Abstract
Background and aims
The hepatic stellate cell, which plays a pivotal role in hepatic fibrosis, contains the filament vimentin which is known to undergo protein citrullination and become immunogenic. The aims of this study were to find out if anti-modified citrullinated vimentin (anti-MCV) antibodies are produced in patients with chronic hepatitis and if such production is associated with liver fibrosis.
Methods
Sera and liver biopsy specimens were collected from 100 patients with chronic hepatitis. Sera were also collected from 100 healthy controls. The liver biopsies were graded according to the Metavir fibrosis scores. The serum concentrations of anti-MCV antibody were measured in both patients and controls by ELISA using commercially available kits.
Results
The mean serum concentration of anti-MCV antibody in patients with chronic hepatitis (54.90 ± 6.09 U/mL) was significantly higher (P = 0.001) than that of controls (17.38 ± 0.56 U/mL). Furthermore, serum anti-MCV antibody titer was able to separate patients with no fibrosis from those with moderate or severe fibrosis or cirrhosis. Using receiver operating characteristic curves, a serum concentration of anti-MCV antibody of 8.82 U/mL was able to diagnose cirrhosis with 60% specificity and 60% sensitivity.
Conclusion
We concluded that serum anti-MCV antibody concentration may be a sensitive noninvasive marker for liver cirrhosis that needs to be investigated further.
Keywords: anti-MCV antibody, serum marker, liver fibrosis
Introduction
Protein citrullination or deimination is a recently described post-translational modification of arginine residues within a peptide sequence into citrulline residues. Deimination is catalyzed by a family of calcium-dependent enzymes, the peptidylarginine deiminases (PADs, EC 3.5.3.15). To date, 5 isoenzymes have been identified. Their genomic organization, subcellular localization, and tissue-specific expression have been determined (for a review, see ref 1). All PAD1 genes are localized in 1 cluster at 1p 36.13 within a 300 kb region.1,2 The product of citrullination is the nonstandard amino acid citrulline, which contributes to the backbone of certain proteins such as histones.3 By decreasing the net positive charge, citrullination can alter the primary, secondary, and tertiary structures of proteins with a potential influence on intermolecular interactions. Proteins known to be citrullinated in vivo include keratin,4 filaggrin,5 trichohyalin,6 vimentin,7 myelin basic proteins,8 histones,9,10 fibrinogen,11,12 fibrins,13 and collagen type I.14 Physiologically, protein citrullination plays an essential role in cell differentiation,4 nerve growth,8,15 embryonic development,16 cell death,17,18 and gene regulation.9,10
Protein citrullation is involved in the pathogenesis of certain human diseases, the best example being rheumatoid arthritis (RA). The most specific family of RA antibodies is the antibodies directed against citrullinated proteins (reviewed in19). These antibodies can be detected in almost 80% of RA with a specificity of 99%.20 Besides being very specific for RA, anti-citrullinated protein antibodies can be detected very early in the disease and appear to predict clinical outcome,21–23 making them a very useful diagnostic tool for rheumatologists. The anti-citrullinated protein antibodies are produced locally in the inflammed synovium.24–26 Since hepatic stellate cells, which play a pivotal role in hepatic fibrosis, contain vimentin, we hypothesized that protein citrullination of vimentin may also occur in chronic hepatitis and may partly explain the fibrosis seen in this disease. The objectives of this study therefore were to find out if production of anti-modified citrullinated vimentin (anti-MCV) antibody is increased in the blood of patients with chronic hepatitis and to find out if there is any relationship between the serum concentrations of these antibodies and the Metavir fibrosis scores.
Materials and methods
Patients and serum samples
One hundred patients with chronic liver disease of varying duration who reported for liver biopsy at the hepatology clinics of Al-Amiri and Mubarak Al-Kabeer University Teaching Hospitals, Kuwait between September 2006 and May 2007 were recruited for this study. They had reported for liver biopsy because of past history of jaundice and persistently elevated serum transaminases. None of these patients fulfilled any of the American Rheumatism Association (ACR) criteria for the diagnosis of rheumatoid arthritis.27 All the patients gave informed consent for use of data and serum for research purposes, and this study was approved by the local research ethical committee.
A fasting blood sample was collected by venipuncture from each patient before liver biopsy. The blood samples were centrifuged and sera separated. The serum samples were kept deep frozen at −80°C until ready for assay.
Controls
One hundred age- and sex-matched healthy controls were recruited from the central blood bank and serum obtained from each person in a similar way. All the volunteers gave informed consent for use of their blood for research purposes.
