Abstract
Background
The well described biological and epidemiologic associations of syphilis and HIV are particularly relevant to the military, as service members are young and at risk for sexually transmitted infections. We therefore used the results of serial serologic testing to determine the prevalence, incidence, and risk factors for incident syphilis in a cohort of HIV-infected Department of Defense beneficiaries.
Methods
Participants with a positive non-treponemal test at HIV diagnosis that was confirmed on treponemal testing were categorized as prevalent cases, whereas participants with an initial negative non-treponemal test who subsequently developed a confirmed positive non-treponemal test as incident cases.
Results
At HIV diagnosis the prevalence of syphilis was 5.8% (n=202). 4239 participants contributed 27,192 person years (PY) to the incidence analysis and 347 (8%) developed syphilis (rate 1.3/100 PY; [1.1, 1.4]). Syphilis incidence was highest during the calendar years 2006 - 2009 (2.5/100 PY; [2.0, 2.9]). In multivariate analyses, younger age (per 10 year increase HR 0.8;[0.8-0.9]); male gender (HR 5.6; [2.3-13.7]); non European-American ethnicity (African-American (HR 3.2; [2.5-4.2]; Hispanic HR 1.9; [1.2-3.0]); history of hepatitis B (HR 1.5; [1.2-1.9]) or gonorrhea (HR 1.4; [1.1 −1.8]) were associated with syphilis.
Conclusions
The significant burden of disease both at and after HIV diagnosis, observed in this cohort, suggests that the cost-effectiveness of extending syphilis screening to at risk military members should be assessed. In addition, HIV infected persons continue to acquire syphilis, emphasizing the continued importance of prevention for positive programs.
Keywords: Seroincidence, Seroprevalence, Risk Factors, Syphilis, HIV infected persons
Background
Syphilis is a sexually transmitted infection (STI) with protean clinical manifestations. In HIV-infected persons syphilis often occurs without overt symptoms 1-2. Historically syphilis has posed a significant threat to military service members 3. With the introduction of penicillin syphilis rates declined in the military. The mid-1980s saw a resurgence of syphilis cases 4, however, rates declined throughout the nineties and in 1999 rates in the US Navy were similar to that observed in civilian settings 5. In the last decade syphilis has reemerged as a significant public health problem, largely fueled by an increase in the number of cases among HIV-infected men who have sex with men (MSM) 6-7. Syphilis increases the risk of both acquiring and transmitting HIV infection 8-9.The biological plausible associations that link syphilis to HIV transmission are of particular relevance to the US military as service members between the ages of 17-24 comprise nearly 40% of the US military, and represent an age group that is at a high risk for STI 10-11. Given the resurgence of syphilis among HIV-infected civilians it is important to define the epidemiology and risk factors associated with the disease in the HIV-infected military population. We utilized serial serologic testing results, to examine the seroprevalence, seroincidence and risk factors associated with incident syphilis in a cohort of HIV-infected US military members.
Material and Methods
To achieve our objectives we examined data from the US Military HIV Natural History Study (NHS) a cohort comprised of HIV-infected Department of Defense (DoD) beneficiaries (active duty service members, retirees, and their dependents) 12-13. HIV infection is documented at NHS entry using a licensed ELISA based assay and confirmed with a Western Blot.
While on active duty HIV positive service members undergo clinical evaluations every 6 months. In keeping with this schedule NHS visits are conducted every 6 months at one of seven military treatment facilities. During these visits participants undergo blood draws (for both study specific tests and tests required for clinical care), individualized risk reduction counseling by a trained technician, are evaluated by a HIV specialist and attend peer education in a group setting. Research personnel collect demographics (including self reported ethnicity), clinical information (including history of STIs), and updated medication history (including treatment for syphilis). Results of all laboratory tests including those performed for clinical care are captured in the NHS database.
Before 2006, screening for syphilis was conducted in accordance with the policies of the treatment facility; since 2006 annual serologic testing has been incorporated as a study-specific test. Syphilis screening is performed using a Nontreponemal (NTr) test primarily the rapid plasma reagin (RPR) test. Positive NTr test results are confirmed using any licensed treponemal (Tr) test. For this sub-study, eligible participants were enrolled in the NHS between 1986 and 2009, and had HIV, NTr and Tr testing results documented in the database. This sub-study was approved by a central institutional review board.
Definitions
To assess the prevalence of syphilis at HIV diagnosis, we examined all participants who had testing performed within a 6-month window of HIV diagnosis. To improve the accuracy of our estimates, we examined a subset of seroconverters (i.e. individuals with documented HIV positive and negative dates within a two-year interval). We next examined the incidence of the first episode of syphilis after HIV diagnosis. All participants with a positive NTr test following an initial negative NTr test that was subsequently confirmed by a positive Tr test result were categorized as an incident case, whereas those with a confirmed positive NTr test at HIV diagnosis were considered a prevalent case. The date of the incident episode was defined as the midpoint between the date of last negative NTr test and date of the first positive NTr test. Non-cases included participants with an initial positive NTr test that was followed by all negative Tr tests (false positives). Syphilis staging have not been captured in the NHS database; hence, we used duration of syphilis infection as an approximation to identify participants early in their course of infection. We defined participants as being “early” in infection if they had a negative NTr test in the 365 days prior to the positive NTr test. Because NTr antibody titer correlates with disease activity, for prevalent cases we considered a titer ≥1:8 as a likely reflection of recent infection 14. Self-reported ethnicity was categorized for analyses as European-American, African-American, Hispanic, or Other. Anti-retroviral therapy (ART) was categorized as none, mono/dual or highly active antiretroviral therapy (HAART). The NHS definition of HAART, defined as the use of three drugs belonging to two or more classes of antiretrovirals or the use of a triple nucleotide regimen, was utilized for this study 15.
