Abstract
Objective
To determine the effect of reducing insulin secretion on hyperandrogenemia in lean normoinsulinemic women with polycystic ovary syndrome (PCOS) and normal metabolic insulin sensitivity.
Design
Transversal assessment at baseline and prospective follow-up of lean PCOS group after 8 days of diazoxide, which reduces insulin secretion, and 1 month of leuprolide, which suppresses LH.
Setting
Clinical research center of an academic hospital.
Patient(s)
Nine lean women (body mass index ≤ 25 kg/m2) with PCOS and normal insulin levels, as well as 17 lean healthy women.
Intervention(s)
Lean PCOS women were reassessed after 8 days of diazoxide and after 1 month of leuprolide, which suppresses LH.
Main Outcome Measure(s)
Androgen levels and insulin-stimulated glucose disposal (metabolic insulin sensitivity), determined by euglycemic-hyperinsulinemic clamp (M-value).
Result(s)
Mean M-value of lean PCOS women (48.5 μmol/kg·min) was similar to lean control subjects (52.9 μmol/kg·min). They also had comparable anthropometric measures, lipids, fibrinogen, and plasminogen activator inhibitor 1. The LH did not change significantly after diazoxide, but was almost suppressed after leuprolide in the PCOS group. Androstenedione decreased significantly after diazoxide and even more after leuprolide. However, free T significantly decreased only after diazoxide in lean PCOS women. Diazoxide also increased SHBG significantly in this group.
Conclusion(s)
In women with typical PCOS and normal insulin levels and metabolic insulin sensitivity, reducing insulin secretion significantly decreased androgen and increased SHBG levels. These results suggest that insulin contributes to hyperandrogenemia even in PCOS women with normal metabolic insulin sensitivity, which might be due to increased sensitivity of their androgenic insulin pathway.
Keywords: Polycystic ovary syndrome, insulin action, hyperandrogenemia, insulin sensitivity
Polycystic ovary syndrome (PCOS) affects 6%–10% of women of childbearing age (1) and is defined by hyperandrogenism, chronic anovulation, and/or polycystic ovaries (2 criteria out of 3) (2). However, it has become apparent that insulin resistance and hyperinsulinemia play a critical role in the syndrome’s pathogenesis (1). Despite advances over the past decade, many questions remain regarding the mechanism by which insulin resistance or insulin produces hyperandrogenemia.
Several studies have demonstrated that insulin stimulates ovarian steroidogenesis in vitro. At physiologic concentrations, insulin stimulates androgen production by cultured ovarian cells to a greater extent in women with PCOS compared with control subjects (3). Moreover, combined stimulation with LH and insulin at physiologic concentrations have been shown to synergistically increase androgen biosynthesis by ovarian tissues from normal and PCOS women (4).
Increased androgen response to LH stimulation in women with PCOS has also been demonstrated in vivo (5). Chronic stimulation by both LH and insulin have been implicated in this ovarian androgen hyper-responsiveness, because most women with PCOS have increased LH and/or insulin levels. To control for chronic stimulation by LH, normal and PCOS women were challenged with hCG before and 4 weeks after LH suppression with a long-acting analog of GnRH (5). Suppression of LH did not alter the typical exaggerated plasma 17α-hydroxyprogesterone response to hCG. Conversely, serum total and nonSHBG-bound testosterone (T) levels decreased significantly after direct suppression of pancreatic insulin release for 10 days with diazoxide in obese PCOS women (6). Moreover, reduction of insulinemia with acarbose, which slows down intestinal absorption of carbohydrates, also reduced serum T levels in PCOS (7). These studies all highlight the importance of insulin in the pathogenesis of PCOS.
Numerous studies have demonstrated that any treatment aimed at improving metabolic insulin resistance in women with PCOS lowers androgen levels and improves ovulatory function (1). The exaggerated steroidogenic response to LH stimulation tests also improves with these treatments (8, 9), suggesting normalization of ovarian androgen hyper-responsiveness. We previously conducted a study using insulin-sensitizing drugs (namely metformin, a biguanine, and rosiglitazone, a peroxisome proliferator-activated receptor γ (PPARγ) agonist) in nonobese women with PCOS and normal insulin levels (10). The results demonstrated a normalization of serum T levels and ovulation in actively treated groups compared with the placebo-treated group. These findings suggest that insulin-sensitizing drugs improve hyperandrogenemia even in nonobese women with PCOS who appear to have normal metabolic insulin sensitivity. Whether it is the correction of abnormal insulin action per se or the reduction of plasma insulin levels that was responsible for these beneficial effects of insulin sensitizers is currently unclear.