Histological analysis of liver biopsies
Liver biopsies were formalin-fixed, paraffin-embedded, and stained with H&E stain as well as other special stains used for liver tissue diagnosis such as Masson’s trichrome stain for collagen, reticulin stain, PAS stain with and without diastase digestion, Perl’s iron stain, and orcein stain. Two histopathologists, completely unaware of patient characteristics, examined the biopsies under the microscope to assess the degree of fibrosis (stage) and the extent of inflammatory activity (grade) according to the Metavir scoring system.28 Fibrosis was staged on a scale of 0 to 4; F0 = no fibrosis, F1 = portal fibrosis without septa, F2 = few septa, F3 = numerous septa without fibrosis, F4 = fibrosis. Activity (based on the intensity of macroinflammatory activity, interface hepatitis and lobulitis) was graded as follows: A0 = no histological activity, A1 = mild activity, A2 = moderate activity, A3 = severe activity. To assess liver biopsy quality, Regev quality criteria29 were used. A biopsy between 10 and 15 mm in length, with fewer than 5 portal tracts or fragmented, was considered as ‘fair quality biopsy’. A ‘poor quality’ biopsy was less than 10 mm in length. All the 6 poor quality biopsies were excluded from the study.
Serum assay of antibodies to modified citrullinated vimentin (anti-MCV)
Concentrations of anti-MCV antibody were measured in the sera of patients and controls using a commercially available ELISA kit (ORGENTIC Diagnostica, GmbH, Mainz, Germany) according to the manufacturer’s instructions. In brief, serum samples were diluted 1:100 and incubated on MCV-coated microtiter wells for 30 minutes at room temperature on a horizontal shaking platform (100/second). Plates were washed 3 times and incubated with peroxidase-labeled anti-human IgG conjugate for 15 minutes. 3′,3′,5,5′-tetramethylbenzidine substrate was added and incubated for 15 minutes after additional washing. Color development was stopped with 1M HCl solution and the optical density of each well was read at 450 nm on an ELISA reader. Results were expressed in U/mL using a sample point–point curve fitting method. The intra-assay and inter-assay coefficients of variation were 6% and 8.5%, respectively, in our laboratory.
Biochemical data
Aspartate aminotransferase (AST), alanine aminotransferase (ALT) and albumin concentrations were measured in patients and control sera on a Beckmann Coulter LX-20 Chemistry Analyser using dedicated reagents.
Statistical analysis of data
Data entry and statistical analysis were performed by using SPSS version 15.0. We evaluated the associations between serum anti-MCV antibody concentrations and Metavir fibrosis and activity scores. We used error bar plots with 95% confidence intervals to display these associations. ROC (receiver operating characteristics) curves were used to identify the optimal serum anti-MCV antibody concentration that would give the maximum sensitivity and specificity for the diagnosis of liver fibrosis.
Results
Patient characteristics
The demographic, biochemical, and histological data, which have been described in previous publications,30,31 are summarized in Table 1.
Table 1.
Demographic and biochemical characteristics of the patients
| Fibrosis stage | n | Male/female | Mean age (years) | Serum ALT (U/L) mean ± SEM | Serum AST (U/L) mean ± SEM | HCV (n) | HBV (n) | NASH (n) |
|---|---|---|---|---|---|---|---|---|
| F0 | 14 | 11/3 | 41.89 ± 4.16 | 69.2 ± 5.77 | 57.75 ± 12.52 | 4 | 2 | 10 |
| F1 | 9 | 8/1 | 46.8 ± 1.65 | 79.85 ± 15.3 | 70.43 ± 16.21 | 4 | 4 | 7 |
| F2 | 13 | 9/4 | 44 ± 1.31 | 84.25 ± 25.23 | 75.71 ± 13.07 | 8 | 4 | 5 |
| F3 | 34 | 24/10 | 45.47 ± 2.05 | 180.6 ± 25.8 | 136.67 ± 84.06 | 16 | 6 | 4 |
| F4 | 24 | 17/7 | 42.8 ± 1.54 | 191.63 ± 78.39 | 145.18 ± 59.23 | 14 | 4 | 2 |
| Total | 94 | 69/25 | 44.1 ± 1.1 | 114.62 ± 22.69 | 93.88 ± 16.46 | 46 | 20 | 28 |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; HCV, hepatitis C virus; HBV, hepatitis B virus; NASH, nonalcoholic steatohepatitis; n, number; SEM, standard error of mean.