Statistical Methods
Descriptive statistics were used to describe the population. Median values are provided with interquartile ranges (IQRs) and compared using the Kruskal-Wallis test. Proportions were compared using either the chi-square or Fisher’s exact tests.
Incidence rates were calculated per 100 person years of follow up (PY), with exact Poisson 95% confidence intervals (CI). Follow up time was calculated from the initial negative NTr test to the time of censoring, defined as the time of incident infection or last NTr test. Poisson regression was used to test for trends in rates by calendar year. Univariate and multivariate Cox proportional hazards methods, stratified by era of diagnosis of HIV infection (pre-1996 vs. 1996 and beyond) and treatment facility, were used to model associations between factors and incident syphilis. Time-updated covariates used all available measurements during the period of observation.
Significance for the multivariate models was defined at P< 0.05; all P values were 2-sided. Age, sex, ethnicity and time from HIV diagnosis were included in the multivariate model, in addition to the variables with a P<0.15 in univariate analyses. Hazard ratios (HRs) and rates are presented with 95% confidence intervals (CIs). The proportional hazards (PH) assumption was assessed for all fixed effects. Analyses were conducted using SAS software, version 9 (SAS Institute). Figures were generated using the R programming language, version 2.10.1. The “loess” and “predict loess” functions in R were used to generate the local linear robust fit smoothing lines and pointwise 95% CIs depicting both the prevalence and incidence of syphilis infection (span=2/3, degree=1) 16 .
Because testing rates varied by enrollment center (supplementary table 1), sensitivity analyses were performed using data from a single center, at which the testing rates have exceeded 90% since the inception of the study.
Results
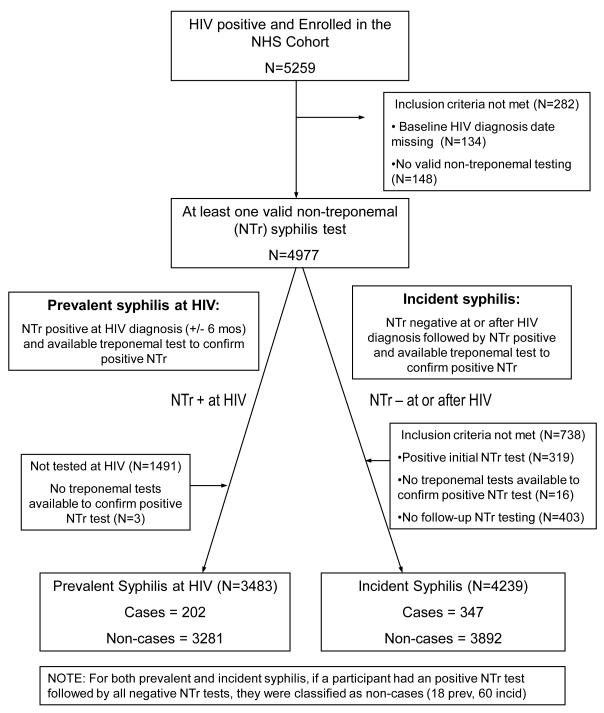
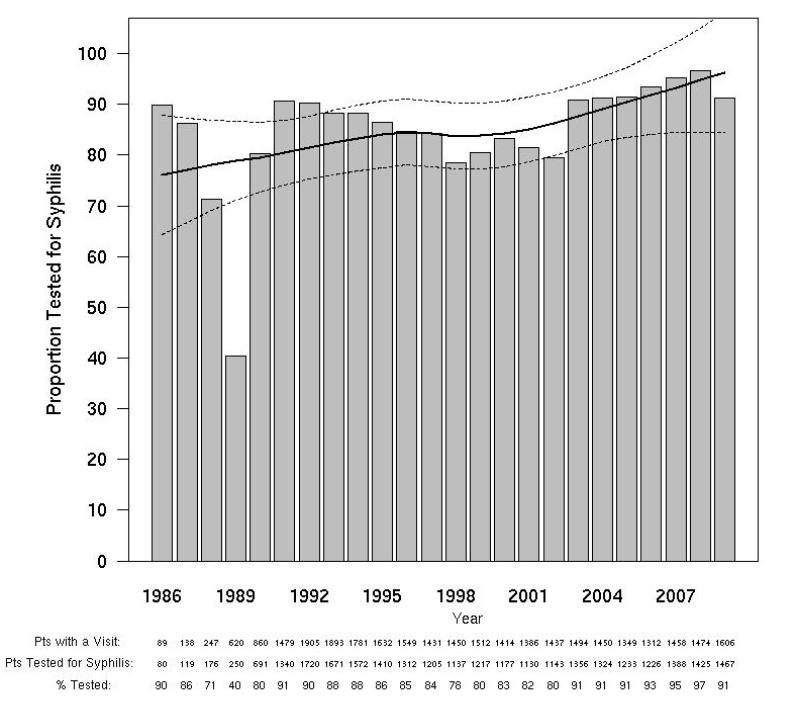
Of the 5125 eligible participants, 4977 (97%) had at least one evaluable NTr test, and were included in this analysis (Figure 1). Study participants were 92% male, 43% European-American, and 44% African-American. The proportion of NHS participants tested for syphilis varied by calendar year and was lowest (78%) in 1998 and since 2003 has exceeded 90% (Figure 2A). The proportion tested for syphilis at the time of HIV diagnosis also varied by calendar year, exceeding 80% since 1993, (Figure 2B).
