B-cell chronic lymphocytic leukemia (CLL) is characterized by actively dividing B-lymphocytes in the lymph nodes and bone marrow, as well as the accumulation of quiescent lymphocytes in the peripheral blood of affected patients. During treatment, the enzyme-mediated repair of DNA damage can induce resistance to chemotherapeutic drugs.1 Even if combinations of chemotherapy with immunotherapy have shown higher response rates and longer duration of responses, they can be associated with poor tolerability characterized by deterioration of immune functions leading to infections.2 More recently, bendamustine, a hybrid agent with a nitrogen mustard moiety and a purine analog, has been tested in clinical trials in CLL, demonstrating that hybrid molecules can result in increased therapeutic efficacy as compared with the nitrogen mustard analog alone.3 We have previously demonstrated that imatinib, an inhibitor of c-abl (c-abl is involved in homologous recombination repair), sensitizes B-CLL lymphocytes to chlorambucil (CLB) in vitro,4 an investigation which led to a phase I clinical trial in CLL patients in which the combination of CLB and imatinib resulted in a 45% response rate in a heavily pretreated population with minimal toxicity.5 These results encouraged us to develop the combi-molecule ZRF4, composed of a bifunctional alkylating moiety with an imatinib-like moiety, designed to degrade to an inhibitor of c-abl tyrosine kinase plus DNA-damaging species.
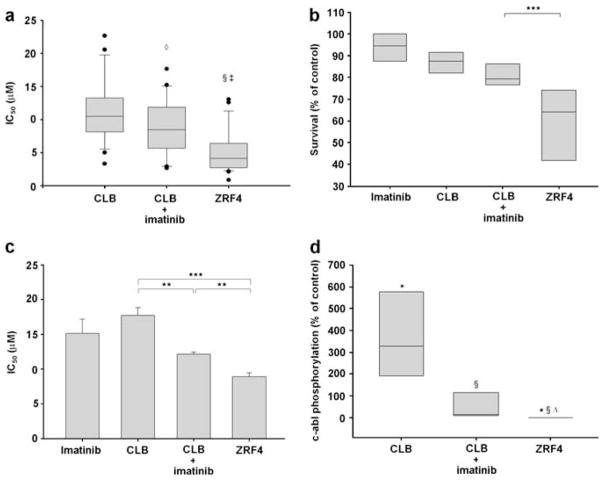
The synthesis of ZRF4 proceeded according to an adaptation of a strategy developed for the synthesis of ZRF1 (Supplementary Figure).6 The cytotoxic effect of ZRF4 was compared with CLB alone or CLB in combination with a non-toxic and clinically achievable concentration of imatinib (5 μM) in B-lymphocytes isolated from the B-CLL patients enrolled in this study (Supplementary Table) utilizing the MTT assay. As previously reported,4 5 μM imatinib sensitized most of the 22 CLL patients’ lymphocytes to CLB (mean IC50 (the drug concentration resulting in 50% of control) CLB=11.5 μM vs mean IC50 of CLB plus 5 μM imatinib=9.0 μM; P=0.044). ZRF4 is more cytotoxic than CLB alone (mean IC50 of CLB=11.5 μM vs mean IC50 of ZRF4=5.3 μM; P<0.001) or in combination with 5 μM imatinib in 88% of the CLL samples (mean IC50 of CLB plus 5 μM imatinib=9.0 μM vs mean IC50 of ZRF4=5.3 μM; P=0.002) (Table 1a, Figure 1a). To confirm the higher cytotoxic effect of ZRF4, we treated B-CLL lymphocytes from six patients with the IC50 of ZRF4 in each CLL sample and equimolar concentrations of CLB and imatinib alone or in combination. At these drug concentrations, imatinib, CLB and the combination were not toxic in CLL lymphocytes. In contrast, consistent with the results described above, ZRF4 was significantly more cytotoxic than the individual components (P<0.001) (Figure 1b). Similar results were obtained when the drugs were tested in B-cells from two patients diagnosed with del 17p13.1 and the p53-deficient B-CLL cell line, MEC2 (Tables 1b and c, Figure 1c). It is noteworthy that the IC50 values for the two patients with del 17p13.1 are higher compared with the non-mutated patient samples tested, resulting in a reduced apparent advantage of ZRF4 compared with the separate drugs.
Table 1.