Serum concentration of anti-MCV antibody
Serum concentrations of anti-MCV antibody in hepatits C virus (HCV) patients (58.80 ± 6.7 U/mL) were significantly higher than those of healthy controls (17.38 ± 0.57 U/mL), patients with hepatitis B virus (HBV) (43.86 ± 7.75 U/mL), or patients with nonalcoholic steatohepatitis (NASH) (23.10 ± 2.88 U/mL) (Table 2). Concentrations in HBV patients were also significantly higher than those in NASH patients and healthy controls. Values in NASH patients did not differ significantly from those of healthy controls.
Table 2.
Mean concentrations of anti-modified citrullinated vimentin (anti-MCV) antibody in patients with HCV, HBV, NASH, and healthy controls
| Group of patients | Anti-MCV concentrations (U/mL) (mean ± SEM) | n |
|---|---|---|
| HCV | 58.80 ± 6.70 | 46 |
| HBV | 43.86 ± 7.75 | 20 |
| NASH | 23.10 ± 2.88 | 28 |
| Controls | 17.38 ± 0.57 | 100 |
Notes: HCV vs controls: P = 0.000; HBV vs controls: P = 0.01; NASH vs controls: P = 0.91; HCV vs HBV: P = 0.151; HCV vs NASH: P = 0.000; HBV vs NASH: P = 0.01.
Abbreviations: HCV, hepatitis C virus; HBV, hepatitis B virus; NASH, nonalcoholic steatohepatitis; n, number; SEM, standard error of mean.
Mean serum anti-MCV antibody concentration in patients with chronic hepatitis (54.90 ± 6.09 U/mL) was significantly (P < 0.01) higher than that of healthy controls (17.38 ± 0.563 U/mL) (Figure 1). None of the healthy controls had a serum concentration greater than 20 U/mL (the upper limit of normal recommended by the manufacturers of the ELISA kit) while 86 of 94 patients with chronic hepatitis had serum anti-MCV antibody concentrations greater than 20 U/mL.
Figure 1.
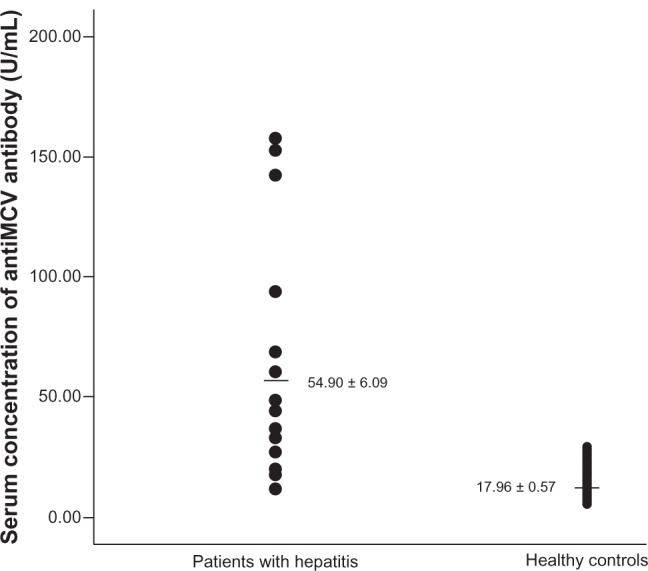
Scatterplot of the serum concentration of anti-modified citrullinated vimentin (anti-MCV) antibody in patients with chronic hepatitis and in healthy controls.
Association among serum concentration of anti-MCV antibody and serum concentrations of liver enzymes and serum albumin
In patients with chronic hepatitis, serum concentration of anti-MCV antibody was positively associated with serum ALT (r = 0.423, P = 0.02) and serum AST (r = 0.44, P = 0.01), and negatively associated with the serum albumin concentration (r = −0.44, P = 0.01).
Relationship between serum anti-MCV antibody concentrations and Metavir fibrosis scores
Mean anti-MCV antibody concentration at stage F0 (no fibrosis) was 25.26 ± 5.25 U/mL (Figure 2), not significantly (P = 0.116) different from the value at stage F1 (30.50 ± 5.82 U/mL) but significantly different from those at F2 (39.11 ± 5.31 U/mL), F3 (40.56 ± 3.40 U/mL), or F4 (78.62 ± 11.16 U/mL). Thus anti-MCV antibody concentration could differentiate patients with no liver fibrosis from those with moderate fibrosis, severe fibrosis, or cirrhosis (F2–F4). The association among serum anti-MCV antibody concentrations and Metavir fibrosis scores are shown in Table 3. Using ROC curves (Figure 3) a cut-off point of 8.82 U/mL of serum anti-MCV antibody concentration was 60% specific and 60% sensitive for predicting liver cirrhosis.