Figure 1.
Flow diagram describing participant selection for the Incidence and Prevalence analyses
Figure 2A. Syphilis Testing Rates by calendar year.
The solid and dotted lines represent the local linear robust fit smoothing line and the pointwise 95% confidence intervals respectively for the prevalence of syphilis at HIV diagnosis. The shaded bars represent testing rates by year.
Figure 2B. Syphilis Testing Rates at HIV Diagnosis by calendar year.
The solid and dotted lines represent the local linear robust fit smoothing line and the pointwise 95% confidence intervals respectively for the prevalence of syphilis at HIV diagnosis. The shaded bars represent testing rates by year.
Syphilis Prevalence
3486 (70.0%) participants had NTr testing performed at HIV diagnosis, 202 (5.8%) participants, 56 European-American and 123 African-American, had concurrent syphilis. Approximately half the prevalent cases (n=98) were likely recently infected. 1676 HIV seroconverters had NTr testing at HIV diagnosis, 4.1% (n=68) had prevalent disease, 42 (67%) seroconverters met our criteria for recent syphilis infection. Since 2003, the proportion of newly diagnosed HIV infected participants who are also co-infected with syphilis has increased (Figure 3). The majority of the participants diagnosed with syphilis (52%) were likely recently infected as evidenced by a titer ≥1:8 (Figure 3).
Figure 3. Prevalence of syphilis cases at the time of HIV diagnosis and by calendar year.
The solid and dotted lines represent the local linear robust fit smoothing line and the pointwise 95% confidence intervals respectively for the prevalence of syphilis at HIV diagnosis. The dark shaded bars represent subjects with early disease.
Syphilis Incidence
Participants with an initial positive NTr test (n=319), no follow up testing (n=403), or no confirmatory Tr tests following an initial negative NTr test (n=16) were excluded from the incidence analysis (Figure 1). The remaining 4239 participants contributed 27,192 person years (PY) of follow up. The median number of NTr tests per subject was 8 (IQR 5, 16) and the median duration of follow up was 4.9 years (IQR 2.2, 9.0). African-American and European-American participants had similar median numbers of NTr tests [European-American: 8 (IQR 5, 15) and African-American: 9 (IQR 5, 17), p=0.23].
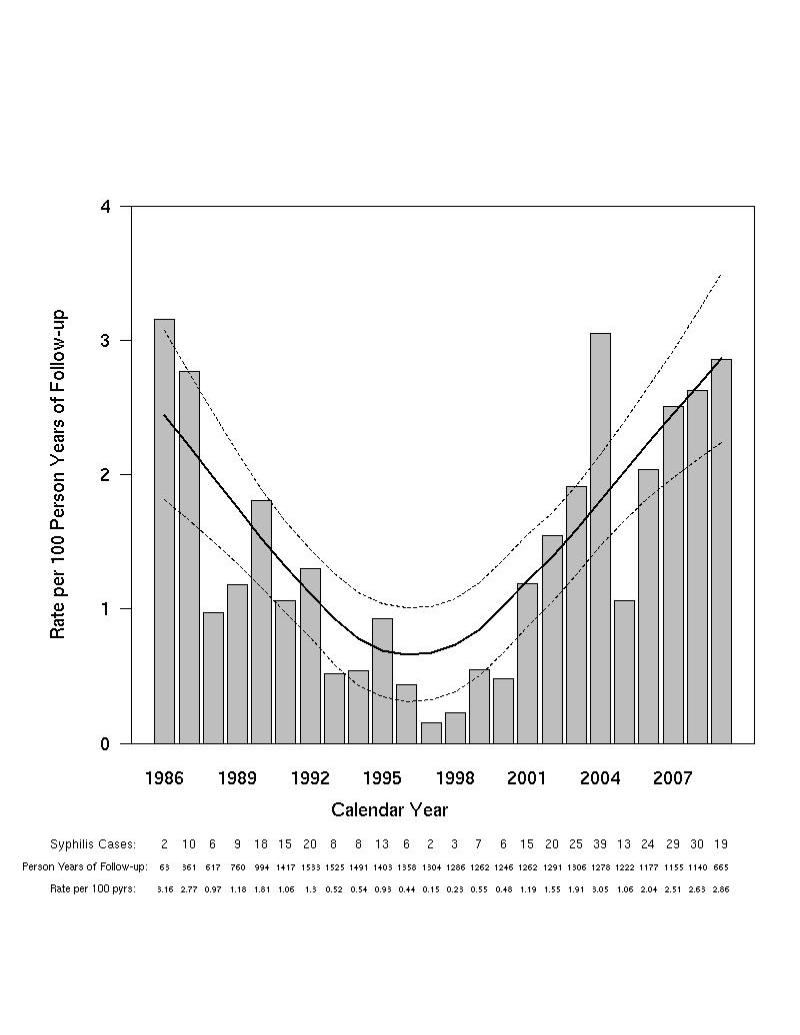
During follow up, 347 participants met the definition of an incident case. Among participants with incident syphilis, 98.6% were male, 68.3% African-American, 21.3% European-American, and 6.7% Hispanic. The median age and CD4 count at syphilis diagnosis were 35.0 years (IQR 28.8, 40.4) and 509 cells/μL (IQR 386, 695) respectively. The median time from HIV diagnosis to syphilis infection was 4.0 years (IQR 1.6, 8.0), (Supplementary Figure 1). Of the participants with incident syphilis, 278 (80.1%) met criteria for early disease. Figure 4 summarizes incidence rates by calendar year for all participants (4A), those with early stage disease (4B), and for African-American and European-American participants (4C).
