Abstract
Background
Genetic variants within the bitter taste receptor gene TAS2R38 are associated with sensitivity to bitter taste and are related to eating behavior in the Amish population. Sensitivity to bitter taste is further related to anthropometric traits in an genetically isolated Italian population. We tested whether the TAS2R38 variants (rs713598; rs1726866 and rs10246939) may be related to eating behavior, anthropometric parameters, metabolic traits and consumer goods intake in the German Sorbs.
Materials and Methods
The three SNPs were genotyped in a total cohort of 1007 individuals (male/female: 405/602). The German version of the three-factor eating questionnaire was completed by 548 individuals. Genetic association analyses for smoking behavior, alcohol and coffee intake, eating behavior factors (restraint, disinhibition and hunger) and other metabolic traits were analyzed. Further, by combining the three SNPs we applied comparative haplotype analyses categorizing PAV (proline-alanine-valine) carriers (tasters) vs. homozygous AVI (alanin-valine-isoleucine) carriers (non-tasters).
Results
Significant associations of genetic variants within TAS2R38 were identified with percentage of body fat, which were driven by associations in women. In men, we observed significant associations with 30 min plasma glucose, and area under the curve for plasma glucose (0–120 min) (all adjusted P≤0.05). Further, we found that carriers of at least one PAV allele show significantly lower cigarette smoking per day (P = 0.002) as well as, albeit non-significant, lower alcohol intake. We did not confirm previously reported associations between genetic variants of TAS2R38 and eating behavior.
Conclusion
Our data suggest that genetic variation in TAS2R38 is related to individual body composition measures and may further influence consumer goods intake in the Sorbs possibly via individual sensitivity to bitter taste.
Introduction
The human gustatory sense is mainly mediated by different taste receptor cells (TRCs) clustered in taste buds predominantly located on the tongue [1]. Further, bitter taste receptors are also expressed in other parts of the gastrointestinal tract [2]. Water-soluble chemicals interact with the apical ends of TRCs resulting in activation of downstream signaling pathways and transmission of signals to the central nervous system [1], [2]. The ability to taste bitterness significantly influences human eating behavior via the development of individual food preferences [2]. In humans, more than 30 different bitter taste receptors have been identified [3]. These G protein-coupled receptors of the TAS2R family are expressed in TRCs on the tongue and in cells of the gastrointestinal mucosa and are involved in energy and glucose metabolism [2], [3]. Sensitivity to bitterness varies between individuals and is associated with genetic variants within genes encoding bitter taste receptors. One of these genes is the well characterized TAS2R38 [4]. Three single-nucleotide polymorphisms (SNPs) (rs713598; rs1726866 and rs10246939) within the TAS2R38 gene, resulting in three amino acid substitutions (A49P; A262V and V296I), are primarily responsible for individual variability in bitter taste perception in response to phenylthiocarbamide (PTC) and 6-n-propyl-2-thiouracil (PROP) [2], [4]. Only two common haplotypes Ala-Val-Ile (AVI) and Pro-Ala-Val (PAV) are known outside sub-Saharan Africa [5], [6]. Heterozygous and homozygous PAV haplotype carriers are more sensitive to PTC and PROP [6], [7] compared to AVI/AVI-carriers. This allows one to classify individuals as tasters or non-tasters based on their genotype [6], [8]. It has been shown that SNPs within TAS2R38 (rs1726866) are nominally associated with the eating behavior phenotype disinhibition in Amish women [8]. Furthermore, in a genetically isolated Italian population it has been shown that among women with low dietary restraint, those classified as non-tasters tend to have higher body mass index (BMI) and waist circumference (WC) than tasters [4].
In addition to the roles outlined above, the family of TAS2R receptors may modulate digestive behavior and glucose homeostasis via signaling pathways e.g. of glucagon like peptide-1 (GLP-1) which is a modulator of insulin biosynthesis and secretion [9], [10]. Dotson and colleagues have shown that genetic variation within several bitter taste receptors significantly influences glucose homeostasis [4], [8].
Lastly, the sensitivity to bitter taste may influence alcohol intake [11] and smoking behavior [12]. As demonstrated by Duffy et al. 2004 [11] homozygous PAV carriers show lower alcohol intake compared to individuals homozygous for AVI. In line with this, Mangold et al. 2008 [13] observed a higher smoking quantity among non-tasters compared to tasters. Further, Cannon et al. 2005 [14] reported differential haplotypic associations and smoking behavior.