Therefore, we hypothesized that insulin contributes to androgen levels even in lean women with PCOS and normal insulin levels owing to hypersensitivity to the androgenic actions of insulin. We sought to determine if a reduction in insulin levels with diazoxide, an agent devoid of any influence on insulin-signaling pathways (11), can reduce androgen production in lean normoinsulinemic women with PCOS and normal metabolic sensitivity to insulin, as demonstrated by the insulin-glucose clamp. Indeed, studied women exhibited normalization of their serum free T levels after treatment with diazoxide, which was more pronounced than after LH suppression with a long-acting agonist of GnRH. These results further support the notion that insulin contributes to hyperandrogenemia even in women with PCOS and normal metabolic sensitivity to insulin.
MATERIALS AND METHODS
Subjects
We recruited women with PCOS and normal control women who were both lean (body mass index [BMI] ≤ 25 kg/m2) and normally insulin sensitive. For practical reasons, a direct measure of metabolic insulin sensitivity could not be performed as a screening procedure before enrollment into the study. Therefore, to increase the probability of enrolling PCOS women without insulin resistance, the inclusion criteria for this group were: PCOS; absence of hypertension or acanthosis nigricans; and normal insulin levels, i.e., fasting serum insulin <15 μIU/mL (104 pmol/L), peak serum insulin during oral glucose tolerance test (OGTT) <100 μIU/mL (695 pmol/L) (12), and fasting serum glucose-to-insulin ratio >4.5 mg·mL/μIU·dL (36 mmol/mmol) (13). The lean control group consisted of normally cycling women with normal weight and normal T levels (as described in the following).
Polycystic ovary syndrome was defined by oligomenorrhea (≤ 8 menstrual periods in the preceding year) or confirmed anovulation, and hyperandrogenemia (serum total T >75 ng/dL [2.6 nmol/L] and calculated free T >1.4 ng/dL [50 pmol/L]). These T normal values were determined by the clinical laboratory of the Centre hospitalier universitaire de Sherbrooke (CHUS). All participants were between 18 and 40 years of age and had normal serum PRL, thyroid-function tests, and glucose tolerance after a 75-g OGTT. Late-onset adrenal hyperplasia was excluded by a serum 17α-OHP of <3.0 μg/L (10 nmol/L). None of the participants ever used insulin-sensitizing drugs, and none were using oral contraceptives or medications that may affect insulin sensitivity. The study protocol was approved by the institutional review board of CHUS, and each woman gave written informed consent.
Experimental Protocol
The PCOS women were studied at least 35 days after their last menstrual period to reduce the possibility that a spontaneous ovulation occurred during the last month, as this may transiently normalize many features of the syndrome. Healthy control women were studied during the midfollicular phase of the menstrual cycle (days 5–10), which most closely mimics the hormonal milieu of anovulatory women with PCOS. Subjects were instructed to use nonhormonal contraceptive methods throughout the period of the study and to remain fasting after 8:00 p.m. the night before each visit.
For the first study visit, subjects from the two groups came to the Metabolic Unit of our Clinical Research Center (CRC) in the morning and weight, height, waist-to-hip ratio (WHR), and supine blood pressure were measured, as well as lean body mass by standing electrical bioimpedance (Tanita weight scale model TBF-300A). Then, fasting blood and urine samples were drawn. At 9:00 a.m. an OGTT was performed by administrating 75 g dextrose orally and collecting blood samples for determination of serum glucose and insulin at 0, 15, 30, 45, 60, 90, and 120 min. Subjects were instructed on following a 300 g/day carbohydrate diet in preparation for the insulin-glucose clamp. At least 2 days later, after a 12-hour overnight fast, an euglycemic-hyperinsulinemic clamp was performed as described by DeFronzo et al. (14) (insulin dose of 40 mU/m2·min). From this clamp, insulin-stimulated glucose disposal (M-value) was calculated as follows: glucose infusion rate during the last 30 minutes of the clamp (μmol/min) divided by the weight of the subject (kg). Because sensitivity to insulin may differ for each of its actions, it is important to note that insulin-stimulated glucose disposal is a measure of metabolic insulin sensitivity.
The following procedures were performed only in the PCOS group. Twenty days after the euglycemic-hyperinsulinemic clamp, the PCOS women began the diazoxide treatment at a dose of 100 mg every 8 hours for 8 days. This duration of therapy was chosen because it was found sufficient to decrease T levels in obese PCOS women by Nestler JE et al. (personal communication) and because the incidence of side effects with diazoxide, essentially swelling, was found to be higher in our lean population. At the end of this treatment period, the PCOS women were admitted to the Metabolic Unit of the CRC to collect fasting blood samples for the determination of plasma insulin, glucose, C-peptide, FSH, LH, sex steroids, and SHBG. The participants then received an IM injection of the long-acting GnRH agonist leuprolide acetate (3.75 mg) and were instructed to return to the CRC 28 days later. This dose and such delay were found to appropriately suppress LH levels and decrease basal T levels in obese PCOS women (5). During this last study visit, the same fasting blood tests were repeated and the weight and waist-to-hip ratio were measured again. C-Peptide was assayed a posteriori on all frozen samples that were still available, i.e., in 14 control and 7 lean PCOS women at baseline and 6 lean PCOS women during interventions.