Figure 4A. Incidence of syphilis after HIV diagnosis and by calendar year.
The solid and dotted lines represent the local linear robust fit smoothing line and the pointwise 95% confidence intervals respectively for the incidence of syphilis after HIV diagnosis.
Figure 4B. Distribution of incident syphilis cases diagnosed with early disease by calendar year.
The solid and dotted lines represent the local linear robust fit smoothing line and the pointwise 95% confidence intervals respectively for the incidence of early syphilis cases after HIV diagnosis.
Figure 4C.
Incidence of syphilis cases after HIV diagnosis among African Americans and European Americans in the NHS
The overall incidence of syphilis was 1.3 /100 PY (95% CI 1.1, 1.4) of follow up. Incidence rates varied across calendar time and race. Rates of incident syphilis were lowest between 1994 and 1999 at 0.5/100 PY (95% CI 0.3, 0.6). A resurgence of syphilis cases was observed during the calendar years 2000-2005 (1.6 /100 PY; 95% CI 1.3, 1.8), and a continued increase has been observed during the calendar years 2006-2009 (2.5 /100 PY; 95% CI 2.0, 2.9), (Figure 4A). The overall incidence of early disease was 1.0/100 PY (95% CI 0.9, 1.1) and exhibited similar trends (Figure 4B). The overall incidence of syphilis was higher among African-Americans (2.1 /100 PY; 95% CI 1.8, 2.4, p<0.0001) and Hispanics (1.1/100 PY; 95% CI 0.7, 1.6, p=0.005) in comparison to European-Americans (0.6 /100 PY; 95% CI 0.4, 0.7). Differences in the incidence of syphilis between European-Americans and African-Americans have been consistently observed over calendar time except more recently, (2008-2009), during which infection rates were not significantly different (European-Americans: 2.0 /100 PY; 95% CI 1.1, 3.0) versus (African-Americans: 3.3 cases/100 PY; 95% CI 1.9, 4.6, p=0.13). Since 2000, syphilis cases have increased in all three ethnic groups, for example, between 2001 and 2009 incident syphilis increased 7 fold among European-Americans (from a rate of 0.53/100 PY to 3.8/100 PY), African-Americans exhibited an approximately 4 fold increase (1.4/100 PY to 5.0/100 PY) and Hispanics had nearly a doubling of the rate (1.1/100 PY to 2.1/100 PY. In a sensitivity analysis, similar trends were observed at the single center where testing rates have exceeded 90% (data not shown).
Risk factors associated with incident syphilis
Because the PH assumption was violated for age, a time-dependent age term was included in the final multivariate model. Risk factors for incident syphilis were younger age (HR 0.82; 95% CI 0.75-0.91), male gender (HR 5.63; 95% CI 2.31-13.73), ethnicity (African-Americans: HR 3.24; 95% CI 2.48-4.24; Hispanic: HR 1.85; 95% CI 1.15-2.96; Other: HR 2.37; 95% CI 1.30-4.29; referent European-Americans), history of hepatitis B (HR 1.52; 95% CI 1.22-1.91), and gonorrhea (HR 1.42; 95% CI 1.12-1.80) (Table 1). HAART use was not associated with disease (HR 0.92; 95% CI 0.69-1.23). HIV specific risk factors including CD4 count, viral RNA levels, and the presence of an AIDS defining illness were not associated with syphilis.
TABLE 1.