Drugs cytotoxicity in primary B-CLL lymphocytes and B-CLL cell line
| Patient | Clinical treatment | IC50 (μM)
|
|||
|---|---|---|---|---|---|
| Imatinib | CLB | ZRF4 | CLB+5 μM imatinib | ||
|
(A) p53-proficient CLL lymphocytes
| |||||
| 1 | Untreated | 34.5 | 17.6 | 9.4 | 14.8 |
| 2 | Untreated | 28.5 | 10.3 | 5.4 | 8.5 |
| 3 | Untreated | 42.0 | 10.1 | 4.8 | 10.2 |
| 4 | Untreated | 33.0 | 11.0 | 2.7 | 7.7 |
| 5 | Untreated | 35.4 | 10.0 | 2.7 | 5.7 |
| 6 | Untreated | 41.9 | 13.3 | 2.4 | 6.4 |
| 7 | Untreated | 25.8 | 9.2 | 0.9 | 8.7 |
| 8 | Untreated | 42.0 | 12.1 | 5.6 | 15.2 |
| 9 | Untreated | 40.7 | 8.1 | 2.2 | 6.5 |
| 10 | Untreated | 31.0 | 10.5 | 12.6 | 8.6 |
| 11 | Untreated | 20.0 | 8.1 | 3.7 | 9.5 |
| 12 | CLB | 18.1 | 11.9 | 13.1 | 13.0 |
| 13 | CLB | 36.3 | 3.3 | 3.7 | 4.3 |
| 14 | CLB | 23.1 | 22.6 | 6.4 | 17.7 |
| 15 | CLB | 25.6 | 8.8 | 3.7 | 11.5 |
| 16 | CLB | 31.4 | 6.3 | 2.4 | 2.7 |
| 17 | CLB | 22.8 | 10.5 | 6.1 | 7.6 |
| 18 | Flu/cytoxan | 34.7 | 18.6 | 9.0 | 13.0 |
| 19 | Flu | 8.0 | 5.0 | 3.9 | 2.9 |
| 20 | CLB/Flu | 33.8 | 7.5 | 4.2 | 5.5 |
| 21 | CLB | 29.1 | 20.6 | 6.4 | 11.9 |
| 22 | CLB | 17.6 | 17.3 | 5.6 | 6.5 |
| Average | Untreated | 34.1 | 10.9 | 4.8 | 9.3 |
| Treated | 25.5 | 12.0 | 5.9 | 8.8 | |
| Total | 29.8 | 11.5 | 5.3 | 9.0 | |
| Median | Untreated | 34.5 | 10.3 | 3.7 | 8.6 |
| Treated | 25.6 | 10.5 | 5.6 | 7.6 | |
| Total | 31.2 | 10.4 | 4.5 | 8.6 | |
| (B) Patients diagnosed with del 17p13.1 | |||||
| 23 | Untreated | 28.6 | 15.5 | 9.3 | 11.1 |
| 24 | Flu | 33.1 | 14.9 | 9.4 | 9.9 |
| (C) p53-deficient B-CLL cell line MEC2 | |||||
| MEC2 | 16.7±4 | 17.8±2.8 | 8.9±1.5 | 12.2±0.7 | |
Abbreviations: IC50, the drug concentration resulting in 50% of control; CLB, chlorambucil; CLL, chronic lymphocytic leukemia; Flu, fludarabine. IC50 values were determined by the MTT assay at 72 h after exposure of primary CLL lymphocytes to CLB, imatinib, ZRF4 or CLB plus 5 μM imatinib.
Clinical treatment: the chemotherapeutics agents the patients were previously treated with at the clinic.
Figure 1.
(a) The MTT assay was utilized to determine the cytotoxicity of ZRF4, CLB alone and CLB plus 5 μM imatinib in cells from 22 B-CLL patients. (b) Equimolar concentrations (IC50 concentration of ZRF4 in each CLL lymphocyte sample) of the drugs were used for treatment of B-cells. (c) Cytotoxic effect of the drugs in the p53 deficient B-CLL cell line MEC2. (d) An enzyme-linked immunosorbent assay was used to determine the c-abl phosphorylation status in six CLL samples at 24 h after incubation with equimolar concentrations of the drugs. Symbols indicate significantly different from: *DMSO (P<0.001), §CLB (P<0.001), ◇CLB (P<0.05), ‡CLB+imatinib (P<0.005), △CLB+imatinib (P<0.05), **P<0.05, ***P<0.001.