Assays
Blood samples were assayed at the biochemistry core laboratory of CHUS, except for fasting C-peptide which was assayed in the laboratory of Dr. Baillargeon. Total T, androstenedione, and 17α-OHP levels were assayed by radioimmunoassay (RIA; Diagnostic Systems Laboratories, Webster, TX). The SHBG was assayed by immunoradiometric assay, and DHEAS and insulin by RIA (Diagnostic Products Corp., Los Angeles, CA). Serum free T was calculated by the method of Sodergard et al. (15) using a serum albumin concentration of 4.0 g/dL (40 g/L). Estradiol, P, FSH, LH, TSH, and PRL were measured by chemiluminescent immunoassay on an automated ADVIA Centaur analyzer (Bayer HealthCare, Toronto, Canada). Glucose, total cholesterol, triglycerides, and high-density lipoprotein–associated cholesterol (HDL-C) were measured by chemiluminescent immunoassay on an automated Vitros analyzer (Ortho-Clinical Diagnostics, Missisauga, Canada). Low-density lipoprotein–associated cholesterol (LDL-C) was calculated using the Friedewald equation (16). Fibrinogen was assayed by the modified Clauss technique using BCS kits (Behring Coagulation System, Newark, DE). Plasminogen activator inhibitor 1 (PAI-1) was determined by ELISA (Diagnostica Stago, Asnières-sur-Seine, France). C-Peptide was measured by RIA (Linco Research, St. Charles, MO). Inter- and intra-assay coefficients of variation were less than 7.5% for insulin, less than 10% for C-peptide and total T, and less than 8.5% for other steroid hormones. The lower limit of detection was 4 μIU/mL (30 pmol/L) for insulin.
Statistical Analysis
Results not normally distributed were log transformed to normalize their distribution for statistical analysis and are reported back-transformed in their original units (geometric mean with 95% confidence intervals). Other results are reported as mean ± SEM. Unpaired t tests were used at baseline to compare variables between the two groups. Diazoxide and leuprolide acetate treatment effects were assessed using paired t tests to compare variables after intervention to baseline within the two PCOS groups.
Two-tailed P values of ≤ .05 were considered to be significant for all analyses, which were performed using JMP 4.0 software (SAS Institute, Cary, NC). Even if the number of subjects was relatively small in each group, paired analyses increased significantly the power of the study. This method also decreases the variability of differences, because each subject is compared to herself, which factors out interindividual variability.
RESULTS
Clinical Characteristics of the Subjects (Table 1)
TABLE 1.
Baseline clinical characteristics and metabolic measurements.
| Characteristic | Control, lean (n = 17) | PCOS, lean (n = 9) | P value |
|---|---|---|---|
| Age (yr) | 31.3 ± 1.6 | 24.3 ± 2.2 | .02 |
| BMI (kg/m2) | |||
| Baseline | 22.0 ± 0.5 | 22.6 ± 0.6 | .45 |
| End of study | 22.2 ± 0.5 | ||
| Waist-to-hip ratio | |||
| Baseline | 0.77 ± 0.01 | 0.79 ± 0.01 | .38 |
| End of study | 0.78 ± 0.02 | ||
| Fat mass (%) | 25.9 ± 1.0 | 26.4 ± 1.4 | .81 |
| Systolic BP (mmHg) | 108 ± 2 | 109 ± 3 | .92 |
| Diastolic BP (mmHg) | 65 ± 2 | 65 ± 2 | .98 |
| Triglycerides (mg/dL)a | 34 (29–41) | 31 (24–39) | .47 |
| HDL-C (mg/dL) | 138 ± 9 | 144 ± 12 | .68 |
| LDL-C (mg/dL) | 200 ± 17 | 213 ± 24 | .66 |
| Chol./HDL-C ratioa | 2.74 (2.36–3.18) | 2.79 (2.27–3.42) | .89 |
| Fibrinogen (g/L) | 2.86 ± 0.13 | 2.87 ± 0.18 | .96 |
| PAI-1a | 17 (12–23) | 19 (12–29) | .61 |
| M-value (μmol/kg·min) | 52.9 ± 4.6 | 48.5 ± 6.3 | .57 |
Note: Plus-minus values are mean ± SEM. BMI = body mass index; BP = blood pressure; HDL-C = high-density lipoprotein–associated cholesterol; LDL-C = low-density lipoprotein–associated cholesterol; Chol./HDL-C ratio = total cholesterol–to–HDL-C ratio; PAI-1 = plasminogen activator inhibitor 1 (by immunoenzymatic assay). To convert values for triglycerides to mmol/L, multiply by 0.0259; and for cholesterol to mmol/L, multiply by 0.0113.