Figure 2.
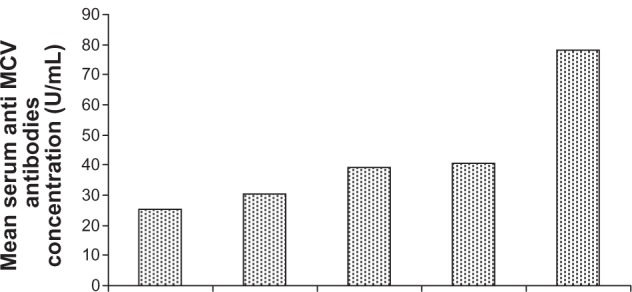
Distribution of serum concentration of anti-modified citrullinated vimentin (anti-MCV) antibody according to Metavir fibrosis score in patients with chronic hepatitis.
Table 3.
Association among serum concentrations of anti-modified citrullinated vimentin (anti-MCV) antibody and degrees of hepatic fibrosis in patients with chronic liver disease
| Metavir fibrosis core | Serum concentration of anti-MCV (U/mL) | Standard error | P value vs F0 |
|---|---|---|---|
| F0 | 25.26 | 5.25 | – |
| F1 | 30.50 | 5.82 | 0.12 |
| F2 | 39.11 | 5.31 | 0.07 |
| F3 | 40.56 | 3.40 | 0.02 |
| F4 | 78.62 | 11.16 | 0.001 |
Figure 3.
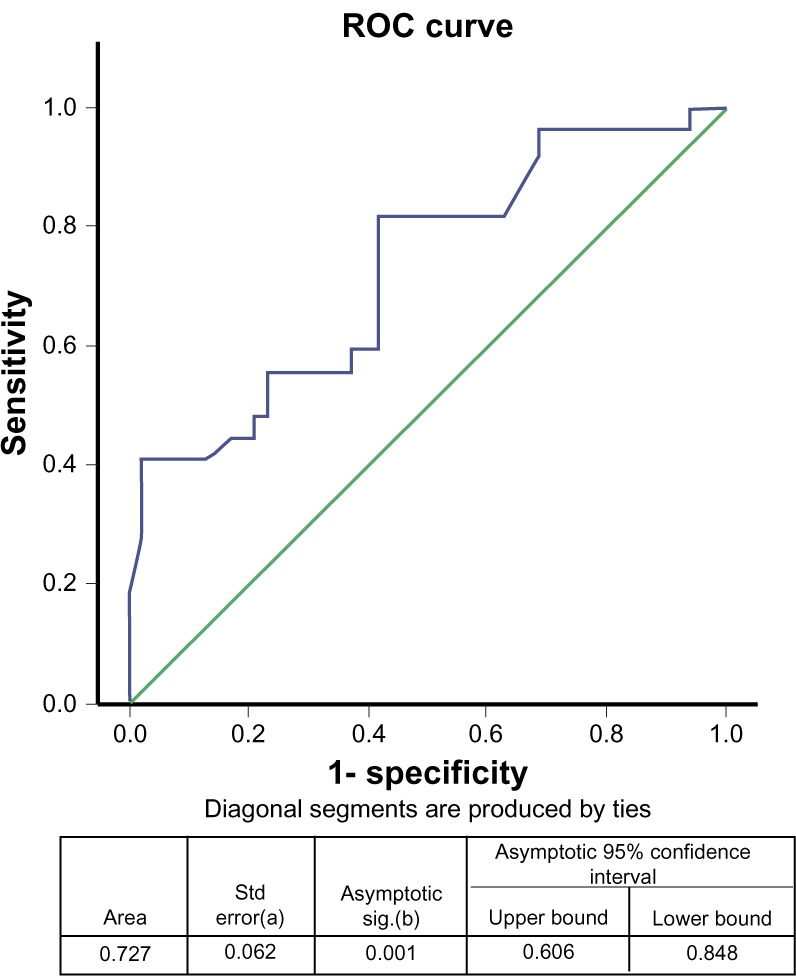
Area under the curve for serum concentration of anti-modified citrullinated vimentin (anti-MCV) antibody and stage of hepatic fibrosis.
Note: The test result variable(s): anti-MCV (U/mL) has at least one tie between the positive actual state group and the negative actual state group. Statistics may be biased aUnder the nonparametric assumption bNullhypothesis true area = 0.5
Abbreviation: ROC, receiver operating characteristics.