Risk Factors Associated With Incident Syphilis
| Characteristic | N | Cases | Person- Years |
Univariate HR |
P | Multivariate HR* |
P |
|---|---|---|---|---|---|---|---|
| Demographics | |||||||
| Age (for every 10-yr increase) |
4239 | 347 (8%) | 27,192 | 0.86 (0.74– 1.00) |
0.05 | 0.82 (0.75– 0.91) |
0.0002 |
| Gender | |||||||
| Male | 3903 | 342 (9%) | 24,951 | 5.97 (2.46– 14.44) |
[lt]0.0001 | 5.63 (2.31– 13.73) |
0.0001 |
| Female | 336 | 5 (1%) | 2241 | Referent | Referent | ||
| Ethnicity | |||||||
| European- American |
1894 | 74 (4%) | 12,886 | Referent | Referent | ||
| African American |
1828 | 237 (13%) |
11,310 | 3.50 (2.69– 4.56) |
[lt]0.0001 | 3.24 (2.48– 4.24) |
[lt]0.0001 |
| Hispanic/Puerto Rican/Mexican |
352 | 23 (7%) | 2033 | 1.85 (1.16– 2.97) |
0.01 | 1.85 (1.15– 2.96) |
0.01 |
| Other | 163 | 13 (8%) | 959 | 2.20 (1.21– 3.97) |
0.009 | 2.37 (1.30– 4.29) |
0.005 |
| HIV-specific characteristics Antiretroviral therapy† |
|||||||
| None | 1185 | 154 (13%) |
3967 | Referent | Referent | ||
| Mono-Dual therapy |
1136 | 45 (4%) | 5593 | 0.65 (0.46– 0.92) |
0.01 | 0.72 (0.50– 1.04) |
0.08 |
| HAART | 1918 | 148 (8%) | 17,632 | 0.75 (0.57–1.0) |
0.05 | 0.92 (0.69– 1.23) |
0.58 |
| CD4 cell count† (cells/uL) |
|||||||
| [lt]200 | 943 | 21 (2%) | 5293 | 0.61 (0.38– 0.97) |
0.04 | — | — |
| 200–499 | 1503 | 146 (10%) |
9583 | 0.96 (0.77– 1.20) |
0.72 | — | — |
| [mtequ]500 | 1756 | 179 (10%) |
12,304 | Referent | |||
| Nadir CD4† (for 100 cell increase) |
4202 | 346 (8%) | 27,180 | 1.06 (1.00– 1.11) |
0.04 | 1.02 (0.96– 1.08) |
0.5 |
| HIV RNA† (log10 copies/mL) (for each log increase) AIDS-defining illness†‡ |
3152 | 247 (8%) | 24,102 | 1.05 (0.94– 1.18) |
0.36 | — | — |
| Yes | 847 | 22 (3%) | 6177 | 0.69 (0.44– 1.07) |
0.10 | 0.69 (0.43– 1.11) |
0.12 |
| No | 3392 | 325(10%) | 21,016 | Referent | — | — | |
| Years from HIV diagnosis |
4239 | 347 (8%) | 27,192 | 0.94 (0.87– 1.02) |
0.13 | 0.93 (0.85– 1.01) |
0.09 |
| Other sexually transmitted infections Hepatitis B† |
|||||||
| Yes | 1890 | 185 (10%) |
14,120 | 1.71 (1.37– 2.13) |
[lt]0.0001 | 1.52 (1.22– 1.91) |
0.0003 |
| No | 2349 | 162 (7%) | 13,072 | Referent | Referent | ||
| Gonorrhea† | |||||||
| Yes | 1129 | 130 (12%) |
8209 | 1.89 (1.50– 2.38) |
[lt]0.0001 | 1.42 (1.12– 1.80) |
0.004 |
| No | 3085 | 216 (7%) | 18,896 | Referent | Referent |
Model is stratified by the era of HIV-positive test (pre-1996, post-1996) and enrollment center.
Time varying covariate, therefore the columns contain the number in each group at censoring (i.e., incident infection or last NTr).
AIDS-defining conditions based on the 1993 CDC criteria excluding CD4 count.
Discussion
Using results of serial serologic testing to identify syphilis cases, this longitudinal twenty five year study among HIV-infected DoD beneficiaries estimated an overall prevalence of syphilis at HIV diagnosis of ~ 6% and subsequent incidence of 1.3 cases/100 PY of follow up.
To our knowledge this is the first study that examines the seroincidence of syphilis over a 25 year period. Compared to other studies in HIV-infected persons, seroincident syphilis rates in the NHS are lower 1, 17-18, and are comparable to case-based estimates from a managed care setting in the US 19. NHS participants have unrestricted access to healthcare and medications, undergo close clinical monitoring, report low rates of illicit drug use, and possess either a minimum of a high school educational equivalent (enlisted) or an undergraduate college degree (officers) 12-13. Further, active duty members of this cohort have stable employment. As low rates of drug use and improved access to health care have been associated with a lower risk of acquiring STIs 20-25; we postulate that the close clinical monitoring and unrestricted access to healthcare and medications that NHS participants enjoy may in part explain the lower incidence of syphilis observed in this study. Reliance on serologic testing to derive estimates reduces the likelihood that the lower incidence is caused by participants seeking care outside the military health care system, as declines in titers following treatment would be captured at subsequent testing, but does not eliminate the possibility. HIV-infected MSM are disproportionately affected by syphilis and have been the primary focus of studies examining the seroincidence of syphilis 1, 17-18. Hence, we compared our rates to published reports among HIV-infected MSM. Although ascertainment of sexual behaviors can be difficult in the military, when last examined, in the early nineties, a third of the surveyed NHS participants reported having sex with women exclusively 12. If the cohort composition has remained the same (namely 2/3rd MSM) it may represent an alternative explanation for our lower syphilis incidence.
In the NHS ethnic minorities shared a greater burden of syphilis, however, these differences are not as pronounced as that observed nationwide 26. For example, African-American men nationwide were 8 times more likely than European-American men to have a diagnosis of primary or secondary syphilis in 2009, whereas in the NHS we found that African-American men were 1.2 times more likely to be diagnosed with syphilis in that year 26. The narrowing of ethnic disparities in the NHS in recent years has not been driven by declines in syphilis cases among African-Americans but rather by increases in syphilis cases among European-Americans. In concordance with the nationwide data we observed increases in incident syphilis rates among Hispanics, however, our sample size for this subset was small 27. Our results suggest to stem the tide of new syphilis infections in the DoD effective prevention must be targeted to at risk groups and appropriate within varying racial/ethnic contexts.