ZRF4 is a combi-molecule designed to target c-abl (through an imatinib moiety) and to induce DNA damage (through a nitrogen mustard-like moiety). The effects of the IC50 of ZRF4 as compared with equimolar concentrations of CLB alone or in combination with imatinib were examined at 24 h after in vitro treatment in nine available CLL lymphocyte samples. Our results indicated that CLB induced c-abl phosphorylation in the CLL lymphocytes tested (P<0.001) (Figure 1d). Moreover, imatinib has no significant effect on the basal c-abl phosphorylation (data not shown) but inhibited CLB-induced c-abl phosphorylation (P<0.001). The combi-molecule ZRF4 completely inhibits c-abl phosphorylation. ZRF4 inhibition of c-abl is significantly different from the basal (P<0.001) and CLB-stimulated (P<0.001) c-abl phosphorylation. Most importantly, ZRF4 is a more potent inhibitor of c-abl phosphorylation than imatinib in combination with CLB (P<0.05) (Figure 1d). Some combi-molecules are more potent inhibitors of the tyrosine kinase than the individual component inhibitor.7 We speculate that this property implies that after treatment of the B-CLL cells with ZRF4, c-abl was inhibited by two different molecules (the intact ZRF4 and the metabolite, which is an imatinib-like molecule) (Supplementary Figure). The effect of these two different molecules is an increase in the inhibition of c-abl as compared with imatinib plus CLB.
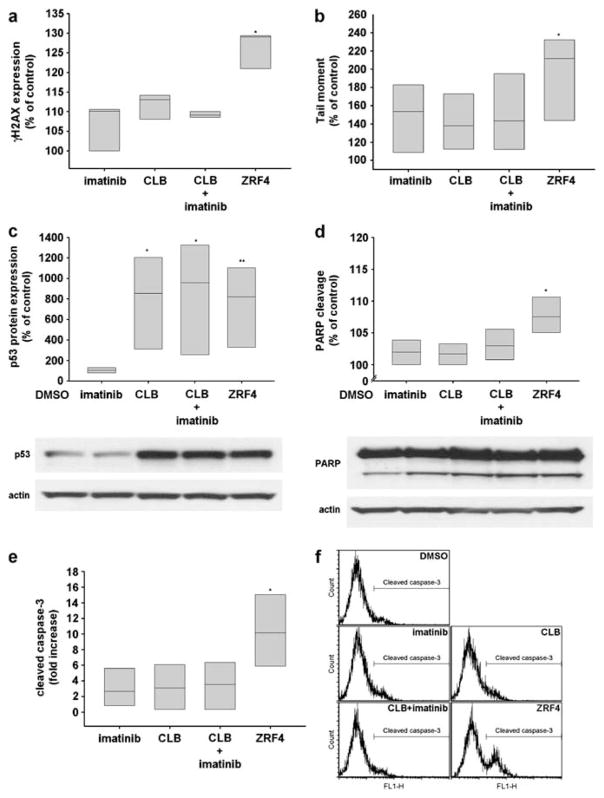
Chemotherapeutic agents that produce DNA damage cause H2AX phosphorylation on serine 139 (γH2AX) within damaged cells.8 In order to verify that the cytotoxic effect of ZRF4 was at least partially mediated throughout its nitrogen mustard-like moiety, we analyzed by flow cytometry, the γH2AX status as a marker of drug-induced DNA damage in B-CLL lymphocytes. ZRF4 produced a 30% increase in γH2AX, whereas CLB alone or CLB in combination with imatinib showed minimal changes in γH2AX (Figure 2a). To confirm the results obtained above, we utilized a more direct assay of DNA damage, the alkaline comet assay. The results demonstrate that the CLL lymphocytes treated with ZRF4 showed more DNA damage than those CLL lymphocytes treated with CLB and imatinib (P=0.011) (Figure 2b).
Figure 2.
The effects of equimolar concentrations (IC50 of ZRF4 in each CLL sample) of the drugs were evaluated at 24 h after treatments by flow cytometry analysis of (a) H2AX phosphorylation and (e and f) caspase-3 cleavage, western blot analysis of (c) p53 protein expression and (d) Poly (ADP-ribose) polymerase cleavage and by (b) alkaline comet assay. *P<0.05, **P<0.005.