Geometric means with 95% confidence interval.
We studied 17 lean normal control subjects and 9 lean PCOS women with normal insulin levels. Our selected group of lean women with PCOS were younger than lean control subjects (P=.02) but had comparable BMI, WHR, and percentage of fat mass. The BMI and WHR did not change significantly in the lean PCOS group throughout the study. Finally, systolic and diastolic blood pressures also were similar between groups.
Metabolic Measures and Insulin Sensitivity
At baseline, fasting insulin (Table 2) and glucose (Fig. 1) levels were comparable between lean PCOS women and control subjects (P=.78 and P=.45, respectively). Fasting levels of triglycerides, HDL-C, LDL-C, fibrinogen, and PAI-1, as well as the total cholesterol–to–HDL-C ratio, also were all comparable between groups.
TABLE 2.
Laboratory results at baseline, after diazoxide, and after leuprolide acetate in lean control (n = 17) and lean PCOS women with normal insulin levels (n = 9).
| Characteristic | Group | Baseline | After diazoxide | Pa | After leuprolide | Pa |
|---|---|---|---|---|---|---|
| Fasting insulin (μU/mL)b | Control, lean | 5.8 (4.3–7.5) | ||||
| PCOS, lean | 6.1 (4.2–9.0) | 5.8 (4.6–7.1) | .60 | 5.8 (4.3–7.8) | .68 | |
| Fasting C-peptide (ng/mL)b | Control, lean | 3.4 (2.6–4.4) (n = 14) | ||||
| PCOS, lean | 3.0 (2.1–4.3) (n = 7) | 2.0 (1.5–2.8) (n = 6) | .02 | 2.5 (2.1–3.0) (n = 6) | .36 | |
| FSH (IU/L) | Control, lean | 4.2 ± 0.3 | ||||
| PCOS, lean | 4.7 ± 0.5 | 4.1 ± 0.5 | .24 | 2.8 ± 0.4d | .003 | |
| 17β-E2 (pg/mL) | Control, lean | 115 ± 22 | ||||
| PCOS, lean | 65 ± 30 | 91 ± 27 | .33 | 18 ± 2d | .001 | |
| Progesterone (ng/mL) | Control, lean | 0.6 ± 0.8 | ||||
| PCOS, lean | 3.7 ± 1.2 | 4.5 ± 1.3 | .71 | 1.2 ± 0.1d | .26 | |
| 17-OHP (μg/L) | Control, lean | 1.7 ± 0.3 (n = 13) | ||||
| PCOS, lean | 2.9 ± 0.4c | 2.5 ± 0.3 | .48 | 1.9 ± 0.2 | .10 | |
| DHEAS (μg/dL) | Control, lean | 130 ± 15 | ||||
| PCOS, lean | 315 ± 19c | 326 ± 22 | .53 | 367 ± 44 | .29 |
Note: Plus-minus values are mean ± SEM. To convert values for insulin to pmol/L, multiply by 6.9; for C-peptide to nmol/L, multiply by 0.333; for 17β-E2 to pmol/L, multiply by 3.671; for progesterone to pmol/L, multiply by 3.18; for 17-OHP to nmol/L, multiply by 3.026; and for DHEAS to μmol/L, multiply by 0.027. The normal ranges for ovulatory women are as follows: insulin 5–20 μIU/mL; FSH 1.5 to 7.6 mIU/mL; 17β-E2 19 to 247 pg/mL; progesterone 0.2 to 1.4 ng/mL; 17-OHP 0.3 to 1.0 μg/L; and DHEAS 40 to 340 μg/dL.
P value vs. baseline (by two-tailed paired t test).
Geometric mean with 95% confidence interval.
P≤ .05 vs. control at baseline (by two-tailed unpaired t test).
P≤ .05 vs. diazoxide (by two-tailed paired t test).
