Abstract
In many species, binding of sperm to the egg initiates cortical granule exocytosis, an event that contributes to a sustained block of polyspermy. Interestingly, cortical granule exocytosis can be elicited in immature Xenopus oocytes by the protein kinase C activator, phorbol-12-myristate-13-acetate. In this study, we investigated the role of cysteine string protein (csp) in phorbol-12-myristate-13-acetate-evoked cortical granule exocytosis. Prior work indicated that csp is associated with cortical granules of Xenopus oocytes. In oocytes exhibiting >20-fold overexpression of full-length Xenopus csp, cortical granule exocytosis was reduced by ~80%. However, csp overexpression did not affect constitutive exocytosis. Subcellular fractionation and confocal fluorescence microscopy revealed that little or none of the overexpressed csp was associated with cortical granules. This accumulation of csp at sites other than cortical granules suggested that mislocalized csp might sequester a protein that is important for regulated exocytosis. Because the NH2-terminal region of csp includes a J-domain, which interacts with constitutively expressed 70-kDa heat shock proteins (Hsc 70), we evaluated the effect of overexpressing the J-domain of csp. Although the native J-domain of csp inhibited cortical granule exocytosis, point mutations that interfere with J-domain binding to Hsc 70 eliminated this inhibition. These data indicate that csp interaction with Hsc 70 molecular chaperones is vital for regulated secretion in Xenopus oocytes.
Eggs of many species exhibit cortical granule exocytosis, an early postfertilization event that leads to a sustained block of polyspermy (1). Although oocytes of Xenopus laevis must undergo maturation before sperm can elicit cortical granule exocytosis, immature oocytes (and eggs) of Xenopus secrete in response to activators of protein kinase C (2). Somewhat unexpectedly (given that the majority of regulated secretory events are initiated by an increase of cytosolic Ca2+, Refs. 3 and 4), cortical granule exocytosis in Xenopus oocytes is insensitive to changes of cytosolic Ca2+. For instance, this secretory event is not triggered by Ca2+ ionophores (using physiological concentrations of Ca2+); it cannot be evoked by direct injection of Ca2+ into the cytoplasm, and it is unaffected by removal of extracellular Ca2+ or by buffers that clamp cytosolic Ca2+ below the resting level (5–7). Thus, oocytes offer an empirical avenue for assessing the function of proteins in a secretory pathway where calcium ions play no direct role. This issue is of considerable importance with respect to cysteine string proteins (csp2(s)), a class of proteins found associated with a broad range of regulated secretory organelles (8–10).
Csp was originally identified as a novel synaptic antigen in Drosophila (11), and several subsequent investigations indicated that csp might be important as a modulator of presynaptic Ca2+ channels (12–17). However, it also became evident that regulation of presynaptic Ca2+ channels was not the sole function of csp (8–10). For instance, with the recognition that the majority of the cysteine residues of csp were fatty acylated (18), it was proposed that this unusual hydrophobic domain of csp might participate directly in membrane fusion (19). Empirical evidence for a role of csp in membrane fusion emerged from amperometric recordings of chromaffin cells overexpressing csp. This study revealed a change in the rise time of amperometric spikes, thereby implicating csp in fusion pore expansion (20). Complementing this work, genetic experiments indicated that the cysteine string of csp could not be truncated or functionally substituted by serine residues (21). Concurrently, strategies to perturb csp function in pancreatic and adrenal cells suggested that csp was involved at an undisclosed step of secretion downstream of Ca2+ entry into the cell (22–24). Studies of a csp null mutant Drosophila yielded a specific hypothesis for this downstream role of csp. Based on an apparent change of Ca2+ homeostasis and the level of Ca2+ needed to trigger quantal secretion, csp was ascribed a role in regulating the Ca2+ sensitivity of the exocytotic machinery (25). In view of these disparate and occasionally conflicting ideas regarding the molecular role(s) of csp, we initiated studies of csp function using Xenopus oocytes.
Xenopus oocytes offer several advantages for studying regulated secretion. As noted, cortical granule exocytosis is a one-time secretory event that can be triggered in a Ca2+-independent manner by the protein kinase C activator, PMA (phorbol-12-myristate-13-acetate). Secretion can be assayed using single oocytes (7, 26). Moreover, oocytes readily express protein from injected mRNA (27–30), and protein expression and secretion can be correlated for the same cell (7, 26). Finally, oocytes possess efficient mechanisms for the delivery of proteins to the plasma membrane (and the extracellular compartment, Refs. 28–30), which enables one to compare the machinery underlying the regulated and constitutive secretory pathways. Thus, we evaluated the impact on cortical granule exocytosis of overexpressing full-length Xenopus csp. Although we surmised that an elevated cellular content of csp might enhance the kinetics of secretion, instead, we observed a profound reduction of cortical granule exocytosis. Further investigation revealed that little or none of the overexpressed csp was localized to cortical granules. This result raised the possibility that the inhibition of cortical granule exocytosis was because of a non-productive association between the overexpressed csp and one or more csp-interacting proteins. We present evidence that the interaction of overexpressed csp with Hsc 70 (constitutively expressed heat shock protein of 70 kDa) figures prominently in the inhibition of cortical granule exocytosis in these cells.
MATERIALS AND METHODS
Isolation and Culturing of Oocytes, RNA Preparation, and Injection
Stage V–VI X. laevis oocytes were obtained by collagenase treatment of fragments of ovary as described (31). RNA transcripts were prepared from linearized constructs subcloned into the plasmid pCS2+ using the SP6 mMessage Machine from Ambion. Oocytes were injected with 30–60 ng of RNA and cultured for 2–4 days at 18 °C prior to the analysis of secretory events.
Preparation of csp Constructs
Linearized (NotI) full-length Xenopus csp cDNA (32) was either used directly to prepare RNA (Xcsp RNA), or it served as a template for the generation by PCR of the J-domain construct (amino acid residues 1–70). Site-directed mutagenesis was used to generate the J-domain mutations H43Q and D45A. The green fluorescent protein (GFP)-csp construct was kindly supplied by H. E. Yu and W. M. Bement (University of Wisconsin, Madison). All constructs were verified by nucleotide sequence analysis.
Regulated Secretion Assay
Cortical granule exocytosis was evoked by exposing single oocytes to PMA (50–100 nm in Barths solution) for 20–30 min followed by Coomassie staining and densitometric analysis of released protein after resolution on SDS-polyacrylamide gels (7, 31).