Here, we describe the analysis of three TAS2R38 variants and the corresponding haplotypes in a self-contained German population, the Sorbs, in regard to consumer goods consumption as well as eating behavior phenotypes. To shed further light on a potential role of TAS2R38 variants in glucose metabolism, we tested for genetic association with a wide range of metabolic phenotypes.
Methods
Subjects
The Sorbs cohort is a self-contained population from Eastern Germany which was extensively phenotyped for a wide range of anthropometric and metabolic phenotypes [15]. Phenotyping included standardized questionnaires for individual medical history, family histories and eating behavior factors (German version of three factor eating questionnaire, FEV) [16]. Weight, height, waist-to-hip-ratio (WHR), body impedance analysis and a 75 g oral glucose tolerance test (OGTT) after an overnight fast were also included. The main characteristics of the study subjects are summarized in Table 1.
Table 1. Main characteristics of the study cohort.
| total | male | female | rs713598 | rs1726866 | rs10246939 | |||||||
| CC | CG | GG | CC | CT | TT | GG | GA | AA | ||||
| N total | 1007 | 405 | 602 | 155 | 509 | 333 | 173 | 530 | 295 | 169 | 517 | 282 |
| Age | 48±16 | 48±16 | 48±15 | 48±15 | 47±16 | 49±17 | 48±16 | 47±17 | 50±16 | 48±16 | 47±16 | 50±16 |
| BMI (kg/m2) | 27.0±4.9 | 27.2±3.9 | 26.8±5.5 | 27.3±4.4 | 26.5±4.7 | 27.4±5.4 | 27.2±4.4 | 26.5±4.8 | 27.6±5.4 | 27.2±4.4 | 26.5±4.8 | 27.5±5.5 |
| WHR | 0.87±0.1 | 0.95±0.1 | 0.83±0.1 | 0.88±0.1 | 0.87±0.1 | 0.88±0.1 | 0.88±0.1 | 0.87±0.1 | 0.88±0.1 | 0.88±0.1 | 0.87±0.1 | 0.88±0.1 |
| Body Fat (%) | 21.3±9.2 | 18.0±6.9 | 23.5±9.9 | 22.0±8.4 | 20.5±8.5 | 21.9±10.3 | 21.9±8.3 | 20.7±8.9 | 22.1±10.2 | 21.9±8.4 | 20.6±8.9 | 22.0±10.3 |
Data are presented as mean ± SD. Reference allele according to HapMap database, standardized to the reverse strand: rs713598 G; rs1726866 C; rs10246939 A. BMI: body mass index; WHR: waist-to-hip ratio.
Individuals with type 2 diabetes (T2D) were excluded when analyzing eating behavior factors and phenotypes of glucose metabolism. A total of 1007 subjects (male/female: 405/602) with mean age of 48±16.2 years and mean BMI 27.0±4.9 kg/m2 were included. All subjects gave written informed consent and the study was approved by the ethics committee of the University of Leipzig.
German Version of the Three-factor Eating Questionnaire
Of the total cohort 548 sorbs completed the German version of the three-factor eating questionnaire (FEV) [16]. The questionnaire measures the factors (dietary restraint, disinhibition and hunger) influencing individual eating behavior, as described for the Sorbs elsewhere [17].
Genotyping
Genomic DNA was extracted using QIAmp DNA Blood Midi Kit (Qiagen Inc., Valencia, CA, USA) according to manufactureŕs instructions. Genotyping of rs1726866 was performed using TaqMan® SNP Genotyping Assay (Applied Biosystems by Life-Technologies Carlsbad, CA, USA). Rs713598 and rs10246939 were genotyped using the KASPar genotyping system (KBioscience allele specific PCR Genotyping System; KBioscience, Teddington, Middlesex, UK). Fluorescence was detected by an ABI 7500 Real-Time PCR system.
Quality Control
All SNPs were in Hardy-Weinberg equilibrium (all P>0.05) To avoid genotyping errors, a random selection (∼5%) of the sample was re-genotyped; all genotypes matched the initially designated genotypes. Water was used as a no template control (NTC). Of the NTCs, 100% were determined as empty samples.