Discussion
Our study showed that significant protein citrullination of vimentin occurs in patients with chronic hepatitis and that the serum concentration of anti-MCV antibody could differentiate patients with no liver fibrosis from those with moderate to severe fibrosis. If this study can be confirmed in a larger sample size, it would seem that serum concentrations of anti-MCV antibody can be used as a sensitive noninvasive marker for staging liver fibrosis in patients with chronic hepatitis. Hitherto, a variety of indirect and direct noninvasive serum markers of liver fibrosis have been evaluated. The indirect markers include derived variables such as the AST/ALT ratio,32–35 platelet count,36 and prothrombin index.37 Other indirect tests include the PGA index which combines measurement of the prothrombin index, gamma glutamyl transferase, and apolipoprotein A1.38–40
More recently, Halfon and colleagues reported on a score derived from 6 markers found to be useful in discriminating between early and more advanced liver fibrosis.41 Other indirect tests include the FibroTest,42 ActiTest,43 Forns test,44 and APRI test.45 The direct markers of liver fibrosis include collagen propeptides (PIIINP),46 hyaluronic acid,47 metalloproteinases and inhibitors of metalloproteinases,48 and a host of others. Unfortunately most indirect and direct serum markers are complex and lack sensitivity. Moreover most studies on their diagnostic usefulness have been limited by their retrospective design and poor reporting on liver biopsy methods. Therefore, there has always been a need to develop new noninvasive markers for liver fibrosis in patients with chronic hepatitis.
This study will be the first to report the presence of anti-MCV antibody in patients with chronic hepatitis and has demonstrated that it can be used to diagnose liver cirrhosis with 60% sensitivity and specificity. It could not differentiate between F0 and F2 or F3 degrees of liver fibrosis. Because of our relatively small sample size, there is a need to confirm this observation in a larger sample size. We are still collecting samples to validate this result and to compare the diagnostic sensitivity and specificity of serum anti-MCV concentrations with other noninvasive markers of liver fibrosis.
The formation of anti-MCV antibody in patients with chronic hepatitis is not difficult to explain. Hepatic stellate cells contain vimentin.49,50 Oxidative stress due to liver injury can modify this vimentin so that it becomes immunogenic stimulating the production of anti-MCV antibody. Our finding that the serum concentration of anti-MCV antibody is associated with the degree of liver fibrosis in patients with chronic hepatitis supports the theory that hepatic stellate cells play a central role in liver fibrosis.
Furthermore, our finding that protein citrullation occurs in patients with chronic hepatitis supports previous reports that protein citrullination is not specific for RA since it has been found to occur in psoriasis,51,52 multiple sclerosis,15,53,54 and various tumors.55 We are now investigating the diagnostic properties of serum anti-MCV antibody concentration for staging liver fibrosis in a large cohort of patients with chronic liver disease, especially viral hepatitis.
Footnotes
Disclosure
The authors declare no conflicts of interest.
References
- 1.Vossenaar ER, Zendman AJ, van Venrooij WJ, Pruijn GJ. PAD, a growing family of citrullinating enzymes: Genes features and involvement in disease. Bioessays. 2003;25:1106–1118. doi: 10.1002/bies.10357. [DOI] [PubMed] [Google Scholar]
- 2.Chavanas S, Mechin MC, Takahara H, Kawada A, Nachat R, Serre G, et al. Comparative analysis of the mouse and human peptidylarginine deiminase gene cluster reveals highly conserved non-coding segments and a new human gene, PAD16. Gene. 2004;330:19–27. doi: 10.1016/j.gene.2003.12.038. [DOI] [PubMed] [Google Scholar]