Independently, we developed a procedure to trigger cortical granule exocytosis that appears to involve a protein-independent effect of Ca2+ at the plasma membrane of the oocyte. This was achieved by exposing immature, stage V–VI oocytes to the calcium ionophore, ionomycin (10 μm), in Barths solution with added calcium chloride (20 mm). Secretion triggered in this manner is unaffected by overexpression of a dominant-negative fragment of Xenopus myosin IC, or by pretreatment of oocytes with N-ethylmaleimide (10 mm) or phenylarsine oxide (50 μm), all of which completely block exocytosis triggered by PMA in immature oocytes (data not shown). We used this high Ca2+-ionomycin method of evoking cortical granule exocytosis to assess whether intact cortical granules were still present in oocytes expressing recombinant csp.
Immunoblot Analysis of Oocytes
To detect the expression of csp, csp constructs or Hsc 70 (using Hsp 70 monoclonal antibody, clone BRM-22 from Sigma) in oocytes, individual cells were extracted in 20–40 μl of 1% Triton X-100 in 0.1 m NaCl, 0.05 m Tris-HCl (pH 7.4), 1 mm EDTA, and the extract was centrifuged at 15,000 × g for 1 min. The supernatant was mixed with 5× concentrated Laemmli sample buffer and resolved on a SDS-polyacrylamide gel for immunoblot analysis as before (7, 26, 31). An antibody specific for the amino terminus of csp (N-end antibody) was obtained by covalently conjugating recombinant Xenopus csp 1–112 to Pierce's aminolink resin (following the manufacturer's instructions) and adsorbing csp antiserum (16) to this matrix, washing with phosphate-buffered saline, and eluting the bound antibody using 0.1 m glycine-HCl (pH 3.0). After adjusting the pH to 7.5 with 1 m Tris base, the antibody capable of binding the carboxyl terminus of csp was removed by passing the N-end antibody through an aminolink resin to which the recombinant carboxyl end of Xenopus csp (residues 137–197) was coupled. This protocol yields an antibody that selectively identifies the amino terminus of csp but not the carboxyl terminus of csp. Quantification (by densitometry) of immunoblot signals used one-dimensional image analysis software from Kodak. Results are expressed as the mean ± S.D., and significant results were assessed by Student's t test. In many experiments, overexpression of csp constructs was sufficiently robust that the signal for csp in control oocytes was only detected when the signal for the overexpressed csp constructs exhibited saturation. Although our routine quantification of the extent of overexpression of csp constructs is likely to be underestimated, independent analyses of diluted extracts from csp overexpressing oocytes set this error at <30%.
Subcellular Fractionation of Oocytes
As described (7), oocytes were gently disrupted and centrifuged (3 min at 720 × g) to yield a pellet enriched in cortical granules, plasma membrane, yolk, and pigment granules, whereas most other subcellular constituents (including β-tubulin, which was detected by immunoblot using monoclonal antibody E7 from the Developmental Studies Hybridoma Bank) remain in the low speed supernatant (7, 26, 31). Surface labeling of oocytes prior to this fractionation protocol used water-soluble, polyethylene oxide-maleimide biotin (0.1 mm in Barths solution; Pierce) for 15 min to alkylate thiol residues that had been reduced by dithiothreitol (2 mm). Detection used streptavidin-peroxidase (Sigma) and enhanced chemiluminescence.
Confocal Fluorescence Microscopy
Isolated cortical regions of oocytes were prepared (as in Ref. 26) from control oocytes or oocytes expressing either GFP-csp or GFP-syntaxin 1a (from rat; kindly provided by Dr. T. Coppola, University of Nice, France) and fixed for 10 min in 3% paraformaldehyde in Barths solution prior to mounting (using Fluoromount) on glass slides. Specimens were examined using a Zeiss 510 Meta laser scanning confocal microscope equipped with an argon laser and a 40× 1.3 na Plan-Neofluar objective (Carl Zeiss Microimaging, Inc., Thornwood, N.Y.) with 488 nm excitation and a band pass filter setting of 505–530 nm. Image stacks (5 μm) were collected in 0.1–1.0-μm z-steps and projected onto a single plane or used to obtain a z-plane image. Images were collected at a resolution of 1024 × 1024 pixels (GFP-csp) or 2048 × 2048 pixels (GFP-syntaxin 1a).
Immunoprecipitation
Oocytes were disrupted in extraction buffer (1% Triton X-100 in phosphate-buffered saline with 2 mm ADP) and centrifuged at 15,000 × g for 1 min. The supernatant was precleared for 15 min with 0.1 volume of protein A/G-agarose (Santa Cruz). Cleared extract from 2–3 oocytes was incubated with or without affinity-purified csp antibody (1–2 μg), and as a separate control, csp antibody was added to extraction buffer alone. After 1 h at 4 °C, protein A/G-agarose (10 μl) was added, and samples were gently mixed by rotation. After 30–40 min, the agarose beads were collected by centrifugation and washed three times with extraction buffer before dispersion in sample buffer. Recovered protein was resolved electrophoretically for immuno-blot analysis using Hsp/Hsc 70 antibody or csp antibody, as above. Blots were also probed for two abundant oocyte proteins, actin and β-tubulin.
Assay of Constitutive Exocytosis
Plasma membrane proteins are efficiently delivered to the surface of the oocyte via a constitutive exocytotic pathway involving carrier vesicles that are ~100 nm in diameter (30). We developed a biochemical assay of this process by expressing GFP-syntaxin 1a in oocytes and monitoring the appearance of GFP-syntaxin 1a immunoreactivity in the low speed pellet fraction of oocytes (generated as in Ref. 7; results from confocal fluorescence microscopy confirm that GFP-syntaxin 1a reaches the plasma membrane of oocytes). To verify that the presence of GFP-syntaxin 1a in the low speed pellet requires intact protein trafficking via the Golgi apparatus, some oocytes were treated with the Golgi-dismantling reagent, brefeldin A (50 μm) subsequent to the injection of RNA for GFP-syntaxin 1a. To assess the impact of csp constructs on the trafficking of GFP-syntaxin, oocytes were injected with csp RNA 2 days prior to injection with GFP-syntaxin 1a RNA.
RESULTS
Functional Consequence of csp Overexpression
Xenopus oocytes typically secrete 1–1.5 μg of cortical granule lectin in response to PMA (2, 7, 26). This is illustrated in Fig. 1A, which shows the broad band of highly glycosylated lectin that is collected from three individual control oocytes exposed to 50 nm PMA. In contrast, secretion of this lectin is greatly diminished (or absent) for cells that overexpress full-length csp (Fig. 1A). Quantification of this inhibitory effect reveals that the mean amount of secreted cortical granule lectin drops to 20 ± 13% of control (for 29 control cells and 26 cells overexpressing csp) in oocytes challenged with PMA 3–4 days after injection of csp RNA (this difference is significant at p < 0.01).