Factors associated with incident syphilis in this study are similar to those reported previously 28-29. Younger age, non European-American ethnicity, male sex, and a prior history of STI were associated with an increased risk of syphilis acquisition. Some studies suggest that in the HAART era HIV-infected men are less concerned about virus transmission thereby increasing sexual risk taking 30-32. Our results did not find a significant association between syphilis acquisition and HAART use and are in agreement with two large studies which also failed to demonstrate an association with HAART use 2, 19.
Historically results of syphilis screening were used to determine military eligibility 3. The changes mandated with the introduction of the Clinical Laboratory Improvement Act led military investigators to examine the cost-effectiveness of universal syphilis screening 33. The study concluded that universal syphilis screening was not cost-effective, and screening was discontinued 33. However, this study was conducted in the nineties when syphilis rates were declining both nationwide and in the US military 5. Since 2000 there has been a resurgence of syphilis cases in HIV-infected persons, our results demonstrate similar trends in the military HIV population 26. A recent analysis of the US military also showed an increase in syphilis rates in 2008-2009 34. Taken together these observations suggest that the current screening policies should be reviewed and the cost-effectiveness of periodic syphilis screening of at risk military members both at and after enlistment needs examination.
One of the strengths of this analysis is the greater precision of our estimates due to the reliance on serologic testing to derive estimates, however, this strategy too has limitations 35. Serologic testing has reduced sensitivity in very early disease and may fail to capture subjects with early treated disease 36. Further, incident syphilis is best characterized by measuring rates of early stage disease (i.e. primary and secondary) as latent infection may have been acquired years earlier. As syphilis staging was not captured in the NHS, we tried to overcome this limitation by defining early and late stage disease by measuring duration of infection but misclassification of cases may have occurred, which might affect our estimates. Other limitations of this study include the fact that the prior policy of “Don’t ask Don’t tell” limited our ability to capture patterns of sexual activity in this cohort as well as the need to estimate the date of syphilis seroconversion due to the periodic nature of syphilis screening. We acknowledge that the failure to adjust for risk behavior might have influenced our point estimates. To address the latter, a parallel analysis of time to incident syphilis with syphilis infection defined as the date of the first positive test found qualitatively similar results. Lastly, the proportion of participants tested in this cohort varied by calendar year and center and this missing data has the potential for introducing bias. Overall 2.5% of the participants never had a syphilis test performed and 30% of the participants did not have a test for syphilis performed within 6 months of HIV diagnosis, although in recent years the proportions of participants tested for syphilis have exceeded 90%. To address this concern, the incidence of syphilis was examined using only data from a center at which the proportion tested exceeded 90% throughout the study period and we found the pattern of incidence over the years to be similar.
In conclusion, rates of incident syphilis, in this cohort, continue to increase despite equal access to care and low rates of drug use. Acquisition of syphilis in HIV-infected persons is indicative of unsafe behavior and points to the continued need for prevention in positive programs in the military. Overall African-Americans shared a disproportionate burden of this disease, however, in recent years there has been a marked increase in new infections within our European-American and Hispanic participants. Additional research to understand what risk reduction interventions will work for this and other HIV-positive populations is warranted. The significant burden of syphilis identified at the time of HIV diagnosis in this cohort suggests that the cost-effectiveness of targeted screening of at-risk military members periodically after enlistment needs study.
Supplementary Material
Supplementary Figure 1- Histogram illustrating the time from HIV diagnosis to onset of Syphilis infection
Acknowledgements
The Infectious Disease Clinical Research Program HIV Working Group is comprised of: Susan Banks RN, Irma Barahona RN, CAPT Mary Bavaro MD, Helen Chun MD, Cathy Decker MD, Lynn Eberly PhD, Conner Eggleston, LTC Tomas Ferguson, COL Susan Fraser MD, MAJ Joshua Hartzell MD, MAJ Joshua Hawley, LTC Gunther Hsue, Arthur Johnson MD, COL Mark Kortepeter MD MPH, Michelle Linfesty, Scott Merritt, LTC Robert O’Connell MD, Cpt Jason Okulicz MD, Sheila Peel PhD, Michael Polis MD, John Powers MD, MAJ Roseanne Ressner MD, , ret Col Edmund Tramont, LT Tyler Warkentien, MAJ Paige Waterman MD, Amy Weintrob MD, Timothy Whitman MD, and LTC Michael Zapor MD. We would also like to specifically acknowledge William Bradley for his assistance with data acquisition.
Support for this work (IDCRP-000-26) was provided by the Infectious Disease Clinical Research Program (IDCRP), a Department of Defense (DoD) program executed through the Uniformed Services University of the Health Sciences. This project has been funded in whole, or in part, with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), under Inter-Agency Agreement Y1-AI-5072.
Footnotes
Disclaimer: The content of this publication is the sole responsibility of the authors and does not necessarily reflect the views or policies of the NIH or the Department of Health and Human Services, the DoD or the Departments of the Army, Navy or Air Force. Mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. Government.
“We certify that all individuals who qualify as authors have been listed; each has participated in the conception and design of this work, the analysis of data (when applicable), the writing of the document, and the approval of the submission of this version; that the document represents valid work; that if we used information derived from another source, we obtained all necessary approvals to use it and made appropriate acknowledgements in the document; and that each takes public responsibility for it.”