Statistics
Non-normally distributed data were logarithmically transformed to approximate a normal distribution. Linear regression models adjusted for sex, age and lnBMI were employed to test for association of the three SNPs/haplotypes with eating behavior factors (restraint, disinhibition and hunger), anthropometric traits as well as glucose and insulin metabolism phenotypes. Moreover, sex stratified analyses were conducted. Linear regression models corrected for sex and age were applied to analyze consumer goods consumption (coffee, smoking and alcohol intake). Coffee consumption was defined as cups per day, smoking behavior as number of cigarettes per day and alcohol intake as number of glasses per week. Additive model of inheritance was tested. P-values <0.05 were regarded as providing nominal evidence for association. Given the directed “a priori hypotheses” of the study results were not corrected for multiple comparisons. Haplotypes were reconstructed for population genotype data using PHASE v2.1.1 [18] raised by rs713598 (A49P), rs1726866 (A262V) and rs10246939 (V296I). Three haplotypes were mainly detected: AVI [alanine-valine-isoleucine (55.9%)], PAV [proline-alanine-valine (40.9%)] and AAV (alanine-alanine-valine (2.9%)]. SPSS statistics version 20.0.1 (SPSS, Inc.; Chicago, IL) was used for all statistical analyses.
Results
Association with Anthropometric Measures and Glucose Metabolism
Using linear regression analysis we identified significant associations between the three TAS2R38 genetic variants and percentage of body fat in non-diabetic women (all P≤0.05, Table 2). Minor allele carriers showed lower body fat content compared to homozygous major allele carriers (Table 2). No significant relationships to BMI and other anthropometric measures, such as WHR, were found. Furthermore, albeit non-significant, we observed slightly decreased BMI and body fat content in PAV haplotype carriers (PAV/PAV+PAV/AVI) compared to homozygous AVI haplotype carriers (Table 3).
Table 2. Association analysis of TAS2R38 SNPs with anthropometric measures and phenotypes of glucose and insulin homeostasis.
| TAS2R38 Genotype | |||||||||
| rs713598 | rs1726866 | rs10246939 | |||||||
| Genotype (N) | CC (137) | CG (460) | GG (294) | CC (153) | CT (478) | TT (261) | GG (149) | GA (467) | AA (249) |
| N male | 54 | 185 | 116 | 59 | 194 | 100 | 57 | 190 | 94 |
| N female | 83 | 275 | 178 | 94 | 284 | 161 | 92 | 277 | 155 |
| BMI (kg/m2) | |||||||||
| male | 26.9±3.3 | 26.5±3.5 | 27.1±3.6 | 26.9±3.3 | 26.5±3.6 | 27.2±3.4 | 26.8±3.4 | 26.4±3.4 | 27.2±3.8 |
| female | 26.7±4.7 | 25.9±5.2 | 26.7±5.7 | 26.6±4.6 | 25.9±5.2 | 26.8±5.7 | 26.7±4.7 | 25.9±5.3 | 26.7±5.7 |
| p-value | 0.873°; 0.939* | 0.947°; 0.534* | 0.723°; 0.627* | ||||||
| Body Fat (%) | |||||||||
| male | 17.4±6.0 | 16.8±5.8 | 17.2±5.9 | 17.4±6.0 | 16.9±5.9 | 17.8±6.1 | 17.3±6.1 | 16.8±5.8 | 17.9±6.5 |
| female | 23.8±8.3 | 22.4±9.2 | 23.2±10.7 | 23.5±8.2 | 22.4±9–4 | 23.3±10.8 | 23.7±8.3 | 22.4±9.4 | 23.0±10.7 |
| p-value | 0.023°; 0.909* | 0.013°;0.680* | 0.012°; 0.832* | ||||||
| WHR | |||||||||
| male | 0.94±0.08 | 0.93±0.08 | 0.94±0.08 | 0.94±0.08 | 0.93±0.08 | 0.94±0.08 | 0.94±0.08 | 0.93±0.08 | 0.95±0.09 |
| female | 0.83±0.07 | 0.81±0.08 | 0.82±0.07 | 0.83±0.07 | 0.81±0.07 | 0.83±0.07 | 0.83±0.07 | 0.81±0.07 | 0.82±0.07 |