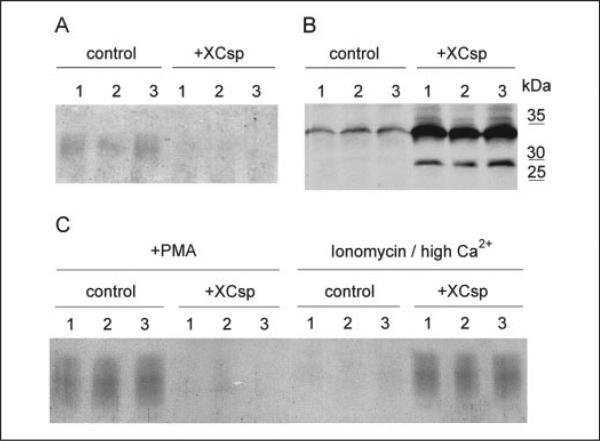
FIGURE 1. Overexpression of Xenopus csp (Xcsp) inhibits PMA-dependent cortical granule exocytosis in Xenopus oocytes.
A, individual control oocytes (three separate cells) and csp-overexpressing oocytes (three separate cells injected with csp RNA) were incubated for 30 min with 50 nm PMA, and released cortical granule lectin was detected by Coomassie staining of samples resolved electrophoretically. Cortical granule lectin runs as a broad band between 35 and 45 kDa. Secretion of this protein was inhibited in cells overexpressing csp (+Xcsp). B, overexpression of csp revealed by immunoblot. Extracts of the same cells depicted in A were subjected to immunoblot for csp. Signal in csp overexpressing cells was appreciably stronger than control at the native mass of csp (~33 kDa). In addition, csp overexpressing cells contain unacylated csp at ~27 kDa. C, three control and three csp overexpressing oocytes were challenged with PMA (50 nm). After 30 min the solution was replaced with ionomycin (10 μm) in high Ca2+ solution for 30 min. Released cortical granule lectin was detected as in A.
Immunoblot analysis revealed that oocytes injected 3–4 days earlier with 30–50 ng of Xcsp RNA expressed 20 ± 15 times more csp than control oocytes (Fig. 1B). The overexpressed csp was present as two distinct species in the RNA-injected oocytes. In addition to csp immunoreactivity at the same mobility of csp in control oocytes (32–33 kDa, see Fig. 1B), there was an additional band at ~27 kDa (Fig. 1B). Because this latter species exhibited mobility that is consistent with the mass of csp prior to fatty acylation (or after deacylation; see Refs. 18, 31, and 32), it is very likely that this form of overexpressed csp lacks lipid modification (indeed, when extracts of csp overexpressing oocytes were treated with the deacylating reagent, hydroxylamine (1 m (pH 7.0), followed by reduction and alkylation) all of the 32–33 kDa csp was converted to the 27-kDa species, data not shown). Together with the data of Fig. 1A these results indicate that csp is overexpressed in oocytes, and this overexpression interferes with the normal pathway by which PMA elicits the secretion of cortical granule lectin. It is also important to note that with less robust overexpression (<10-fold) of csp there is proportionately less inhibition of cortical granule exocytosis (data not shown).
The results of Fig. 1A could be explained by an effect of overexpressed csp that either impairs the exocytotic pathway or that alters the pool of cortical granule lectin available for secretion (for instance, by promoting degradation of cortical granules or by prematurely evoking secretion). We excluded an effect of overexpressed csp on the available pool of cortical granules via three separate avenues. First, individual oocytes were transferred to 12 μl of Barths solution 2 h after injection of csp RNA and after 2 days the fluid was collected and assayed for secreted cortical granule lectin. No lectin was detected (data not shown), indicating that csp overexpression does not, by itself, trigger secretion from cortical granules (in contrast, overexpression of protein kinase C η does trigger cortical granule exocytosis; see Ref. 31). Second, we exposed oocytes (control or csp overexpressing) to PMA (50 nm for 30 min) and verified that secretion was significantly diminished in oocytes overexpressing csp (Fig. 1C). Then, the same oocytes were incubated in a solution containing 10 μm ionomycin plus 20 mm added Ca2+ (this procedure promotes secretion via an apparently protein-independent effect on oocyte membranes; see “Materials and Methods”). This second incubation culminated in the discharge of very little cortical granule lectin from the control oocytes, because these cells had discharged most of the available lectin in response to PMA (Fig. 1C). However, the csp overexpressing oocytes released essentially as much cortical granule lectin during the high Ca-ionomycin incubation as control oocytes had released in response to PMA (Fig. 1C). These data indicate that csp overexpressing oocytes retain cortical granule lectin in a pool that is accessible to the nonspecific secretory effect of high Ca2+ plus ionomycin. For a final control, we evaluated oocyte extracts for cortical granule lectin immunoreactivity after 30 min in 50 nm PMA. Under these conditions, control oocytes typically secrete ~80% of their cortical granule lectin (data not shown; see Ref. 31). In contrast, csp overexpressing oocytes retained a level of cortical granule lectin immunoreactivity that was indistinguishable from cells that had not been treated with PMA (data not shown; however, images in Fig. 3B reveal that cortical granules are present in oocytes expressing high levels of GFP-csp). Taken together, these experiments indicate that cortical granules persist in oocytes that overexpress csp, but there is a block of the pathway by which PMA elicits cortical granule exocytosis.
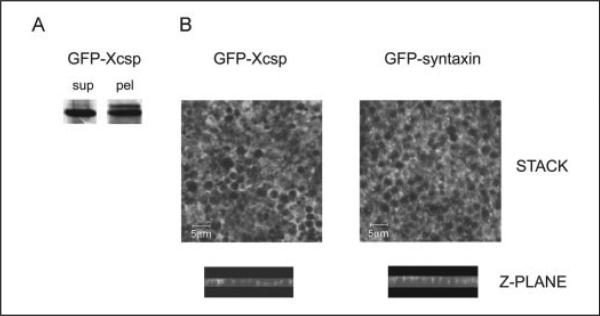
FIGURE 3. Distribution of GFP-csp and GFP-syntaxin 1a in oocytes.
A, an oocyte expressing GFP-csp was fractionated as in Fig. 2, and fractions were analyzed by immunoblot for GFP-csp, which migrates at ~50 kDa. B, image stacks of z-plane sections (obtained by confocal fluorescence microscopy) through cortical region fragments from GFP-csp or GFP-syntaxin 1a expressing oocytes reveal fluorescence surrounding the cortical granules. Representative z-plane sections (side views) show undulating fluorescence associated with the pole of cortical granules closest to the plasma membrane (but not encircling the granules). Scale bar is 5 μm. sup, supernatant; pel, pellet.