- 3.Fabrizio G, Mastronardi D, Wood D, et al. Increased citrullination of histone H3 in multiple sclerosis brain and animal models of demyelination: a role for tumor necrosis factor-induced peptidylarginine deiminase 4 translocation. J Neurosci. 2006;26:11387–11396. doi: 10.1523/JNEUROSCI.3349-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Senshu T, Akiyama K, Ishigami A, Nomura K. Studies on specificity of peptidylarginine deiminase reactions using an immunochemical probe that recognizes an enzymatically deiminated partial sequence of mouse keratin K1. J Dermatol Sci. 1999;21:113–126. doi: 10.1016/s0923-1811(99)00026-2. [DOI] [PubMed] [Google Scholar]
- 5.Ishida-Yamamoto A, Senshu T, Eady RA, et al. Sequential reorganization of cornified cell keratin filaments involving filaggrin-mediated compaction and keratin 1 deimination. J Invest Dermatol. 2002;118:282–287. doi: 10.1046/j.0022-202x.2001.01671.x. [DOI] [PubMed] [Google Scholar]
- 6.Tarcsa E, Marekov LN, Meri G, Melino G, Lee SC, Steinert PM. Protein unfolding by peptidylarginine deiminase. Substrate specificity and structural relationships of the natural substrates trichohyalin and filaggrin. J Biol Chem. 1996;271:30709–30716. doi: 10.1074/jbc.271.48.30709. [DOI] [PubMed] [Google Scholar]
- 7.Vossenaar ER, Després N, Lapointe E, et al. Rheumatoid arthritis specific anti-Sa antibodies target citrullinated vimentin. Arthritis Res Ther. 2004;6:R142–R150. doi: 10.1186/ar1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moscarello MA, Wood DD, Ackerley C, Boulias C. Myelin in multiple sclerosis is developmentally immature. J Clin Invest. 1994;94:146–154. doi: 10.1172/JCI117300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Wysocka J, Sayegh J, et al. Human PAD4 regulates histone arginine methylation. Science. 2004;306:279–283. doi: 10.1126/science.1101400. [DOI] [PubMed] [Google Scholar]
- 10.Cuthbert GL, Daujat S, Snowden AW, et al. Histone deimination antagonizes arginine methylation. Cell. 2004;1118:545–553. doi: 10.1016/j.cell.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 11.Vossenaar ER, Nijenhuis S, Helsen MM, et al. Citrullination of synovial proteins in murine models of rheumatoid arthritis. Arthritis Rheum. 2003;48:2489–2500. doi: 10.1002/art.11229. [DOI] [PubMed] [Google Scholar]
- 12.Caponi L, Petit-Teixeira E, Sebbag M, et al. A family based study shows no association between rheumatoid arthritis and the PAD14 gene in a white French population. Ann Rheum Dis. 2005;64:587–593. doi: 10.1136/ard.2004.026831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masson-Bessière C, Sebbag M, Girbal-Neuhauser E, et al. The major synovial targets of the rheumatoid arthritis-specific antifilaggrin autoantibodies are deiminated forms of the alpha and beta chains of fibrin. J Immunol. 2001;166:4177–4184. doi: 10.4049/jimmunol.166.6.4177. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki A, Yamada R, Ohtake-Yamanaka M, Okazaki Y, Sawada T, Yamamato K. Anti-citrullinated collagen type I antibody is a target of autoimmunity in rheumatoid arthritis. Biochem Biophys Res Commun. 2005;333:418–426. doi: 10.1016/j.bbrc.2005.05.137. [DOI] [PubMed] [Google Scholar]
- 15.Wood DD, Bilbao JM, O’Connors P, Moscarello MA. Acute multiple sclerosis (Marburg type) is associated with developmentally immature myelin basic protein. Ann Neurol. 1996;40:18–24. doi: 10.1002/ana.410400106. [DOI] [PubMed] [Google Scholar]
- 16.Wright PW, Bolling LC, Calvert ME, et al. ePAD, an oocyte and early embryo-abundant peptidylarginine deiminase-like protein that localizes to egg cytoplasmic sheets. Dev Biol. 2003;256:73–88. doi: 10.1016/s0012-1606(02)00126-4. [DOI] [PubMed] [Google Scholar]
- 17.Asaga H, Yamada M, Senshu T. Selective deimination of vimentin in calcium ionophore-induced apoptosis of mouse peritoneal macrophages. Biochem Biophys Res Commun. 1998;243:641–646. doi: 10.1006/bbrc.1998.8148. [DOI] [PubMed] [Google Scholar]
- 18.Mizoguchi M, Manabe M, Kawamura Y, et al. Deimination of 70-kD nuclear protein during epidermal apoptotic events in vitro. J Histochem Cytochem. 1998;46:1303–1309. doi: 10.1177/002215549804601110. [DOI] [PubMed] [Google Scholar]
- 19.Van Boekel MA, Vossenaar ER, van den Hoogen FH, van Venrooij WJ. Autoantibody systems in rheumatoid arthritis: specificity, sensitivity and diagnostic value. Arthritis Res. 2002;4:87–93. doi: 10.1186/ar395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Venrooij WJ, Hazes JM, Visser H. Anticitrullinated protein/peptide antibody and its role in the diagnosis and prognosis of early rheumatoid arthritis. Neth J Med. 2002;60:383–388. [PubMed] [Google Scholar]