| p-value | 0.938°; 0.368* | 0.670°; 0.993* | 0.852°; 0.963* | ||||||
| Fasting Plasma Glucose (mmol/L) | |||||||||
| male | 5.44±0.5 | 5.46±0.5 | 5.43±0.5 | 5.44±0.4 | 5.46±0.5 | 5.46±0.4 | 5.41±0.4 | 5.44±0.5 | 5.46±0.4 |
| female | 5.17±0.5 | 5.13±0.5 | 5.21±0.6 | 5.14±0.4 | 5.14±0.5 | 5.22±0.6 | 5.17±0.5 | 5.14±0.5 | 5.21±0.6 |
| p-value | 0.517°; 0.448* | 0.478°; 0.668* | 0.757°; 0.979* | ||||||
| Plasma Glucose 30 min (mmol/L) | |||||||||
| male | 9.13±1.8 | 9.05±1.6 | 8.71±1.4 | 9.08±1.7 | 9.06±1.6 | 8.72±1.4 | 9.02±1.8 | 9.00±1.6 | 8.75±1.4 |
| female | 7.99±1.5 | 8.00±1,6 | 8.28±1.7 | 7.9±1.5 | 8.03±1.6 | 8.30±1.7 | 7.97±1.5 | 8.02±1.6 | 8.26±1.6 |
| p-value | 0.168°; 0.045 * | 0.123°; 0.062* | 0.224°; 0.161* | ||||||
| 2 hr Plasma Glucose (mmol/L) | |||||||||
| male | 5.39±2.0 | 5.23±1.8 | 5.00±1.7 | 5.41±2.1 | 5.11±1.8 | 5.08±1.8 | 5.40±2.1 | 5.08±1.8 | 5.08±1.8 |
| female | 5.78±1.7 | 5.44±1.7 | 5.71±1.6 | 5.72±1.7 | 5.44±1.7 | 5.75±1.6 | 5.78±1.6 | 5.40±1.7 | 5.77±1.6 |
| p-value | 0.836°; 0.052* | 0.747°; 0.153* | 0.759°; 0.979* | ||||||
| Fasting Plasma Insulin (pmol/L) | |||||||||
| male | 40.07±25.9 | 36.55±22.8 | 36.36±21.3 | 39.42±25.2 | 36.45±22.5 | 37.14±23.0 | 38.92±25.1 | 36.33±22.6 | 37.27±22.9 |
| female | 37.87±18.7 | 38.26±23.5 | 41.71±25.0 | 37.03±17.6 | 38.85±23.9 | 42.09±25.4 | 37.60±18.0 | 39.15±24.1 | 41.46±25.4 |
| p-value | 0.314°; 0.150* | 0.226°; 0.109* | 0.402°; 0.172* | ||||||
| Plasma Insulin 30 min (pmol/L) | |||||||||
| male | 273.8±154.0 | 283.9±168.1 | 294.3±208.5 | 275.6±153.4 | 289.4±167.4 | 291.2±220.1 | 267.5±150.8 | 289.6±167.9 | 295.8±222.7 |
| female | 284.4±141.5 | 300.7±179.5 | 298.8±197.1 | 289.8±151.1 | 300.2±182.0 | 299.1±196.0 | 291.3±149.8 | 303.5±185.0 | 291.6±194.3 |
| p-value | 0.898; 0.910* | 0.972°; 0.403* | 0.609°; 0.783* | ||||||
| 2 hrs Plasma Insulin (pmol/L) | |||||||||
| male | 176.1±225.8 | 144.4±160.8 | 128.3±141.7 | 173.4±218.7 | 140.2±160.4 | 135.9±155.1 | 167.8±219.8 | 139.7±160.8 | 136.7±155.4 |
| female | 197.7±133.6 | 187.6±147.6 | 195.4±139.2 | 195.3±128.7 | 187.3±147.1 | 205.3±166.3 | 198.4±127.8 | 189.9±161.4 | 196.2±143.1 |
| p-value | 0.754°; 0.162* | 0.839°; 0.263* | 0.471°; 0.416* | ||||||
| AUC Glucose 0–120 min (mmol/L) | |||||||||
| male | 14.53±2.3 | 14.34±2.5 | 13.82±2.2 | 14.50±2.3 | 14.26±2.5 | 13.89±2.2 | 14.43±2.3 | 14.18±2.4 | 13.92±2.3 |
| female | 13.61±2.6 | 13.36±2.4 | 13.87±2.6 | 13.48±2.5 | 13.39±2.5 | 13.93±2.6 | 13.59±2.5 | 13.35±2.5 | 13.90±2.5 |
| p-value | 0.266°; 0.003 * | 0.191°; 0.013 * | 0.291°; 0.032 * | ||||||
| AUC Insulin 0–120 min (pmol/L) | |||||||||
| male | 397.8±274.2 | 401.5±233.5 | 416.0±289.1 | 415.6±279.5 | 403.9±232.1 | 402.4±290.5 | 403.2±278.7 | 403.6±233.3 | 407.7±298.7 |
| female | 456.7±245.3 | 451.7±250.9 | 442.1±208.6 | 445.5±211.7 | 450.1±248.6 | 463.6±264.9 | 449.5±209.0 | 455.8±260.4 | 449.1±248.1 |
| p-value | 0.820°; 0.377* | 0.845°; 0.226* | 0.698°; 0.415* | ||||||
Data are presented as mean ± SD. Type 2 diabetics were excluded. P-values were calculated using additive model of inheritance using linear regression adjusted for age, sex and lnBMI (except for BMI); ° = female;
= male; BMI: body mass index; WHR: waist-to-hip ratio; AUC: area under the curve. Reference allele according to HapMap database, standardized to the reverse strand: rs713598 G; rs1726866 C; rs10246939 A.