Distribution of Overexpressed csp in Oocytes
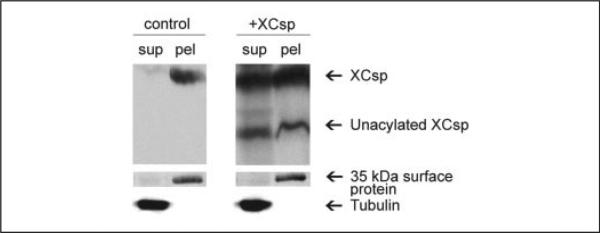
To assess further the basis of the secretory inhibition in oocytes overexpressing csp, we evaluated the subcellular distribution of the newly produced csp using biochemical and cell biological approaches. As reported previously (26), when control oocytes are gently disrupted and subjected to low speed centrifugation, 80–90% of the endogenous csp immunoreactivity (and >90% of the cortical granule lectin immunoreactivity; Ref. 26) sediments with the low speed pellet (Fig. 2). (The utility of this fractionation procedure for discriminating oocyte compartments is further evidenced by the fact that β-tubulin immunoreactivity is detected only in the low speed supernatant of control and csp overexpressing oocytes (Fig. 2). Conversely, when oocytes that had been incubated with a membrane-impermeant alkylating agent are fractionated via this protocol, the labeled proteins, exemplified by the 35-kDa species in Fig. 2, are detected only in the pellet). However, fractionation of oocytes that overexpress csp yielded a pattern that was qualitatively and quantitatively different from control oocytes. In addition to there being abundant csp in the low speed supernatant (overall, csp in the low speed supernatant of csp overexpressing cells was >20-fold higher than csp in the supernatant of control oocytes), the 27-kDa form of csp (that lacks lipid modification) was distributed approximately equally between the low speed supernatant and pellet (Fig. 2). Although these data indicate that csp is differentially distributed in oocytes that overexpress this protein, the results do not distinguish where in the cell csp is localized. Because csp is predominantly associated with cortical granules in control oocytes (26), a functionally important issue is whether any of the overexpressed csp is targeted to these granules.
FIGURE 2. csp distribution in control and csp overexpressing oocytes.
Single control or csp overexpressing oocytes were fractionated to yield a supernatant (sup) and low speed pellet (pel) and analyzed by immunoblot for csp and tubulin (tubulin signal in the pellet was undetectable). In addition, a 35-kDa protein (labeled using a membrane-impermeant alkylating agent) partitions almost exclusively in the pellet.
To evaluate whether overexpressed csp associates with cortical granules, we expressed GFP-tagged csp in oocytes and assessed its subcellular distribution biochemically (Fig. 3A). Of particular interest was the fact that nearly 50% of the GFP-csp partitioned in the low speed pellet (Fig. 3A). Because this pellet includes most of the cortical granules, as well as the plasma membrane, this result indicates that GFP-csp associates with one or both of these structures. To resolve this issue, we used confocal fluorescence microscopy to examine isolated cortical fragments of oocytes expressing GFP-csp (Fig. 3B). As the image stack of Fig. 3B indicates, GFP-csp yielded a “Swiss cheese” pattern of fluorescence, where the “holes” had the dimensions of cortical granules (the diameter of these holes was between 1.2 and 2.9 μm for n = 100; a range typical for cortical granules; Ref. 1). For comparison, we also examined oocytes expressing GFP-syntaxin 1a (syntaxin 1a is a t-SNARE that is prominently associated with the plasma membrane of a wide variety of regulated secretory cells; Ref 33). The pattern of GFP-syntaxin 1a fluorescence was essentially indistinguishable from the pattern of GFP-csp (Fig. 3B). Oblate structures (whose diameter ranged from 1.1 to 2.7 μm for n = 100) were devoid of GFP-syntaxin 1a fluorescence and surrounded by interstitial zones of strong fluorescence (Fig. 3B). Similar patterns of GFP-csp and GFP-syntaxin 1a fluorescence were also apparent in the z-plane (Fig. 3B). Because GFP-csp is distributed similarly to GFP-syntaxin 1a (which should traffic exclusively to the plasma membrane), these data indicate that the csp in the low speed pellet fraction of oocytes is predominantly associated with the plasma membrane and is not detectably present on the cortical granules.
Overexpression of the J-domain of csp Inhibits Cortical Granule Exocytosis
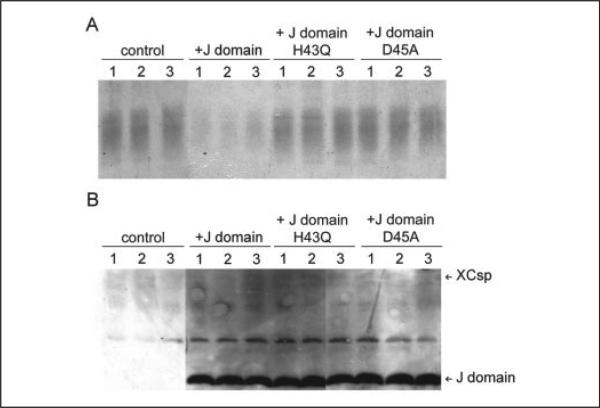
The preceding results raise the possibility that csp overexpression inhibits cortical granule exocytosis, because mislocalized csp interacts non-productively with one or more proteins that are necessary for the secretory cascade. Although csp has been reported to interact in vitro with a large number of other proteins (9, 10), its affiliation with members of the Hsp 70 and Hsc 70 family is indisputable (34–38,40). Thus, we tested whether expression of the J-domain of csp, the region that binds Hsp/Hsc 70 proteins (9, 10, 39), suffices to inhibit cortical granule exocytosis. The results (Fig. 4A) indicate that J-domain overexpression inhibits cortical granule exocytosis. Across a series of trials (18 control and 20 J-domain-expressing cells), secretion fell significantly (p < 0.01) to 30 ± 17% of control (Fig. 4A). Concomitantly, using antibodies that target only the NH2-terminal region of csp (N-end antibody), we observed a dramatic overexpression of the csp J-domain in the cells that exhibit the secretory inhibition (Fig. 4B). Densitometry reveals that the average signal for the overexpressed J-domain exceeds that of the endogenous csp by 36 ± 16-fold. Thus, by itself, the Hsc 70-interacting domain of csp is capable of blunting cortical granule exocytosis.
FIGURE 4. Cortical granule exocytosis is inhibited by overexpression of the J-domain of csp but not by mutated J-domains.
A, PMA-evoked cortical granule exocytosis was assayed (as in Fig. 1) using control oocytes or oocytes expressing the native J-domain or mutant J-domains (either H43Q or D45A). B, immunoblot analysis of extracts of the oocytes in A used antibody that selectively binds to the amino terminus of csp. It revealed strong signal for J-domain constructs (at 12–13 kDa) only in oocytes injected with J-domain RNAs. At this exposure, the endogenous csp (at ~33 kDa) signal is very faint. The band between csp and the J-domains is nonspecific immunoreactivity.