- 21.Visser H, Le Cessie S, Vos K, Breedveld FC, Hazes JM. How to diagnose rheumatoid arthritis early? A prediction model for persistent (erosive) arthritis. Arthritis Rheum. 2002;46:357–365. doi: 10.1002/art.10117. [DOI] [PubMed] [Google Scholar]
- 22.Kroot EJ, de Jong BA, van Leeuwen MA, et al. The prognostic value of anti-cyclic citrullinated peptide antibody in patients with recent-onset rheumatoid arthritis. Arthritis Rheum. 2000;43:1831–1835. doi: 10.1002/1529-0131(200008)43:8<1831::AID-ANR19>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 23.Meyer O, Labarre C, Dougados M, et al. Anticitrullinated protein/peptide antibody assays in early rheumatoid arthritis to predicting five year radiographic damage. Ann Rheum Dis. 2003;62:120–126. doi: 10.1136/ard.62.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masson-Bessiere C, Sebbag M, Durieux JJ, et al. In the rheumatoid pannus, anti-filaggrin autoantibodies are produced by local plasma cells and constitute a higher propotion of IgG than in synovial fluid and serum. Clin Exp Immunol. 2000;119:544–552. doi: 10.1046/j.1365-2249.2000.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reparon-Schuijt CC, van Esch WJ, Breedveld FC, Verweij CL. Secretion of anti-citrulline-containing peptide antibody by B lymphocytes in rheumatoid arthritis. Arthritis Rheum. 2001;44:41–47. doi: 10.1002/1529-0131(200101)44:1<41::AID-ANR6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 26.Smeets TJ, Vossenaar ER, Kraan MC, et al. Expression of citrulline-containing antigens in RA synovium.[abstract] Arthritis Res. 2002;3:P4. [Google Scholar]
- 27.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 28.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 29.Regev A, Molina E, Moua R, Bejarano P, et al. Reliability of histopathologic assessment for differentiation of recurrent hepatitis C from acute rejection after liver transplantation. Liver Transpl. 2004;10:1233–1239. doi: 10.1002/lt.20245. [DOI] [PubMed] [Google Scholar]
- 30.Abdeen SM, Olusi SO, Askar HA, Thalib L, Al-Azemi A, George S. The predictive value of CD38 positive hepatic stellate cell count for assessing disease activity and fibrosis in patients with chronic hepatitis. Acta Histochem. 2009;111:520–530. doi: 10.1016/j.acthis.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 31.Abdeen SM, Olusi SO. Peptidyl arginine deiminase: a novel immunohistochemical marker for liver fibrosis in patients with chronic hepatitis. Acta Histochem. 2010;112:592–603. doi: 10.1016/j.acthis.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Zlobec I, Steele R, Michel RP, Compton CC, et al. Scoring of P 53, VEG F, BCl-2 and APAF-1 immunohistochemistry and inter-observer reliability in colorectal cancer. Mod Pathol. 2006;19:1236–1242. doi: 10.1038/modpathol.3800642. [DOI] [PubMed] [Google Scholar]
- 33.Imperiale TF, Said AT, Cummings OW, Born LJ. Need for validation of clinical decision aids: use of the AST/ALT ratio in predicting cirrhosis in chronic hepatitis C. Am J Gastroenterol. 2000;95:2328–2332. doi: 10.1111/j.1572-0241.2000.02322.x. [DOI] [PubMed] [Google Scholar]
- 34.Sheth SG, Flamm SL, Gordon FD, Chopra S. AST/ALT ratio predicts cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 1998;93:44–48. doi: 10.1111/j.1572-0241.1998.044_c.x. [DOI] [PubMed] [Google Scholar]
- 35.Giannini E, Risso D, Botta F, et al. Validity and clinical utility of the aspartate aminotransferase-alanine aminotransferase ratio in assessing disease severity and prognosis in patients with hepatitis C virus-related chronic liver disease. Arch Intern Med. 2003;163:218–224. doi: 10.1001/archinte.163.2.218. [DOI] [PubMed] [Google Scholar]
- 36.Croquet V, Vuillemin E, Ternisien C, et al. Prothrombin index is an indirect marker of severe liver fibrosis. Eur J Gastroenterol Hepatol. 2002;14:1133–1141. doi: 10.1097/00042737-200210000-00015. [DOI] [PubMed] [Google Scholar]
- 37.Poynard T, Aubert A, Bedossa P, et al. A simple biological index for detection of alcoholic liver disease in drinkers. Gastroenterology. 1991;100:1397–1402. [PubMed] [Google Scholar]