Table 3. TAS2R38 haplotypes: anthropometric measures and phenotypes of glucose and insulin homeostasis.
| AVI/AVI | PAV/AVI+PAV/PAV | P-value | |
| N total (m/f) | 262 (111/151) | 572 (218/354) | |
| BMI (kg/m2) | 26.7±4.8 | 26.2±4.5 | 0.379 |
| Body fat (%) | 20.6±9.3 | 20.5±8.4 | 0.056 |
| WHR | 0.87±0.09 | 0.86±0.09 | 0.724 |
| Fasting plasma glucose(mmol/L) | 5.31±0.58 | 5.25±0.48 | 0.654 |
| Plasma glucose 30 min(mmol/L) | 8.59±1.58 | 8.33±1.67 | 0.112 |
| 2 hr plasma glucose(mmol/L) | 5.56±1.75 | 5.32±1.72 | 0.066 |
| AUC glucose(0–120 min) | 14.10±2.46 | 13.65±2.48 | 0.036 |
| Fasting plasma insulin(pmol/L) | 38.63±22.63 | 38.00±23.54 | 0.994 |
| Plasma insulin 30 min(pmol/L) | 288.54±195.92 | 291.65±170.34 | 0.250 |
| 2 hr plasma insulin(pmol/L) | 176.16±160.83 | 169.73±150.12 | 0.484 |
| AUC insulin(0–120 min) | 430.27±255.47 | 428.32±241.24 | 0.672 |
Data are presented as mean ± S.D. Type 2 diabetics were excluded. P-values were calculated using linear regression adjusted for age, sex and lnBMI (except for BMI). AVI = alanine-valine-isoleucine; PAV = proline-alanine-valine, BMI: body mass index; WHR: waist-to-hip ratio; AUC: area under the curve.
Next, we tested whether TAS2R38 genetic variants influence phenotypes of glucose and insulin metabolism. In non-diabetic men, we found a relationship between genetic variants and 30 min plasma glucose levels as well as area under the curve (AUC) of plasma glucose (0–120 min). We observed increased AUC and glucose levels among minor allele carriers, applying additive model of inheritance (adjusted for sex, age and lnBMI, Table 2). Similar results were obtained when adjusting for age, sex and percentage of body fat. Moreover, we observed decreased 30 min plasma glucose levels and AUC values in PAV allele carriers (Table 3). No relationship was detected between TAS2R38 haplotypes and markers of insulin metabolism.
TAS2R38 May Influence Smoking Behaviour, Alcohol and Coffee Consumption
We tested for associations between SNPs within TAS2R38 and smoking behaviour. No significant relationship was observed. However, carriers of at least one PAV haplotype showed significantly decreased cigarette smoking per day compared to homozygous AVI-carriers (Figure 1, Table 4).
Figure 1. TAS2R38 haplotypes and smoking.