Based on the preceding results, we predicted that specific mutations of the J-domain (which have been shown in other J-domain proteins (39) and in csp (35, 40) to interfere with binding to Hsp/Hsc 70 isoforms) should largely eliminate the secretory inhibition associated with J-domain overexpression. As is evident in Fig. 4A, despite substantial overexpression (relative to csp), the H43Q- or D45A-mutated versions of the J-domain had little impact on cortical granule exocytosis. For cumulative evaluation of these data, we analyzed secretory results from oocytes that exhibited at least a 20-fold overexpression of the H43Q or D45A construct (this threshold was chosen, because >20-fold overexpression of the wild-type J-domain was necessary to inhibit cortical granule exocytosis by ~70%; Fig. 4B). Thus, for 8 oocytes (which overexpressed the D45A mutant construct by a mean of 34-fold) secretion was 81 ± 12% of control. Because of variability among control oocytes, this apparent reduction of secretion was not significant (p > 0.10) indicating that the construct with the D45A mutation negligibly affects cortical granule exocytosis. The more important comparison is between the mutant and wild-type J-domain, where the small reduction of secretion with the D45A mutant construct was significantly less (p < 0.001) than the 70% decline observed with native J-domain. Extending this analysis to the H43Q mutation of the J-domain (where 17 oocytes reached the criterion of >20-fold overexpression of this construct), we found that secretion was 99 ± 9% of control. This represents a significant (p < 0.001) loss of inhibitory effect relative to wild-type J-domain.
Csp Overexpression Alters the Subcellular Distribution of Hsc 70 in Oocytes
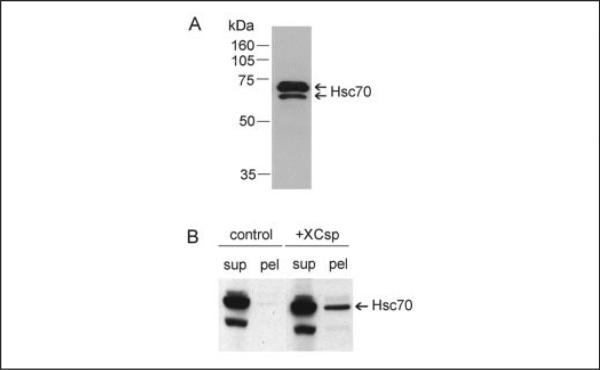
In Fig. 5A, the immunoblot shows the pattern of Hsp/Hsc 70 immunoreactivity obtained for an extract of a single oocyte using the broad spectrum (in terms of species recognition) Hsp 70 monoclonal antibody BRM-22. In addition to a pair of very closely overlapping bands at 70 kDa (which were also detected using Hsp/Hsc 70 monoclonal antibody N27F3-4 from Stressgen; results not shown), there was a lower mass species at ~67 kDa (the abundance of this 67-kDa species varied considerably among batches of oocytes; data not shown). Although prior work (40, 41) has documented the presence in Xenopus oocytes of two Hsc 70 isoforms, we have not resolved further the identity of the immunoreactive species in Fig. 5A. However, as expected for soluble proteins, when oocytes were fractionated (as in Fig. 2), most (>90%) of the immunoreactive Hsp/Hsc 70 remained in the low speed supernatant (Fig. 5B). Interestingly, in csp overexpressing oocytes, appreciably more Hsc 70 (but, not the 67-kDa species) was detected in the low speed pellet (Fig. 5B, csp). Quantifying this effect in three separate experiments, we observed that the level of Hsp/Hsc 70 immunoreactivity in the pellet of csp overexpressing cells was >5-fold the level in control cells (this difference was significant at p < 0.01), arguing that the abundant csp in the pellet of csp overexpressing cells (Fig. 2) contributes to a redistribution of Hsp/Hsc 70.
FIGURE 5. Overexpression of csp influences the subcellular distribution of Hsp/Hsc 70 in oocytes.
A, Hsp/Hsc 70 immunoreactivity in a single control oocyte. Detergent extract was prepared from a single oocyte and resolved electrophoretically for immunoblot analysis using Hsp/Hsc 70 antibody BRM-22. B, a single control oocyte and a csp overexpressing oocyte were fractionated (as in Fig. 2) to yield low speed supernatants (sup) and pellets (pel), which were subjected to immunoblot analysis for Hsp/Hsc 70.
Co-immunoprecipitation of csp and Hsp/Hsc 70
As independent evidence that csp and Hsp/Hsc 70 interact in uninjected control oocytes, we immunoprecipitated csp and tested for co-immunoprecipitation of Hsp/Hsc 70. Results in Fig. 6 indicate that Hsp/Hsc 70 coimmunoprecipitates with csp. (Controls for this experiment included samples of csp antibody alone or extract alone to which were added protein A/G-agarose; no csp or Hsp/Hsc 70 signal was detected in these samples; independently, we found that neither actin nor tubulin coimmunoprecipitated with csp; data not shown). However, perhaps because of limited access of the Hsp/Hsc 70 monoclonal antibody to the antigen, we observed a very low efficiency (<5%) of immunoprecipitation of Hsp/Hsc 70 from oocyte extracts and no detectable co-immunoprecipitation of csp (data not shown).
FIGURE 6. Hsp/Hsc 70 co-immunoprecipitates with csp.
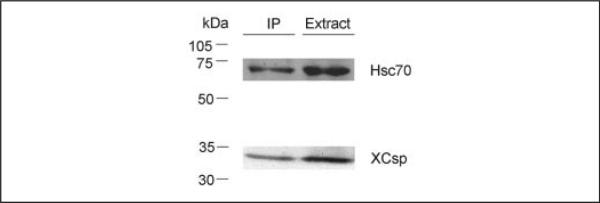
Oocyte extract was subjected to immunoprecipitation (IP) using csp antibody and protein A/G-agarose to capture immune complexes. Samples were resolved electrophoretically for sequential immunoblot analysis using Hsp/Hsc 70 antibody followed by csp antibody. Immunoblot signals in the lane labeled extract correspond to 25% of the oocyte extract used for immunoprecipitation.