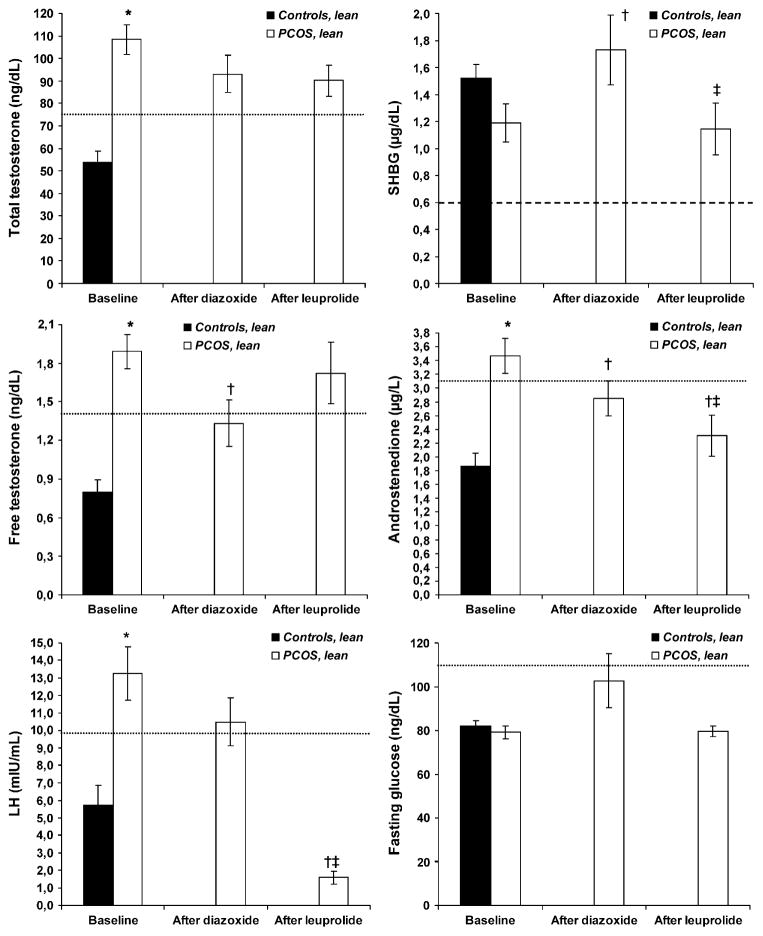
FIGURE 1.
Selected laboratory results at baseline and after treatment with diazoxide and leuprolide acetate in lean control (solid bars; n = 17) and lean PCOS (clear bars; n = 9) women. Results are presented as mean with SEM. To convert values for total T to nmol/L, multiply by 0.0347; for SHBG to nmol/L, multiply by 34.7; for free T to pmol/L, multiply by 34.7; and for androstenedione to nmol/L, multiply by 3.492; and for glucose to mmol/L, multiply by 0.0556. Free T was calculated by the method of Sodergard et al. (15). The normal ranges for ovulatory women are: total T, 20–80 ng/dL; SHBG, 0.6–3.0 μg/dL; free T, <1.4 ng/dL; androstenedione, 0.7–3.1 μg/L; LH, 0.5–9.8; and fasting glucose, 60–110 mg/dL. *P≤ .05 vs. control at baseline (by two-tailed paired t test); †P≤ .05 vs. baseline (by two-tailed paired t-test); ‡P≤ .05 vs. diazoxide (by two-tailed paired t test).
Metabolic insulin sensitivity (M-value), directly measured by the euglycemic-hyperinsulinemic clamp, was 52.9 μmol/kg·min in lean normal women, with 95% of the results ranging between 24.9 μmol/kg·min and 105.9 μmol/kg·min. The mean M-value of lean women with PCOS and normal insulin levels (mean 48.5 μmol/kg·min) was very close to that of control women, and all M-values (range 29.5–73.3 μmol/kg·min) in lean PCOS women were within the 95% interval range of controls.
Effects of Reduction of Insulin or LH Secretion (Table 2 and Fig. 1)
Fasting insulin levels did not change significantly with interventions in lean PCOS women (Table 2). However, fasting C-peptide levels reduced significantly after diazoxide in lean PCOS women (P=.02) but not after leuprolide (P=.36). The FSH and 17β-E2 levels were comparable between the two groups at baseline and were not affected by diazoxide, but they were significantly reduced by leuprolide acetate in the lean PCOS group (P=.003 and P=.001, respectively). However, progesterone levels, which were comparable between groups at baseline, did not change with diazoxide or leuprolide. The 17α-OHP and DHEAS levels were significantly higher in lean PCOS women than in control subjects at baseline (P=.02 and P<.001, respectively) and were not significantly altered by treatment with diazoxide or leuprolide acetate.
At baseline, total T levels were significantly higher in lean PCOS women than in control women (P<.001), as expected (Fig. 1). Total T levels decreased after both diazoxide and leuprolide acetate in PCOS women, but these effects were not significant (P=.18 and P=.14, respectively). The SHBG concentrations were slightly lower in lean PCOS women at baseline, although this was not significant (P=.06). Treatment with diazoxide increased significantly the levels of SHBG in lean PCOS women (P=.04), but they were reduced back to baseline levels after leuprolide acetate (P=.78 vs. baseline; P=.02 vs. diazoxide). At baseline, free T was significantly higher in lean PCOS women than in control women (P<.001), and treatment with diazoxide significantly normalized free T levels in lean PCOS women (P=.03). However, treatment with leuprolide acetate led to elevation of free T back to baseline levels (P=.57 vs. baseline).