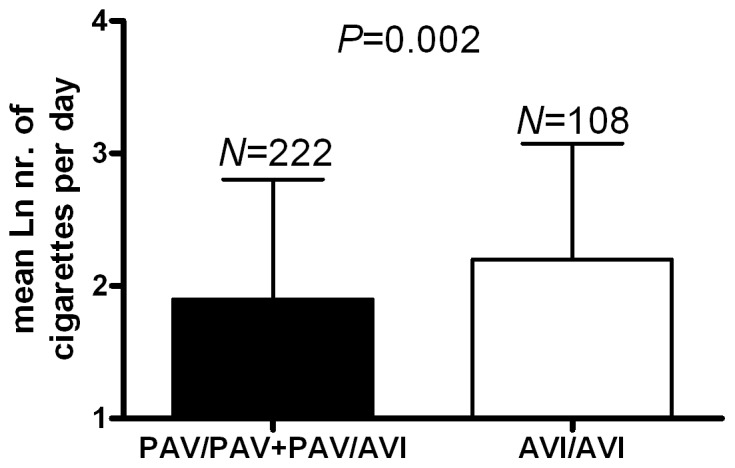
Data are presented as mean ± S.D. P-value was calculated using linear regression adjusted for age and sex. AVI = alanine-valine-isoleucine; PAV = proline-alanine-valine.
Table 4. TAS2R38 haplotypes and consumer goods.
| AVI/AVI | PAV/AVI+PAV/PAV | P-value | |
| N | 108 | 222 | |
| smoking | 12.5±10.1 | 9.3±6.9 | 0.002 |
| N | 306 | 665 | |
| alcohol | 2.87±1.28 | 2.83±1.18 | 0.903 |
| N | 250 | 558 | |
| coffee | 1.82±0.71 | 1.82±0.78 | 0.994 |
Data are presented as mean.± S.D. P-values were calculated using linear regression adjusted for age and sex. AVI = alanine-valine-isoleucine; PAV = proline-alanine-valine.
We did not find effects of single genetic variants on alcohol consumption, however, categorizing individuals carrying at least one PAV haplotype vs. AVI/AVI homozygous carriers, we observed lower alcohol intake per week in the PAV group (Table 4). No associations were found for SNPs/haplotypes and coffee consumption.
TAS2R38 and Eating Behaviour
Linear regression analyses for association of SNPs and haplotypes with the three different eating behaviour factors (restraint, disinhibition and hunger) did not show significant associations (Table S1). However, we observed a non-significant trend for increased restraint scores in PAV allele carriers as well as decreased disinhibition values (Table S2).
Discussion
The main findings of the present study are: (i) a significant relationship of TAS2R38 genetic variation was found with percentage of body fat in women and further, an association with phenotypes related to glucose homeostasis in men. Moreover, we observed (ii) that PAV haplotype carriers show significantly lower tobacco intake per day compared to homozygous AVI haplotype carriers. (iii) We did not confirm the previously reported significant relationship of genetic variation in TAS2R38 with eating-related disinhibition [8].
Our findings in the Sorbs cohort corroborate the previously suggested relationship with body composition measures observed in the isolated residents of Carlantino in southern Italy [4]. Our results are also in line with glucose homeostasis associations demonstrated in the Amish Family Diabetes Study [19]. Besides the relationship between the TAS2R38 variants and body fat content we also found that the three SNPs are related to glucose and insulin metabolism phenotypes as similarly reported for TAS2R7 and TAS2R9 [19]. While we assume these effects are mediated by the bitter taste receptor TAS2R38 on the tongue we cannot rule out the possibility that the receptors expression in other parts of the gastrointestinal tract plays a role and may, in part, mediate the observed relationships of genetic variants and quantitative traits [9], [20].
Supporting the hypothesis that glucose metabolism may be influenced by bitter taste sensitivity, PAV allele carriers showed consistently lower 30 min plasma glucose levels and AUC values compared to homozygous AVI carriers. While non-significant, we observed slightly decreased BMI and percentage of body fat in PAV haplotype carriers. Given that the PAV haplotype has been dubbed ‘PROP tasters’ [5], [6], [11] PAV allele carriers might be more sensitive to PROP tasting than non-carriers. Unfortunately, since PROP tasting sensitivity has not been tested in the Sorbs, we could not specifically test this hypothesis in our cohort. Nevertheless, individuals more sensitive to PROP or PTC (PAV allele carriers) may potentially show more selective eating behavior strategies. We found that individuals more sensitive to bitter taste show, albeit non-significant, higher restraint scores in eating behavior suggesting higher cognitive control. Thus, our data suggest the ability to taste bitterness might potentially influence glucose homeostasis via a more cognitive-based route (i.e. through eating behavior).
