy of chronic hepatic encephalopathy: results of a placebo-controlled double-blind study. J Hepatol. 1998;28:856–864. doi: 10.1016/s0168-8278(98)80237-7. [DOI] [PubMed] [Google Scholar]
- 134.Kircheis G, Nilius R, Held C, et al. Therapeutic efficacy of L-ornithine-L-aspartate infusions in patients with cirrhosis and hepatic encephalopathy: results of a placebo-controlled, double-blind study. Hepatology. 1997;25:1351–1360. doi: 10.1002/hep.510250609. [DOI] [PubMed] [Google Scholar]
- 135.Acharya SK, Bhatia V, Sreenivas V, Khanal S, Panda SK. Efficacy of L-ornithine L-aspartate in acute liver failure: a doubleblind, randomized, placebo-controlled study. Gastroenterology. 2009;136:2159–2168. doi: 10.1053/j.gastro.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 136.Ferenci P. Critical evaluation of the role of branched chain amino acids in liver disease. In: Thomas JC, Jones EA, editors. Recent Advances in Hepatology. New York: Churchill Livingstone; 1986. p. 137. [Google Scholar]
- 137.Naylor CD, O’Rourke K, Detsky AS, Baker JP. Parenteral nutrition with branched-chain amino acids in hepatic encephalopathy. A meta-analysis. Gastroenterology. 1989;97:1033–1042. doi: 10.1016/0016-5085(89)91517-5. [DOI] [PubMed] [Google Scholar]
- 138.Horst D, Grace ND, Conn HO, et al. Comparison of dietary protein with an oral, branched chain-enriched amino acid supplement in chronic portal-systemic encephalopathy: a randomized controlled trial. Hepatology. 1984;4:279–287. doi: 10.1002/hep.1840040218. [DOI] [PubMed] [Google Scholar]
- 139.Basile AS, Hughes RD, Harrison PM, et al. Elevated brain concentrations of 1,4-benzodiazepines in fulminant hepatic failure. N Engl J Med. 1991;325:473–478. doi: 10.1056/NEJM199108153250705. [DOI] [PubMed] [Google Scholar]
- 140.Goulenok C, Bernard B, Cadranel JF, et al. Flumazenil vs. placebo in hepatic encephalopathy in patients with cirrhosis: a meta-analysis. Aliment Pharmacol Ther. 2002;16:361–372. doi: 10.1046/j.1365-2036.2002.01191.x. [DOI] [PubMed] [Google Scholar]
- 141.Loomba V, Pawar G, Dhar KL, Setia MS. Serum zinc levels in hepatic encephalopathy. Indian J Gastroenterol. 1995;14:51–53. [PubMed] [Google Scholar]
- 142.Marchesini G, Fabbri A, Bianchi G, Brizi M, Zoli M. Zinc supplementation and amino acid-nitrogen metabolism in patients with advanced cirrhosis. Hepatology. 1996;23:1084–1092. doi: 10.1053/jhep.1996.v23.pm0008621138. [DOI] [PubMed] [Google Scholar]
- 143.Van der Rijt CC, Schalm SW, Schat H, Foeken K, de Jong G. Overt hepatic encephalopathy precipitated by zinc deficiency. Gastroenterology. 1991;100:1114–1118. doi: 10.1016/0016-5085(91)90290-2. [DOI] [PubMed] [Google Scholar]
- 144.Riggio O, Ariosto F, Merli M, et al. Short-term oral zinc supplementation does not improve chronic hepatic encephalopathy. Results of a double-blind crossover trial. Dig Dis Sci. 1991;36:1204–1208. doi: 10.1007/BF01307509. [DOI] [PubMed] [Google Scholar]
- 145.Steindl PE, Finn B, Bendok B, Rothke S, Zee PC, Blei AT. Disruption of the diurnal rhythm of plasma melatonin in cirrhosis. Ann Intern Med. 1995;123:274–277. doi: 10.7326/0003-4819-123-4-199508150-00005. [DOI] [PubMed] [Google Scholar]
- 146.Lewy AJ, Ahmed S, Jackson JM, Sack RL. Melatonin shifts human circadian rhythms according to a phase-response curve. Chronobiol Int. 1992;9:380–392. doi: 10.3109/07420529209064550. [DOI] [PubMed] [Google Scholar]
- 147.Runyon BA, Antillon MR, Montano AA. Effect of dieresis versus therapeutic paracentesis on ascitic fluid opsonic activity and serum complement. Gastroenterology. 1989;97:158–162. doi: 10.1016/0016-5085(89)91430-3. [DOI] [PubMed] [Google Scholar]
- 148.Felisart J, Rimola A, Arroyo V, et al. Cefotaxime is more effective than is ampicillin-tobramycin in cirrhotics with severe infections. Hepatology. 1985;5:457–462. doi: 10.1002/hep.1840050319. [DOI] [PubMed] [Google Scholar]
- 149.Runyon BA, McHutchison JG, Antillon MR, Akriviadis EA, Montano AA. Short-course versus long-course antibiotic treatment of spontaneous bacterial peritonitis. A randomized controlled study of 100 patients. Gastroenterology. 1991;100:1737–1742. doi: 10.1016/0016-5085(91)90677-d. [DOI] [PubMed] [Google Scholar]
- 150.Kalambokis G, Economou M, Fotopoulos A, et al. The effects of chronic treatment with octreotide versus octreotide plus Midodrine on systemic hemodynamics and renal hemodynamics and function in nonazotemic cirrhotic patients with ascites. Am J Gastroenterol. 2005;100:879–885. doi: 10.1111/j.1572-0241.2005.40899.x. [DOI] [PubMed] [Google Scholar]
- 151.Pomier-Layrargues G, Paquin SC, Hassoun Z, Lafortune M, Tran A. Octreotide in hepatorenal syndrome: a randomized, doubleblind, placebo-controlled, crossover study. Hepatology. 2003;38:238–243. doi: 10.1053/jhep.2003.50276. [DOI] [PubMed] [Google Scholar]
- 152.Guevara M, Gines P, Fernandez-Esparrach G, et al. Reversibility of hepatorenal syndrome by prolonged administration of ornipressin and plasma volume expansion. Hepatology. 1998;27:35–41. doi: 10.1002/hep.510270107. [DOI] [PubMed] [Google Scholar]
- 153.Martin-Llahi M, Pepin MN, Guevara M, et al. Terlipressin and albumin vs albumin in patients with cirrhosis and hepatorenal syndrome: a randomized study. Gastroenterology. 2008;134:1352–1359. doi: 10.1053/j.gastro.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 154.Sanyal AJ, Boyer T, Garcia-Tsao G, et al. A randomized, prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology. 2008;134:1360–1368. doi: 10.1053/j.gastro.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