Effect of csp Overexpression on Constitutive Exocytosis
Oocytes exhibit robust trafficking of plasma membrane components, and the entire plasma membrane apparently turns over within 24 h (30). To ascertain whether csp overexpression interferes with this process, we fractionated oocytes to monitor the subcellular distribution of co-expressed GFP-syntaxin 1a. In control oocytes, ~60% of the GFP-syntaxin 1a immunoreactivity was associated with the low speed pellet of oocytes, and this percentage was unchanged in oocytes overexpressing csp (Fig. 7, the GFP-syntaxin 1a that remained in the low speed supernatant was presumably associated with Golgi apparatus and endoplasmic reticulum, as it sedimented after 1 h at 100,000 × g; data not shown). Cumulatively, in two experiments involving 12 control and 12 csp overexpressing oocytes, the percentage of GFP-syntaxin 1a in the pellet was 57 ± 5% for control and 60 ± 3% for csp overexpressing cells (this difference was not significant; in addition, the J-domain of csp did not affect GFP-syntaxin 1a distribution; data not shown). However, if oocytes were maintained in brefeldin A (50 μm), a reagent that disrupts the Golgi apparatus (43), then no GFP-syntaxin 1a appeared in the low speed pellet (Fig. 7). Collectively, these results indicate that Golgi-dependent membrane trafficking (including constitutive exocytosis) is necessary for the normal distribution of GFP-syntaxin 1a in oocytes and that csp overexpression does not affect this process.
FIGURE 7. Brefeldin A affects the subcellular distribution of GFP-syntaxin 1a, whereas overexpression of csp does not.
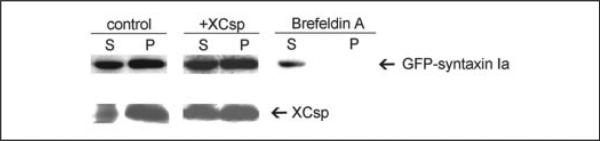
Control oocytes, oocytes overexpressing csp, or oocytes maintained in brefeldin A (50 μm) were injected with RNA encoding GFP-syntaxin 1a. After 2 days (to permit expression of GFP-syntaxin 1a), single oocytes were fractionated to yield low speed supernatants (S) and pellets (P), and the extracts were subjected to immunoblot analysis to detect GFP-syntaxin 1a (which migrates at 60 kDa). Blots were reprobed to verify csp overexpression (only acylated csp is shown).
DISCUSSION
This study was initiated with the goal of evaluating the role of csp in a regulated exocytotic pathway that is independent of Ca2+. This is an important issue, because it has been argued, based on studies of csp null mutant Drosophila, that csp plays a vital role in controlling the Ca2+ sensitivity of the regulated secretory machinery (25). Although the current data do not exclude the possibility that csp displays additional functions at nerve terminals (where Ca2+ sensing is important for the stimulus-evoked release of transmitter), our results indicate that csp participates in the regulated exocytotic cascade at a step that is independent of Ca2+. This conclusion is based on the fact that, as reviewed in the Introduction, PMA-triggered cortical granule exocytosis in Xenopus oocytes does not involve changes of cytosolic Ca2+ and proceeds normally even when cytosolic Ca2+ is buffered (5–7, 31). However, cortical granule exocytosis is significantly disrupted by the overexpression of full-length csp, and the remainder of this investigation was aimed at clarifying the basis of this secretory blockade.
It was our original hypothesis that csp overexpression in frog oocytes might augment cortical granule exocytosis. This expectation was based on results from several other investigations. For instance, absence of csp in csp null mutant Drosophila leads to a decline of stimulus-dependent transmitter release (44), and csp antibody causes a reduction of evoked transmitter release at embryonic frog motor nerve terminals (16). Together, these results imply that loss of csp function correlates with an impaired secretory response. Conversely, stable overexpression of csp in PC12 cells culminates in enhanced secretion of dopamine in response to Ca2+ or GTPγS (22), and Li+-induced up-regulation of csp is also associated with elevated dopamine secretion from PC12 cells (46). However, contrary to our expectation, csp overexpression in oocytes strongly inhibited cortical granule exocytosis (note that inhibitory effects of transient csp overexpression have independently been reported in several other systems, Refs. 20, 23, 24, and 47). The inhibition of secretion in oocytes also relied on the extent of csp overexpression. Thus, little or no impact on cortical granule exocytosis was observed when csp overexpression was <10 × the level of csp in control oocytes (data not shown), but profound inhibition was seen when csp exceeded the control level by >20-fold.
In seeking to explain the inhibition of cortical granule exocytosis in oocytes overexpressing csp, we initially evaluated the possibility that overexpressed csp failed to reach its normal destination, the cortical granules. This would presumably leave a large pool of mislocalized csp to interfere with exocytosis. Subcellular fractionation of csp overexpressing oocytes (Fig. 2) revealed a substantial increase of csp in the low speed supernatant, a fraction that typically contains very little csp and is essentially devoid of intact cortical granules (26, 31). Concomitantly, results obtained by confocal fluorescence microscopy showed that GFP-csp fluorescence was effectively excluded from cortical granules. Instead, GFP-csp was clearly associated with the plasma membrane of the oocyte, exhibiting fluorescence that was indistinguishable from the pattern of GFP-syntaxin 1a, a plasma membrane protein (33). Thus, in contrast to control oocytes, where most of the csp is associated with cortical granules (26), oocytes overexpressing csp appear to accumulate this protein at sites other than cortical granules. In retrospect, this result is consistent with what is known about cortical granule biogenesis in oocytes. Balinsky and Devis (48) reported that production of cortical granules by the Golgi apparatus ceases once oocytes reach 0.5–0.6 mm in diameter. Similarly, Dumont (49) observed that a few small cortical granules remained in the cytoplasm of stage IV (0.6–1-mm diameter) oocytes, whereas by stage V–VI, the granules uniformly assumed a position immediately beneath the plasma membrane. These results (48, 49) suggest that the turnover of cortical granules is negligible in stage V–VI oocytes (supporting this conclusion, we have detected no decline over 3 days in the amount of cortical granule lectin in oocytes incubated with cycloheximide at a level (100 μg/ml) that blocks ~98% of protein synthesis; data not shown), and would explain why very little of the overexpressed csp is found in association with these relatively quiescent structures. Nevertheless, because our prior work on oocytes documented that soluble csp becomes membrane-associated as a consequence of fatty acylation (18), the current data imply that cortical granule membranes lack the capability to bind and fatty acylate newly produced csp.