As previously reported by others, the ability to taste bitter compounds (PROP or PTC) predicted by TAS2R38 haplotype (PAV) may also influence smoking and alcohol intake [11], [12], [13], [14]. We show that individuals carrying homozygous or heterozygous PAV haplotypes smoke significantly less cigarettes per day than homozygous AVI carriers, in line with the suggestion that these individuals avoid both bitterness and tobacco. Furthermore, our results are consistent with Duffy and colleagues [11], identifying decreased alcohol consumption in individuals carrying at least one PAV haplotype.
In regard to the eating behavior factors restraint, disinhibition and hunger we also confirmed the findings of Tepper et al. [4] which showed lower dietary restraint in non-tasting females. We find the same tendency for lower mean restraint values in the AVI/AVI group, which may be cautiously interpreted as individuals classified as non-tasters. However, we did not find any significant association between the TAS2R38 SNPs or haplotypes and eating behavior factors. Further, we could not confirm the previously reported relationship between rs1726866 and reduced disinhibition scores in women from an Amish population [8]. However, we cannot entirely rule out that differences between the TFEQ [21] and FEV [16] regarding the interpretation of the disinhibition scale may have an impact on the relationship between genetic variation and eating behavior factors. When using the TFEQ there are difficulties in interpreting the factor disinhibition since the theoretical basis of disinhibition is not entirely clarified. According to Westenhöfer [22] disinhibited eating behavior requires former cognitively controlled eating behavior. Therefore, in the German version (FEV) the disinhibition scale is interpreted more as the interference of eating behaviour through emotional and situational circumstances eliminating this shortcoming of the TFEQ questionnaire. However, the same three dimensions of eating behavior factors are measured in both questionnaires.
Additionally, the current study also has several other limitations. The sample size of our cohort, especially the number of individuals with eating behavior data, is limited. Unfortunately, data were not available regarding phenotypic sensitivity to PROP or other bitter compounds, meaning a direct link between bitter tasting and consumer goods consumption cannot be made. Nonetheless, the data in regard to smoking and alcohol intake are in line with data previously reported [11], [12], [13].
In conclusion, our data suggest that, in Sorbs, genetic variation within TAS2R38 may be related to anthropometric measures and glucose homeostasis. Moreover, the ability to taste bitterness may influence the amount of alcohol and tobacco intake.
Supporting Information
TAS2R38 genetic variants and eating behavior factors. Data are presented as means ± SD. Type 2 diabetics were excluded. P-values were calculated using linear regression model adjusted for age, sex and lnBMI.
(DOC)
TAS2R38 haplotypes and eating behavior factors. Data are presented as means ± SD. Type 2 diabetics were excluded. P-values were calculated using linear regression model adjusted for age, sex and lnBMI. AVI = alanine-valine-isoleucine; PAV = proline-alanine-valine.
(DOC)
Acknowledgments
We thank all those who participated in the studies. We would like to acknowledge excellent technical assistance by Ines Müller.
Funding Statement
This work was supported by grants from the German Diabetes Association (to Y.B., A.T., P.K.) and from the DDS Foundation to (Y.B.). A.T. and Y.B. were supported by the IFB AdiposityDiseases; ADI-K50D and ADI-K7-45 (to Y.B.); K7-37 and K403 (to A.T.). M.K. is funded by ADI-K7-39. IFB AdiposityDiseases is supported by the Federal Ministry of Education and Research (BMBF), Germany, FKZ: 01EO1001. X.L. is financed by a research grant from the DFG (BO/3147-4 to Y.B.). This work was further supported by a Collaborative Research Center granted by the DFG (CRC 1052 “Obesity Mechanisms”): A01 (to. M.S.); B03 (to P.K.) and C01 (to A.T.). K.R is funded by a research grant from the Boehringer Ingelheim Foundation. The publication was supported by a publication allowance from the Graduate Center LIFE Sciences of the Research Academy Leipzig at the University of Leipzig, Germany. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Sainz E, Cavenagh MM, Gutierrez J, Battey JF, Northup JK, et al. (2007) Functional characterization of human bitter taste receptors. Biochem J 403: 537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bachmanov AA, Beauchamp GK (2007) Taste receptor genes. Annu Rev Nutr 27: 389–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Janssen S, Laermans J, Verhulst P, Thijs T, Tack J, et al. (2011) Bitter taste receptors and -gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proc Natl Acad Sci U S A 108: 2094–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tepper BJ, Koelliker Y, Zhao L, Ullrich NV, Lanzara C, et al. (2008) Variation in the Bitter-taste Receptor Gene TAS2R38, and Adiposity in a Genetically Isolated Population in Southern Italy. Obesity 16: 2289–2295. [DOI] [PubMed] [Google Scholar]