At baseline, androstenedione levels were significantly higher in lean PCOS women than in control women (P<.001). In this group, androstenedione levels significantly decreased after diazoxide (P=.05) and decreased further after leuprolide acetate (P=.002 vs. baseline; P=.002 vs. diazoxide treatment), to levels within the normal range. At baseline, LH levels were significantly higher in lean PCOS women than in control women (P<.001). They tended to decrease after diazoxide (P=.17) and were almost completely suppressed after leuprolide acetate (P<.001 vs. baseline; P<.001 vs. diazoxide). Finally, treatment with diazoxide tended to increase fasting glucose levels in lean PCOS women compared with baseline, though not significantly (P=.06). Fasting glucose did not change after treatment with leuprolide acetate (P=.84).
DISCUSSION
This study assessed for the first time the in vivo effect of insulin in lean PCOS women with normal metabolic insulin sensitivity and insulin levels. The results demonstrated that reduction of insulin secretion with diazoxide in these women significantly decreased levels of free T and androstenedione and significantly increased SHBG (Fig. 1). Importantly, it has been earlier determined that suppression of serum insulin secretion by diazoxide does not alter serum T or SHBG levels in healthy nonobese women (11). The significant improvement of free T and SHBG levels observed after treatment with diazoxide were in contrast to the absence of change observed after treatment with the long-acting GnRH agonist leuprolide acetate, despite near total suppression of LH levels.
The present results underscore the preponderant role of insulin action over LH on the hyperandrogenemia of normoinsulinemic lean PCOS women. Furthermore, these results are not solely explained by increased SHBG levels in the PCOS women, because insulin reduction with diazoxide also significantly decreased androstenedione levels, which is not bound to SHBG. Based on our results, it appears that LH suppression is more effective than insulin reduction to decrease androgen biosynthesis, as assessed by androstenedione levels; but insulin lowering is more effective to reduce hyperandrogenemia, as assessed by free T levels, because it improves both androgenesis and SHBG levels.
We succeeded in recruiting nine women with typical PCOS, normal insulin levels during both fasting and after an OGTT, and normal sensitivity to insulin-stimulated glucose metabolism, as assessed by the euglycemic-hyperinsulinemic clamp technique. Indeed, the M-values of these lean PCOS women were all within the 95% interval range of the 17 lean healthy controls (Table 1) and even within or above the normal range for lean normal women published by Dunaif at al. (whole range 29.1–46.8 μmol/kg·min; n = 8) (17). Adjusting M-value for fat-free mass instead of weight in the present population resulted in the same conclusion (PCOS: 66.2 μmol/kg·min [range 36.2–98.0]; control: 71.6 μmol/kg·min [range 37.4–147.1]). Furthermore, the present lean PCOS group was characterized by normal anthropometric and metabolic parameters which were comparable to those in the lean healthy control group (Table 1).
Fasting insulin levels were only slightly reduced by diazoxide in the present study. This finding is consistent with results from Nestler et al. (18), who did not find significant change of fasting insulin levels in obese PCOS women after 10 days of diazoxide but demonstrated significantly blunted insulin response to glucose during OGTT. However, diazoxide significantly reduced fasting C-peptide levels in the present group of PCOS women, demonstrating that insulin secretion was indeed reduced by this intervention. C-Peptide is cosecreted with insulin, minimally extracted by the liver, and excreted mainly unchanged in urine. Therefore, multiple studies have determined that it is a better index of insulin secretion than fasting insulin (19, 20). Furthermore, fasting glucose levels were increased with diazoxide, and maintained in the normal range, which is consistent with clinically significant insulin reduction and with earlier findings (18, 21).
The lack of suppression of total and free T levels after treatment with a GnRH agonist in the present study, despite near suppression of LH, was in contrast to previous studies (5, 22, 23). This discrepancy might be explained by shorter treatment period (4 weeks) as compared with Dunaif et al. (22) and Lasco et al. (23) (12 weeks). However, although Gilling-Smith et al. (5) found some reduction of T levels after 4 weeks, androgen responses to hCG stimulation were not decreased. Another important difference is the selection of predominantly obese women in those earlier studies (5, 22, 23). It is therefore possible that GnRH contribution to hyperandrogenemia differs in lean insulin-sensitive PCOS women. Even if such a conclusion cannot be ascertained in the present study, the post-GnRH results are important to conclude with certitude that the improvement of androgen levels after diazoxide is not explained by the small decrease in LH levels (Fig. 1). Finally, because the duration of action of diazoxide is short (8 hours) and was used for only 8 days, we do not believe that it interfered with leuprolide action.
A possible weakness of the present study is the small number of lean insulin-sensitive PCOS women. This drawback reduces the power of the study to determine the absence of difference between groups or after versus before treatments. However, we have used robust nonparametric statistical tests that are independent from the number of subjects to show statistically significant differences. Thus, significant results are internally valid even if the number of subjects is relatively small.