“Some authors on this paper are military service members and/or employees of the U.S. Government. As such, this work was prepared as part of official duties. Title 17 U.S.C. 105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C. 101 defines a United States Government work as a work prepared by a military service member or employee of the United States Government as part of that person’s official duties.”
This work was presented in part at the 16th Conference on Retrovirus and Opportunistic Infections, Montreal, February 16-19, 2009; Abstract number 1057.
Author Contributions: All authors have reviewed and approved this manuscript.
Anuradha Ganesan and Ann Fieberg had full access to all the data and take responsibility for the accuracy of the data.
Study concept and design: Anuradha Ganesan, Brian Agan
Acquisition of the data: Ganesan, Crum-Cianflone, Landrum, Lalani, Wortmann
Drafting of the manuscript: Ganesan, Fieberg, Agan, and Macalino
Critical review of the manuscript: Wortmann, Landrum, Lalani, Crum-Cianflone, and Lifson
Obtaining funding: Ganesan and Agan
Study supervision: Ganesan
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Branger J, van der Meer JT, van Ketel RJ, Jurriaans S, Prins JM. High incidence of asymptomatic syphilis in HIV-infected MSM justifies routine screening. Sex Transm Dis. 2009;36:84–5. doi: 10.1097/OLQ.0b013e318186debb. [DOI] [PubMed] [Google Scholar]
- 2.Thurnheer MC, Weber R, Toutous-Trellu L, et al. Occurrence, risk factors, diagnosis and treatment of syphilis in the prospective observational Swiss HIV Cohort Study. AIDS. 2010;24:1907–16. doi: 10.1097/QAD.0b013e32833bfe21. [DOI] [PubMed] [Google Scholar]
- 3.Rasnake MS, Conger NG, McAllister K, Holmes KK, Tramont EC. History of U.S. military contributions to the study of sexually transmitted diseases. Mil Med. 2005;170:61–5. doi: 10.7205/milmed.170.4s.61. [DOI] [PubMed] [Google Scholar]
- 4.Dembert ML, Finney LA, Berg SW. Epidemiology of reported syphilis among U.S. Navy and Marine Corps personnel, 1985-1987. Sex Transm Dis. 1990;17:95–8. doi: 10.1097/00007435-199004000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Thomas RJ, MacDonald MR, Lenart M, Calvert WB, Morrow R. Moving toward the eradication of syphilis. Mil Med. 2002;167:489–95. [PubMed] [Google Scholar]
- 6.Williams LA, Klausner JD, Whittington WL, Handsfield HH, Celum C, Holmes KK. Elimination and reintroduction of primary and secondary syphilis. Am J Public Health. 1999;89:1093–7. doi: 10.2105/ajph.89.7.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Primary and secondary syphilis among men who have sex with men--New York City, 2001. MMWR Morb Mortal Wkly Rep. 2002;51:853–6. [PubMed] [Google Scholar]
- 8.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75:3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchacz K, Klausner JD, Kerndt PR, et al. HIV incidence among men diagnosed with early syphilis in Atlanta, San Francisco, and Los Angeles, 2004 to 2005. J Acquir Immune Defic Syndr. 2008;47:234–40. [PubMed] [Google Scholar]
- 10.Weinstock H, Berman S, Cates W., Jr. Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health. 2004;36:6–10. doi: 10.1363/psrh.36.6.04. [DOI] [PubMed] [Google Scholar]
- 11.Lee SE, Nauschuetz W, Jordan N, et al. Survey of sexually transmitted disease laboratory methods in US Army laboratories. Sex Transm Dis. 2010;37:44–8. doi: 10.1097/OLQ.0b013e3181b66dd6. [DOI] [PubMed] [Google Scholar]
- 12.Brodine SK, Starkey MJ, Shaffer RA, et al. Diverse HIV-1 subtypes and clinical, laboratory and behavioral factors in a recently infected US military cohort. AIDS. 2003;17:2521–7. doi: 10.1097/00002030-200311210-00016. [DOI] [PubMed] [Google Scholar]
- 13.Marconi VC, Grandits GA, Weintrob AC, et al. Outcomes of highly active antiretroviral therapy in the context of universal access to healthcare: the U.S. Military HIV Natural History Study. AIDS Res Ther. 2010;7:14. doi: 10.1186/1742-6405-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottlieb SL, Pope V, Sternberg MR, et al. Prevalence of syphilis seroreactivity in the United States: data from the National Health and Nutrition Examination Surveys (NHANES) 2001-2004. Sex Transm Dis. 2008;35:507–11. doi: 10.1097/OLQ.0b013e3181644bae. [DOI] [PubMed] [Google Scholar]
- 15.Weintrob AC, Grandits GA, Agan BK, et al. Virologic response differences between African Americans and European Americans initiating highly active antiretroviral therapy with equal access to care. J Acquir Immune Defic Syndr. 2009;52:574–80. doi: 10.1097/QAI.0b013e3181b98537. [DOI] [PubMed] [Google Scholar]
- 16.Cleveland W. Robust locally weighted regression and smoothing scatterplots. J Amer Statist Assoc. 74:829–36. [Google Scholar]