Because most of the overexpressed csp in oocytes appears to be mislocalized (being present in the cytosol or associated with membranous structures other than cortical granules), it was reasonable to postulate that the mislocalized csp was sequestering one or more proteins that are necessary for cortical granule exocytosis. Abundant work has shown that csp interacts via its J-domain with members of the Hsp 70 family of molecular chaperones (34–38, 40). In addition, oocytes constitutively express at least two Hsp/Hsc 70 isoforms (Refs. 41 and 42 and Fig. 5; independently, cDNAs encoding two closely related Hsc 70 isoforms have been reported for Xenopus; Refs. 45 and 50), and we found that oocyte Hsp/Hsc 70 co-immunoprecipitates with csp. Moreover, subcellular fractionation of oocytes reveals a significant redistribution of Hsp/Hsc 70 to the fraction that contains the newly synthesized csp (Fig. 5B). Thus, it was reasonable to consider the possibility that overexpressed csp interferes with an interaction between Hsp/Hsc 70 and granule-associated csp that is important for regulated exocytosis. Two results support this conclusion. First, by itself, overexpression of the J-domain of csp significantly blunts cortical granule exocytosis. Because the J-domain underlies the interaction of csp with members of the Hsp/Hsc 70 family of proteins (34–38), this result supports a role for csp-Hsp/Hsc 70 interaction in cortical granule exocytosis. Second, mutation of the J-domain of csp (to produce constructs that have been shown not to associate with Hsp/Hsc 70; see Refs. 35, 40, and 41) significantly reduces the inhibitory effect of these constructs on cortical granule exocytosis. Collectively, these results suggest that the normal pathway for cortical granule exocytosis involves an interaction between granule-associated csp and Hsp/Hsc 70. However, additional work will be necessary to obtain a more detailed understanding of the role of this interaction in the secretory cascade. As a final comment on this issue, recent work (47) concluded that the J-domain of csp was not intrinsically vital for insulin secretion from insulinoma cells. A possible explanation for the apparent discrepancy between this finding and our results is that overexpression of the J-domain in the insulin-secreting cells may have been insufficient to produce the inhibition seen in oocytes.
Although csp overexpression inhibits cortical granule exocytosis, the constitutive exocytotic pathway for the delivery of proteins to the plasma membrane remains intact. This conclusion is supported by the observation that syntaxin 1a trafficking to the plasma membrane is unaltered by csp overexpression. Interestingly, the finding that GFP-csp is also targeted to the plasma membrane of oocytes (Fig. 3) suggests that this pool of csp may also reach the plasma membrane via ongoing constitutive exocytosis. Several prior investigations have noted that perturbation of csp function in the regulated pathway does not affect spontaneous neurotransmitter release at nerve endings (16, 44) or the basal secretory process in adrenal or pancreatic cells (22–24, 47). These results support the hypothesis that csps evolved to perform a function important for the regulated secretory pathway. Concomitantly, the current data indicate that the role of csp in regulated exocytosis depends on its interaction with Hsc 70.
Acknowledgments
We thank Dr. C. Schietroma for helpful discussions, Drs. T. Coppola, W. M. Bement, and H. Y. Yu for GFP constructs and Dr. N. Brecha for access to the confocal microscope in the UCLA Department of Neurobiology. The tubulin antibody, E7 (developed by M. Klymkowsky) was obtained from the Developmental Studies Hybridoma Bank at the University of Iowa.
Footnotes
This work was supported by Grant NS31934 from the National Institutes of Health (to J. A. U.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The abbreviations used are: csp, cysteine string protein; PMA, phorbol-12-myristate-13-acetate; GFP, green fluorescent protein; GTPγS, guanosine 5′-3-O-(thio)triphosphate.
An investigation of the role of the J-domain of csp in Drosophila appeared recently (Bronk, P., Nie, Z., Klose, M. K., Dawson-Scully, K., Zhang, J., Robertson, R. M., Atwood, H. L., and Zinsmaier, K. E. (2005) J. Neurosci. 25, 2204–2214).
REFERENCES
- 1.Schuel H. Gamete Res. 1978;1:299–382. [Google Scholar]
- 2.Bement WM, Capco DG. J. Cell Biol. 1989;108:885–892. doi: 10.1083/jcb.108.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burgoyne RD, Morgan A. Biochem. J. 1993;293:305–316. doi: 10.1042/bj2930305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Augustine GJ, Burns ME, DeBello WM, Pettit DL, Schweizer FE. Annu. Rev. Pharmacol. Toxicol. 1996;36:659–701. doi: 10.1146/annurev.pa.36.040196.003303. [DOI] [PubMed] [Google Scholar]
- 5.Bement WM, Capco DG. Cell Regul. 1990;1:315–326. doi: 10.1091/mbc.1.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charbonneau M, Grey RD. Dev. Biol. 1984;102:90–97. doi: 10.1016/0012-1606(84)90177-5. [DOI] [PubMed] [Google Scholar]
- 7.Kohan SA, Gundersen CB. J. Exp. Zool. 2003;300:113–125. doi: 10.1002/jez.a.10317. [DOI] [PubMed] [Google Scholar]
- 8.Buchner E, Gundersen CB. Trends Neurosci. 1997;20:223–227. doi: 10.1016/s0166-2236(96)10082-5. [DOI] [PubMed] [Google Scholar]
- 9.Chamberlain LH, Burgoyne RD. J. Neurochem. 2001;74:1781–1789. doi: 10.1046/j.1471-4159.2000.0741781.x. [DOI] [PubMed] [Google Scholar]
- 10.Zinsmaier KL, Bronk P. Biochem. Pharmacol. 2001;62:1–11. doi: 10.1016/s0006-2952(01)00648-7. [DOI] [PubMed] [Google Scholar]
- 11.Zinsmaier KE, Hofbauer A, Heimbeck G, Pflugfelder GO, Buchner S, Buchner E. J. Neurogenet. 1990;7:15–29. doi: 10.3109/01677069009084150. [DOI] [PubMed] [Google Scholar]
- 12.Gundersen CB, Umbach JA. Neuron. 1992;9:527–537. doi: 10.1016/0896-6273(92)90190-o. [DOI] [PubMed] [Google Scholar]