In summary, the present results suggest that some women with typical PCOS are insulin sensitive to glucose metabolism and normoinsulinemic and that insulin contributes significantly to the hyperandrogenemia of these women. Therefore, women with PCOS might develop hyperandrogenemia because of increased sensitivity of their androgenic pathway to insulin. Because SHBG was also significantly affected by diazoxide-induced insulin reduction in these women, it is probable that insulin actions on liver production of SHBG are also increased. A pituitary effect is not excluded either, because LH tended to decrease after treatment with diazoxide.
Therefore, we hypothesize that women develop PCOS in part because of a selective and tissue-specific hypersensitivity to insulin (24). In a minority of women, this defect is sufficiently severe to cause typical PCOS without insulin resistance. However, it is important to recognize that in most women with PCOS the severity of this androgenic hypersensitivity to insulin is such that concomitant development of insulin resistance and hyperinsulinemia is necessary for phenotypic expression of the syndrome.
Our hypothesis probably explains the results of our previous clinical trial (10). Subjects in that trial were recruited using the same inclusion criteria as in the present study, which highly suggests that they were normally insulin sensitive as well (for glucose metabolism). Even if insulin sensitive, those PCOS women significantly improved T levels and ovulation after treatment with insulin-sensitizing drugs metformin or rosiglitazone. Metformin, but not rosiglitazone, significantly reduced insulin levels during that trial. We therefore propose that metformin decreased hyperandrogenemia mainly by reducing insulin levels, as did diazoxide in the present study, and that rosiglitazone may have been beneficial by directly improving the androgenic hypersensitivity to insulin.
A recent study (25) highlighted a selective defect in insulin activity in granulosa cells from women with PCOS, i.e., resistance in the metabolic pathway associated with an increase in mitogenic activity. Moreover, this study demonstrated that troglitazone, a PPARγ agonist, can correct this insulin hypersensitivity of the mitogenic pathway along with a significant improvement of the insulin-resistant metabolic pathway. Therefore, these results support the hypothesis that PPARγ may directly improve the androgenic hypersensitivity to insulin in PCOS. The observation that insulin-signaling pathways may express differential, and even divergent, activity levels under various circumstances also supports the possibility of a selective defect of the insulin androgenic pathway in women with PCOS.
In conclusion, reducing insulin secretion in women with typical PCOS, normal insulin levels and normal metabolic insulin sensitivity significantly decreased androgen and significantly increased SHBG levels. These results suggest that insulin contributes to hyperandrogenemia even in PCOS women with normal metabolic insulin sensitivity, which may be due to increased sensitivity of their androgenic insulin pathway. Liver insulin pathways may also be implicated through reduced secretion of SHBG. The characterization of this potential defect could have important implications for the development of specific and more effective treatments for PCOS.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (Operating Grant Program MOP-62946) and the Fonds de la Recherche en Santé du Québec (Young Clinical Investigator Establishment Grant #2834). Dr. Baillargeon is a Junior 1 Clinical Investigator of the Fonds de la Recherche en Santé du Québec (#3158) and Dr. Carpentier is a New Investigator of the Canadian Institutes of Health Research.
The authors thank John E. Nestler, M.D., Professor and Chair, Division of Endocrinology and Metabolism, Medical College of Virginia, Virginia Commonwealth University, for his critical review of the manuscript and helpful comments.
References
- 1.Baillargeon JP, Iuorno MJ, Nestler JE. Insulin sensitizers for polycystic ovary syndrome. Clin Obstet Gynecol. 2003;46:325–40. doi: 10.1097/00003081-200306000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–7. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 3.Nestler JE, Jakubowicz DJ, de Vargas AF, Brik C, Quintero N, Medina F. Insulin stimulates testosterone biosynthesis by human thecal cells from women with polycystic ovary syndrome by activating its own receptor and using inositolglycan mediators as the signal transduction system. J Clin Endocrinol Metab. 1998;83:2001–5. doi: 10.1210/jcem.83.6.4886. [DOI] [PubMed] [Google Scholar]
- 4.Willis D, Mason H, Gilling-Smith C, Franks S. Modulation by insulin of follicle-stimulating hormone and luteinizing hormone actions in human granulosa cells of normal and polycystic ovaries. J Clin Endocrinol Metab. 1996;81:302–9. doi: 10.1210/jcem.81.1.8550768. [DOI] [PubMed] [Google Scholar]