- 17.Ivens D, Patel M. Incidence and presentation of early syphilis diagnosed in HIV-positive gay men attending a central London outpatients’ department. Int J STD AIDS. 2005;16:201–2. doi: 10.1258/0956462053420202. [DOI] [PubMed] [Google Scholar]
- 18.Jin F, Prestage GP, Zablotska I, et al. High incidence of syphilis in HIV-positive homosexual men: data from two community-based cohort studies. Sex Health. 2009;6:281–4. doi: 10.1071/SH09060. [DOI] [PubMed] [Google Scholar]
- 19.Horberg MA, Ranatunga DK, Quesenberry CP, Klein DB, Silverberg MJ. Syphilis epidemiology and clinical outcomes in HIV-infected and HIV-uninfected patients in Kaiser Permanente Northern California. Sex Transm Dis. 2010;37:53–8. doi: 10.1097/OLQ.0b013e3181b6f0cc. [DOI] [PubMed] [Google Scholar]
- 20.Adimora AA, Schoenbach VJ. Social context, sexual networks, and racial disparities in rates of sexually transmitted infections. J Infect Dis. 2005;191(Suppl 1):S115–22. doi: 10.1086/425280. [DOI] [PubMed] [Google Scholar]
- 21.Edlin BR, Irwin KL, Faruque S, et al. Intersecting epidemics--crack cocaine use and HIV infection among inner-city young adults. Multicenter Crack Cocaine and HIV Infection Study Team. N Engl J Med. 1994;331:1422–7. doi: 10.1056/NEJM199411243312106. [DOI] [PubMed] [Google Scholar]
- 22.Fullilove MT, Golden E, Fullilove RE, 3rd, et al. Crack cocaine use and high-risk behaviors among sexually active black adolescents. J Adolesc Health. 1993;14:295–300. doi: 10.1016/1054-139x(93)90177-q. [DOI] [PubMed] [Google Scholar]
- 23.Geisler WM, Chyu L, Kusunoki Y, Upchurch DM, Hook EW., 3rd Health insurance coverage, health care-seeking behaviors, and genital chlamydial infection prevalence in sexually active young adults. Sex Transm Dis. 2006;33:389–96. doi: 10.1097/01.olq.0000194584.80513.4a. [DOI] [PubMed] [Google Scholar]
- 24.Hogben M, Leichliter JS. Social determinants and sexually transmitted disease disparities. Sex Transm Dis. 2008;35:S13–8. doi: 10.1097/OLQ.0b013e31818d3cad. [DOI] [PubMed] [Google Scholar]
- 25.Characteristics associated with HIV infection among heterosexuals in urban areas with high AIDS prevalence --- 24 cities, United States, 2006-2007. MMWR Morb Mortal Wkly Rep. 2011;60:1045–9. [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention [accessed May 20, 2011];Sexually Transmitted Diseases Surveillance-Syphilis. 2009 http://www.cdc.gov/std/stats09/tables/34b.htm.
- 27.Su JR, Beltrami JF, Zaidi AA, Weinstock HS. Primary and secondary syphilis among black and Hispanic men who have sex with men: case report data from 27 States. Ann Intern Med. 2011;155:145–51. doi: 10.7326/0003-4819-155-3-201108020-00004. [DOI] [PubMed] [Google Scholar]
- 28.Baffi CW, Aban I, Willig JH, Agrawal M, Mugavero MJ, Bachmann LH. New syphilis cases and concurrent STI screening in a southeastern U.S. HIV clinic: a call to action. AIDS Patient Care STDS. 2010;24:23–9. doi: 10.1089/apc.2009.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li D, Jia Y, Ruan Y, et al. Correlates of incident infections for HIV, syphilis, and hepatitis B virus in a cohort of men who have sex with men in Beijing. AIDS Patient Care STDS. 2010;24:595–602. doi: 10.1089/apc.2010.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katz MH, Schwarcz SK, Kellogg TA, et al. Impact of highly active antiretroviral treatment on HIV seroincidence among men who have sex with men: San Francisco. Am J Public Health. 2002;92:388–94. doi: 10.2105/ajph.92.3.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stolte IG, Dukers NH, Geskus RB, Coutinho RA, de Wit JB. Homosexual men change to risky sex when perceiving less threat of HIV/AIDS since availability of highly active antiretroviral therapy: a longitudinal study. AIDS. 2004;18:303–9. doi: 10.1097/00002030-200401230-00021. [DOI] [PubMed] [Google Scholar]
- 32.Ostrow DE, Fox KJ, Chmiel JS, et al. Attitudes towards highly active antiretroviral therapy are associated with sexual risk taking among HIV-infected and uninfected homosexual men. AIDS. 2002;16:775–80. doi: 10.1097/00002030-200203290-00013. [DOI] [PubMed] [Google Scholar]
- 33.Clark KL, Kelley PW, Mahmoud RA, et al. Cost-effective syphilis screening in military recruit applicants. Mil Med. 1999;164:580–4. [PubMed] [Google Scholar]
- 34.Armed Forces Health Surveillance. Medical Surveillance Monthly report. Aug, 2010.
- 35.Center for Disease Control [accessed on June 17,2011];Program Operation Guidelines for STD prevention, surveillance and data management. http://www.cdc.gov/std/program/Surveillance.pdf.
- 36.Creegan L, Bauer HM, Samuel MC, Klausner J, Liska S, Bolan G. An evaluation of the relative sensitivities of the venereal disease research laboratory test and the Treponema pallidum particle agglutination test among patients diagnosed with primary syphilis. Sex Transm Dis. 2007;34:1016–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1- Histogram illustrating the time from HIV diagnosis to onset of Syphilis infection