The tumor suppressor protein p53 accumulates in cells treated with drugs that create DNA damage and provides an important link between DNA damage and apoptosis. Before assessing the effect of drug treatments on apoptosis, we investigated the p53 protein expression in B-CLL lymphocytes at 24 h after treatment. B-CLL lymphocytes treated with ZRF4, CLB alone or CLB in combination with imatinib resulted in an increase in p53 protein expression (Figure 2c). We then investigated the cleavage of poly (ADP-ribose) polymerase by western blot analysis and caspase-3 by flow cytometry analysis as markers of apoptosis to determine the effect of ZRF4-induced cell death in primary B-CLL cells. Results demonstrated that ZRF4 induced a small, but significant increase in poly (ADP-ribose) polymerase cleavage and a more significant increase in caspase-3 cleavage than CLB or CLB in combination with imatinib (Figures 2d–f).
Taken together, these data sustain the idea that the higher potency of ZRF4 is mediated by a greater inhibition of c-abl along with its stronger DNA-damaging potential. The greater inhibition of c-abl in the presence of DNA damage results in more apoptosis and thus, cytotoxicity in B-CLL lymphocytes. This should translate into a more efficient treatment of CLL.
Supplementary Material
Acknowledgments
We thank the Manitoba CLL tissue bank for the IgVH mutation analysis. LP and RA are members of the Quebec Clinical Research Organization in Cancer consortium. This study was supported by grants from the Leukemia Lymphoma society (RA and LP), Canadian Institutes of Health Research (LP, RA and BJC) and Leukemia Lymphoma Society (Canada) (BJC).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
References
- 1.Sampath D, Plunkett W. The role of DNA repair in chronic lymphocytic leukemia pathogenesis and chemotherapy resistance. Curr Oncol Rep. 2007;9:361–367. doi: 10.1007/s11912-007-0048-6. [DOI] [PubMed] [Google Scholar]
- 2.Robak T, Dmoszynska A, Solal-Celigny P, Warzocha K, Loscertales J, Catalano J, et al. Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J Clin Oncol. 2010;28:1756–1765. doi: 10.1200/JCO.2009.26.4556. [DOI] [PubMed] [Google Scholar]
- 3.Dennie TW, Kolesar JM. Bendamustine for the treatment of chronic lymphocytic leukemia and rituximab-refractory, indolent B-cell non-Hodgkin lymphoma. Clin Ther. 2009;31(Part 2):2290–2311. doi: 10.1016/j.clinthera.2009.11.031. [DOI] [PubMed] [Google Scholar]
- 4.Aloyz R, Grzywacz K, Xu ZY, Loignon M, Alaoui-Jamali MA, Panasci L. Imatinib sensitizes CLL lymphocytes to chlorambucil. Leukemia. 2004;18:409–414. doi: 10.1038/sj.leu.2403247. [DOI] [PubMed] [Google Scholar]
- 5.Hebb J, Assouline S, Rousseau C, Desjardins P, Caplan S, Egorin MJ, et al. A phase I study of imatinib mesylate in combination with chlorambucil in previously treated chronic lymphocytic leukemia patients. Cancer Chemother Pharmacol. 2010 doi: 10.1007/s00280-010-1530-7. e-pub ahead of print 1 December 2010. [DOI] [PubMed] [Google Scholar]
- 6.Rachid Z, Katsoulas A, Williams C, Larroque AL, McNamee J, Jean-Claude BJ. Optimization of novel combi-molecules: identification of balanced and mixed bcr-abl/DNA targeting properties. Bioorg Med Chem Lett. 2007;17:4248–4253. doi: 10.1016/j.bmcl.2007.05.067. [DOI] [PubMed] [Google Scholar]
- 7.Rachid Z, Katsoulas A, Brahimi F, Jean-Claude BJ. Synthesis of pyrimidinopyridine-triazene conjugates targeted to abl tyrosine kinase. Bioorg Med Chem Lett. 2003;3:3297–3300. doi: 10.1016/s0960-894x(03)00553-5. [DOI] [PubMed] [Google Scholar]
- 8.Riballo E, Kuhne M, Rief N, Doherty A, Smith GC, Recio MJ, et al. A pathway of double-strand break rejoining dependent upon ATM, Artemis, and proteins locating to gamma-H2AX foci. Mol Cell. 2004;16:715–724. doi: 10.1016/j.molcel.2004.10.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.