- 38.Naveau S, Poynard T, Benattar C, Bedossa P, Chaput JC. Alpha-2-macroglobulin and hepatic fibrosis. Diagnostic interest. Dig Dis Sci. 1994;39:2426–2432. doi: 10.1007/BF02087661. [DOI] [PubMed] [Google Scholar]
- 39.Teare JP, Sherman D, Greenfield SM, et al. Comparison of serum procollagen III peptide concentrations and PGA index for assessment of hepatic fibrosis. Lancet. 1993;342:895–898. doi: 10.1016/0140-6736(93)91946-j. [DOI] [PubMed] [Google Scholar]
- 40.Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T, MULTIVIRC Group Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069–1075. doi: 10.1016/S0140-6736(00)04258-6. [DOI] [PubMed] [Google Scholar]
- 41.Halfon P, Imbert-Bismut F, Messous D, et al. A prospective assessment of the inter-laboratory variability of biochemical markers of fibrosis (FibroTest) and activity (ActiTest) in patients with chronic liver disease. Comp Hepatol. 2002;1:3. doi: 10.1186/1476-5926-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poynard T, McHutchison J, Manns M, Myers RP, Albrecht J. Biochemical surrogate markers of liver fibrosis and activity in a randomized trial of peginterferon alfa-2b and ribavirin. Hepatology. 2003;38:481–492. doi: 10.1053/jhep.2003.50319. [DOI] [PubMed] [Google Scholar]
- 43.Forns X, Ampurdanès S, Llovet JM, et al. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36:986–992. doi: 10.1053/jhep.2002.36128. [DOI] [PubMed] [Google Scholar]
- 44.Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 45.Guéchot J, Laudat A, Loria A, Serfaty L, Poupon R. Giboudeau Diagnostic accuracy of hyaluronan and type III procollagen amino-terminal peptide serum assays as markers of liver fibrosis in chronic viral hepatitis C evaluated by ROC curve analysis. Clin Chem. 1996;42:558–563. [PubMed] [Google Scholar]
- 46.Murawaki Y, Ikuta Y, Nishimura Y, Koda M, Kawasaki H. Serum markers for connective tissue turnover in patients with chronic hepatitis B and chronic hepatitis C: a comparative analysis. J Hepatol. 1995;23:145–152. doi: 10.1016/0168-8278(95)80328-9. [DOI] [PubMed] [Google Scholar]
- 47.Arthur MJ. Fibrogenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2000;279:G245–G249. doi: 10.1152/ajpgi.2000.279.2.G245. [DOI] [PubMed] [Google Scholar]
- 48.Chang X, Yamada R, Suzuki A, et al. Localization of peptidylarginine deiminase 4 (PADI4) and citrullinated protein in synovial tissue of rheumatoid arthritis. Rheumatology. 2005;44:40–50. doi: 10.1093/rheumatology/keh414. [DOI] [PubMed] [Google Scholar]
- 49.Dejaco C, Klotz W, Larcher H, Duftner C, Schirmer M, Herold M. Diagnostic value of antibodies against a modified citrullinated vimentin in rheumatoid arthritis. Arthritis Res Ther. 2006;8:R119. doi: 10.1186/ar2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Niki T, De Bleser PJ, Xu G, Van Den Berg K, Wisse E, Geerts A. Comparison of glial fibrillary acidic protein and desmin staining in normal and CCl4-induced fibrotic rat livers. Hepatology. 1996;23:1538–1545. doi: 10.1002/hep.510230634. [DOI] [PubMed] [Google Scholar]
- 51.Niki T, Pekny M, Hellemans K, et al. Class VI intermediate filament protein nestin is induced during activation of rat hepatic stellate cells. Hepatology. 1999;29:520–527. doi: 10.1002/hep.510290232. [DOI] [PubMed] [Google Scholar]
- 52.Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85:307–310. doi: 10.1016/s0092-8674(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 53.Boggs JM, Rangaraj G, Koshy KM, Ackerley C, Wood DD, Moscarello MA. Highly deiminated isoform of myelin basic protein from multiple sclerosis brain causes fragmentation of lipid vesicles. J Neurosci Res. 1999;57:529–535. [PubMed] [Google Scholar]
- 54.Wood DD, Moscarello MA. The isolation, characterization, and lipid-aggregating properties of a citrulline containing myelin basic protein. J Biol Chem. 1989;264:5121–5127. [PubMed] [Google Scholar]
- 55.Pritzker LB, Joshi S, Gowan JJ, Harauz G, Moscarello MA. Deimination of myelin basic protein. 1. Effect of deimination of arginyl residues of myelin basic protein on its structure and susceptibility to digestion by cathepsin D. Biochemistry. 2000;39:5374–5381. doi: 10.1021/bi9925569. [DOI] [PubMed] [Google Scholar]