- 5. Wooding S, Kim U, Bamshad MJ, Larsen J, Jorde LB, et al. (2004) Natural selection and molecular evolution in PTC, a bitter-taste receptor gene. Am J Hum Genet 74: 637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim U, Jorgenson E, Coon H, Leppert M, Risch N, et al. (2003) Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science 299: 1221–1225. [DOI] [PubMed] [Google Scholar]
- 7. Bufe B, Breslin PAS, Kuhn C, Reed DR, Tharp CD, et al. (2005) The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Curr Biol 15: 322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dotson CD, Shaw HL, Mitchell BD, Munger SD, Steinle NI (2010) Variation in the gene TAS2R38 is associated with the eating behavior disinhibition in Old Order Amish women. Appetite 54: 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, et al. (2007) Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A 104: 15069–15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grimm ER, Steinle NI (2011) Genetics of eating behavior: established and emerging concepts. Nutrition Reviews 69: 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Duffy VB, Davidson AC, Kidd JR, Kidd KK, Speed WC, et al. (2004) Bitter receptor gene (TAS2R38), 6-n-propylthiouracil (PROP) bitterness and alcohol intake. Alcohol Clin Exp Res 28: 1629–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Enoch MA, Harris C, Goldman D (2001) Does a reduced sensitivity to bitter taste increase the risk of becoming nicotine addicted. Addict Behav 26: 399–404. [DOI] [PubMed] [Google Scholar]
- 13. Mangold JE, Payne TJ, Ma JZ, Chen G, Li MD (2008) Bitter taste receptor gene polymorphisms are an important factor in the development of nicotine dependence in African Americans. J Med Genet 45: 578–582. [DOI] [PubMed] [Google Scholar]
- 14. Cannon D, Baker T, Piper M, Scholand MB, Lawrence D, et al. (2005) Associations between phenylthiocarbamide gene polymorphisms and cigarette smoking. Nicotine Tob Res 7: 853–858. [DOI] [PubMed] [Google Scholar]
- 15. Veeramah KR, Tönjes A, Kovacs P, Gross A, Wegmann D, et al. (2011) Genetic variation in the Sorbs of eastern Germany in the context of broader European genetic diversity. Eur J Hum Genet 19: 995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pudel D, Westenhöfer J (1989) Fragebogen zum Eßverhalten (FEV). Handanweisung. Göttingen: Hogrefe.
- 17. Breitfeld J, Tönjes A, Gast M, Schleinitz D, Blüher M, et al. (2013) Role of vaspin in human eating behaviour. PLoS ONE 8: e54140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stephens M, Donnelly P (2003) A comparison of bayesian methods for haplotype reconstruction from population genotype data. Am J Hum Genet 73: 1162–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dotson CD, Zhang L, Xu H, Shin Y, Vigues S, et al. (2008) Bitter Taste Receptors Influence Glucose Homeostasis. PLoS ONE 3: e3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Janssen S, Depoortere I (2013) Nutrient sensing in the gut: new roads to therapeutics. Trends Endocrinol Metab 24: 92–100. [DOI] [PubMed] [Google Scholar]
- 21. Stunkard AJ, Messick S (1985) The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res 29: 71–83. [DOI] [PubMed] [Google Scholar]
- 22. Westenhoefer J (1991) Dietary restraint and disinhibition: Is restraint a homogeneous construct. Appetite 16: 45–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TAS2R38 genetic variants and eating behavior factors. Data are presented as means ± SD. Type 2 diabetics were excluded. P-values were calculated using linear regression model adjusted for age, sex and lnBMI.
(DOC)
TAS2R38 haplotypes and eating behavior factors. Data are presented as means ± SD. Type 2 diabetics were excluded. P-values were calculated using linear regression model adjusted for age, sex and lnBMI. AVI = alanine-valine-isoleucine; PAV = proline-alanine-valine.
(DOC)