- 13.Umbach JA, Gundersen CB. J. Neurosci. 1997;17:7203–7209. doi: 10.1523/JNEUROSCI.17-19-07203.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Umbach JA, Saitoe M, Kidokoro Y, Gundersen CB. J. Neurosci. 1998;18:3233–3240. doi: 10.1523/JNEUROSCI.18-09-03233.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leveque C, Pupier S, Marqueze B, Geslin L, Kataoka M, Takahashi M, DeW-aard M, Seagar M. J. Biol. Chem. 1998;273:13488–13492. doi: 10.1074/jbc.273.22.13488. [DOI] [PubMed] [Google Scholar]
- 16.Poage RL, Meriney SL, Gundersen CB, Umbach JA. J. Neurophysiol. 1999;82:50–59. doi: 10.1152/jn.1999.82.1.50. [DOI] [PubMed] [Google Scholar]
- 17.Chen S, Zheng X, Schulze KL, Morris T, Bellen H, Stanley E. J. Physiol. 2002;538:383–389. doi: 10.1113/jphysiol.2001.013397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gundersen CB, Mastrogiacomo A, Faull K, Umbach JA. J. Biol. Chem. 1994;269:19197–19199. [PubMed] [Google Scholar]
- 19.Gundersen CB, Mastrogiacomo A, Umbach JA. J. Theor. Biol. 1995;172:269–277. doi: 10.1006/jtbi.1995.0023. [DOI] [PubMed] [Google Scholar]
- 20.Graham ME, Burgoyne RD. J. Neurosci. 2000;20:1281–1289. doi: 10.1523/JNEUROSCI.20-04-01281.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnold C, Reisch N, Leibold C, Becker S, Prufert K, Sautter K, Palm D, Jatzke S, Buchner S, Buchner E. J. Exp. Zool. 2004;207:1323–1334. doi: 10.1242/jeb.00898. [DOI] [PubMed] [Google Scholar]
- 22.Chamberlain LH, Burgoyne RD. Mol. Biol. Cell. 1998;9:2259–2267. doi: 10.1091/mbc.9.8.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown H, Larsson O, Branstrom R, Yang S, Leibiger B, Leibiger I, Fried G, Moede T, Deeney JT, Brown GR, Jacobsson G, Rhodes CJ, Braun JEA, Scheller RH, Corkey BE, Berggren P, Meister B. EMBO J. 1998;17:5048–5058. doi: 10.1093/emboj/17.17.5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang H, Kelley WL, Chamberlain LH, Burgoyne RD, Wollheim C, Lang J. FEBS Lett. 1998;437:267–272. doi: 10.1016/s0014-5793(98)01233-2. [DOI] [PubMed] [Google Scholar]
- 25.Dawson-Scully K, Bronk P, Atwood HL, Zinsmaier KE. J. Neurosci. 2000;20:6039–6047. doi: 10.1523/JNEUROSCI.20-16-06039.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gundersen CB, Aguado F, Sou S, Mastrogiacomo A, Coppola T, Kornblum HI, Umbach JA. Cell Tissue Res. 2001;303:211–219. doi: 10.1007/s004410000314. [DOI] [PubMed] [Google Scholar]
- 27.Gurdon JB, Lane CD, Woodland HR, Marbaix G. Nature. 1971;233:177–182. doi: 10.1038/233177a0. [DOI] [PubMed] [Google Scholar]
- 28.Lane CD, Colman A, Mohun T, Morser J, Champion J, Kourides I, Craig R, Higgins S, James TC, Applebaum SW, Ohlsson RI, Paucha E, Houghton M, Matthews J, Miflin BJ. Eur. J. Biochem. 111:225–235. doi: 10.1111/j.1432-1033.1980.tb06097.x. !980. [DOI] [PubMed] [Google Scholar]
- 29.Gundersen CB, Miledi R, Parker I. Nature. 1984;308:421–424. doi: 10.1038/308421a0. [DOI] [PubMed] [Google Scholar]
- 30.Zampighi GA, Loo DDF, Kreman M, Eskandari S, Wright EM. J. Gen. Physiol. 1999;113:507–523. doi: 10.1085/jgp.113.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gundersen CB, Kohan SA, Chen Q, Iagnemma J, Umbach JA. J. Cell Sci. 2002;115:1313–1320. doi: 10.1242/jcs.115.6.1313. [DOI] [PubMed] [Google Scholar]
- 32.Mastrogiacomo A, Kornblum HI, Umbach JA, Gundersen CB. Biochim. Biophys. Acta. 1998;1401:239–241. doi: 10.1016/s0167-4889(97)00160-2. [DOI] [PubMed] [Google Scholar]
- 33.Bennett MK, Calakos N, Scheller RH. Science. 1992;257:255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- 34.Braun JEA, Wilbanks SM, Scheller RH. J. Biol. Chem. 1996;271:25989–25993. doi: 10.1074/jbc.271.42.25989. [DOI] [PubMed] [Google Scholar]
- 35.Chamberlain LH, Burgoyne RD. Biochem. J. 1997;322:853–858. doi: 10.1042/bj3220853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chamberlain LH, Burgoyne RD. J. Biol. Chem. 1997;272:31420–31426. doi: 10.1074/jbc.272.50.31420. [DOI] [PubMed] [Google Scholar]
- 37.Stahl B, Tobaben S, Sudhof TC. Eur. J. Cell Biol. 1999;78:375–381. doi: 10.1016/S0171-9335(99)80079-X. [DOI] [PubMed] [Google Scholar]
- 38.Tobaben S, Thakur P, Fernandez-Chacon R, Sudhof TC, Rettig J, Stahl B. Neuron. 2001;31:987–999. doi: 10.1016/s0896-6273(01)00427-5. [DOI] [PubMed] [Google Scholar]
- 39.Kelley WL. Trends Biochem. Sci. 1998;23:222–227. doi: 10.1016/s0968-0004(98)01215-8. [DOI] [PubMed] [Google Scholar]
- 40.Zhang H, Kelley WL, Chamberlain LH, Burgoyne RD, Lang J. J. Cell Sci. 1999;112:1345–1351. doi: 10.1242/jcs.112.9.1345. [DOI] [PubMed] [Google Scholar]
- 41.Mandell RB, Feldherr CM. J. Cell Biol. 1990;111:1775–1783. doi: 10.1083/jcb.111.5.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herberts C, Moreau N, Angelier N. Int. J. Dev. Biol. 1993;37:397–406. [PubMed] [Google Scholar]
- 43.Klausner RD, Donaldson JG, Lippincott-Schwartz J. J. Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Umbach JA, Zinsmaier KE, Eberle KK, Buchner E, Benzer S, Gundersen CB. Neuron. 1994;13:899–907. doi: 10.1016/0896-6273(94)90255-0. [DOI] [PubMed] [Google Scholar]
- 45.Ali A, Salter-Cid L, Flajnik M, Heikkila JJ. Biochim. Biophys. Acta. 1996;1309:174–178. doi: 10.1016/s0167-4781(96)00156-x. [DOI] [PubMed] [Google Scholar]
- 46.Cordeiro ML, Gundersen CB, Umbach JA. Neuropsychopharmacology. 2004;29:39–44. doi: 10.1038/sj.npp.1300288. [DOI] [PubMed] [Google Scholar]
- 47.Boal F, Zhang H, Tessier C, Scotti P, Lang J. Biochemistry. 2004;43:16212–16223. doi: 10.1021/bi048612+. [DOI] [PubMed] [Google Scholar]
- 48.Balinsky BI, Devis R. Acta Embryol. Morphol. Exper. 1963;6:55–108. [Google Scholar]
- 49.Dumont JN. J. Morphol. 1972;136:153–180. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- 50.Ali A, Salter-Cid L, Flajnik MJ, Heikkila JJ. Comp. Biochem. Physiol. 1996;113:681–687. doi: 10.1016/0305-0491(95)02081-0. [DOI] [PubMed] [Google Scholar]