- 5.Gilling-Smith C, Story H, Rogers V, Franks S. Evidence for a primary abnormality of thecal cell steroidogenesis in the polycystic ovary syndrome. Clin Endocrinol (Oxf) 1997;47:93–9. doi: 10.1046/j.1365-2265.1997.2321049.x. [DOI] [PubMed] [Google Scholar]
- 6.Nestler JE, Barlascini CO, Matt DW, Steingold KA, Plymate SR, Clore JN, et al. Suppression of serum insulin by diazoxide reduces serum testosterone levels in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1989;68:1027–32. doi: 10.1210/jcem-68-6-1027. [DOI] [PubMed] [Google Scholar]
- 7.Ciotta L, Calogero AE, Farina M, De Leo V, la Marca A, Cianci A. Clinical, endocrine and metabolic effects of acarbose, an alpha-glucosidase inhibitor, in PCOS patients with increased insulin response and normal glucose tolerance. Hum Reprod. 2001;16:2066–72. doi: 10.1093/humrep/16.10.2066. [DOI] [PubMed] [Google Scholar]
- 8.Nestler JE, Jakubowicz DJ. Decreases in ovarian cytochrome P450c17 alpha activity and serum free testosterone after reduction of insulin secretion in polycystic ovary syndrome. N Engl J Med. 1996;335:617–23. doi: 10.1056/NEJM199608293350902. [DOI] [PubMed] [Google Scholar]
- 9.Ehrmann DA, Schneider DJ, Sobel BE, Cavaghan MK, Imperial J, Rosenfield RL, et al. Troglitazone improves defects in insulin action, insulin secretion, ovarian steroidogenesis, and fibrinolysis in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82:2108–16. doi: 10.1210/jcem.82.7.4069. [DOI] [PubMed] [Google Scholar]
- 10.Baillargeon JP, Jakubowicz DJ, Iuorno MJ, Jakubowicz S, Nestler JE. Effects of metformin and rosiglitazone, alone and in combination, in lean women with polycystic ovary syndrome and normal indices of insulin sensitivity. Fertil Steril. 2004;82:893–902. doi: 10.1016/j.fertnstert.2004.02.127. [DOI] [PubMed] [Google Scholar]
- 11.Nestler JE, Singh R, Matt DW, Clore JN, Blackard WG. Suppression of serum insulin level by diazoxide does not alter serum testosterone or sex hormone-binding globulin levels in healthy, nonobese women. Am J Obstet Gynecol. 1990;163(4 Pt 1):1243–6. doi: 10.1016/0002-9378(90)90698-7. [DOI] [PubMed] [Google Scholar]
- 12.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–70. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 13.Legro RS, Finegood D, Dunaif A. A fasting glucose to insulin ratio is a useful measure of insulin sensitivity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1998;83:2694–8. doi: 10.1210/jcem.83.8.5054. [DOI] [PubMed] [Google Scholar]
- 14.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–23. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 15.Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–10. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 16.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 17.Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;38:1165–74. doi: 10.2337/diab.38.9.1165. [DOI] [PubMed] [Google Scholar]
- 18.Nestler JE, Powers LP, Matt DW, Steingold KA, Plymate SR, Rittmaster RS, et al. A direct effect of hyperinsulinemia on serum sex hormone–binding globulin levels in obese women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 1991;72:83–9. doi: 10.1210/jcem-72-1-83. [DOI] [PubMed] [Google Scholar]
- 19.Hoekstra JB, van Rijn HJ, Erkelens DW, Thijssen JH. C-Peptide. Diabetes Care. 1982;5:438–46. doi: 10.2337/diacare.5.4.438. [DOI] [PubMed] [Google Scholar]
- 20.Faber OK, Binder C. C-Peptide: an index of insulin secretion. Diabetes Metab Rev. 1986;2:331–45. doi: 10.1002/dmr.5610020307. [DOI] [PubMed] [Google Scholar]
- 21.Nestler JE, Barlascini CO, Matt DW, Steingold KA, Plymate SR, Clore JN, et al. Suppression of serum insulin by diazoxide reduces serum testosterone levels in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1989;68:1027–32. doi: 10.1210/jcem-68-6-1027. [DOI] [PubMed] [Google Scholar]
- 22.Dunaif A, Green G, Futterweit W, Dobrjansky A. Suppression of hyper-androgenism does not improve peripheral or hepatic insulin resistance in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1990;70:699–704. doi: 10.1210/jcem-70-3-699. [DOI] [PubMed] [Google Scholar]
- 23.Lasco A, Cucinotta D, Gigante A, Denuzzo G, Pedulla M, Trifiletti A, et al. No changes of peripheral insulin resistance in polycystic ovary syndrome after long-term reduction of endogenous androgens with leuprolide. Eur J Endocrinol. 1995;133:718–22. doi: 10.1530/eje.0.1330718. [DOI] [PubMed] [Google Scholar]
- 24.Baillargeon JP, Nestler JE. Polycystic ovary syndrome: a syndrome of ovarian hypersensitivity to insulin? J Clin Endocrinol Metab. 2006;91:22–4. doi: 10.1210/jc.2005-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu XK, Zhou SY, Liu JX, Pollanen P, Sallinen K, Makinen M, et al. Selective ovary resistance to insulin signaling in women with polycystic ovary syndrome. Fertil Steril. 2003;80:954–65. doi: 10.1016/s0015-0282(03)01007-0. [DOI] [PubMed] [Google Scholar]