Abstract
Studies of conceptual processing have revealed that the prefrontal cortex is implicated in close‐ended, deliberate memory retrieval, especially the left ventrolateral prefrontal regions. However, much of human thought—particularly that which is characterized as creative—requires more open‐ended, spontaneous memory retrieval. To explore the neural systems that support conceptual processing under these two distinct circumstances, we obtained functional magnetic resonance images from 24 participants either while retrieving the common use of an everyday object (e.g., “blowing your nose,” in response to a picture of a tissue) or while generating a creative (i.e., uncommon but plausible) use for it (e.g., “protective padding in a package”). The patterns of activation during open‐ and closed‐ended tasks were reliably different, with regard to the magnitude of anterior versus posterior activation. Specifically, the close‐ended task (i.e., Common Use task) reliably activated regions of lateral prefrontal cortex, whereas the open‐ended task (i.e., Uncommon Use task) reliably activated regions of occipito‐temporal cortex. Furthermore, there was variability across subjects in the types of responses produced on the open‐ended task that was associated with the magnitude of activation in the middle occipital gyrus on this task. The present experiment is the first to demonstrate a dynamic tradeoff between anterior frontal and posterior occipitotemporal regions brought about by the close‐ or open‐ended task demands. Hum Brain Mapp, 2011. © 2010 Wiley‐Liss, Inc.
Keywords: cognitive flexibility, lateral occipital‐temporal cortex, inferior frontal gyrus, creative object use, semantic knowledge
INTRODUCTION
The left prefrontal cortex (PFC) is believed to facilitate the formation of experience‐derived schemas and hypotheses [i.e., mental models; Rumelhart and Ortony, 1977] that are critical for everyday cognition by allowing the prediction of future events. On the other hand, one's “habitual ways” of organizing the world can occasionally impede flexible thought. Thus, although ordinary cognitive processes are characterized by remarkable flexibility and are thought to lie at the heart of all creative endeavors [Klahr and Simon, 1999; Weisberg, 2006], under certain circumstances, there appears to be a tradeoff between one's tendency to employ mental models and one's ability to adopt optimal alternative strategies for goal achievement [Heilman et al., 2003]. One such example is the case of probability matching: While guessing the probability that one of two events will occur (e.g., whether a red light will appear on the left or right side of a screen), healthy adults tend to frequency‐match (i.e., match the probability of the responses to that of the events) and not maximize (i.e., constantly choose the most frequent option), even though maximizing guarantees higher success [Wolford et al., 2000]. For example, if the target appears on the right of the screen three quarters of the time, healthy adult subjects will tend to “match” those probabilities and guess that it appears on the right 75% of the time and on the left 25% of the time; however, to provide the maximum number of correct responses, the best strategy would be to select “right” for all the trials. Interestingly, in similar tasks, patients with left PFC damage and split‐brain patients (with information presented to the right hemisphere) follow the optimal maximizing strategy and do not probability match [Wolford et al., 2000; see also Apperly et al., 2004]. Similarly, patients with focal PFC injuries have been reported to outperform normal participants on certain aspects of problem‐solving that require a reassessment of predetermined hypotheses about task constraints [Reverberi et al., 2005].
Why would damage to PFC be associated with better performance on certain tasks? It has been argued that during PFC development, the construction of mental models is supported by the dynamic inhibition of low‐level, raw data—processed in primary cortical regions—that results from increased top–down influences [Snyder et al., 2003; see also Bunge et al., 2002 and Fair et al., 2007]. In the absence of such inhibition due to PFC underdevelopment or injury, this low‐level information may prevail and facilitate performance on tasks that would benefit from such information. For example, atypical development of the left PFC (e.g., in autism) is linked with access to normally unavailable low‐level information (e.g., absolute pitch) that may allow some autistic children to become artistic, musical, or mathematical savants [Snyder, 2009; Snyder et al., 2003]. In fact, temporarily disrupting top–down influences through rapid transcranial magnetic stimulation over the left frontotemporal lobe has elicited short‐term savant‐like skills in normal subjects [e.g., absolute number estimation; Snyder et al., 2003, 2006]. The spontaneous development of such abilities has also been reported in patients after the onset of frontotemporal dementia (FTD). At the early stages of FTD, some patients paradoxically develop new artistic and musical skills not possessed before the onset of the disease [Finney and Heilman, 2007; Seeley et al., 2008].
The link between flexible thinking and diminished PFC functioning (henceforth “hypofrontality”) has its roots in early electroencephalogram (EEG) studies with healthy adults using tasks of ideational fluency [e.g., the Alternative Uses Task (AUT); Christensen and Guilford, 1958], successful performance at which has been associated with higher alpha‐band activity [i.e., lower arousal; Martindale and Hasenfus, 1978]. Recent work has confirmed these findings, by demonstrating that such tasks induce more complex EEG response patterns in central and posterior cortical regions, but less complex patterns in PFC regions that might serve as evidence for lower attentional control [Mölle et al., 1999]. Changes in PFC functioning may also explain some pharmacologically induced enhancements on tasks requiring broad associations [e.g., with beta adrenergic blockers; Alexander et al., 2007]. Current neuroscientific studies with normal participants have explored various aspects of creative cognition, including the extent of right‐lateralized hemispheric asymmetry and lower cortical arousal in insight solutions [Kounios et al., 2006, 2008], the relationship between hemispheric asymmetry and performance in open‐ended tasks [Carlsson et al., 2000; Howard‐Jones et al., 2005], and semantic priming in creative association tasks [Faust and Lavidor, 2003]. These studies report either higher or lower unilateral or bilateral PFC activations, results that are likely due to tasks requiring different degrees of cognitive control over conceptual knowledge networks as facilitated by the left ventrolateral PFC [Badre and Wagner, 2007; Thompson‐Schill et al., 1997, 1999]. However, the link between flexible knowledge retrieval and hypofrontality in healthy adults has not been addressed directly. Importantly, previous studies have not examined how the PFC is dynamically engaged or, critically, disengaged during conceptual retrieval depending on the requirements of a given task. One exception is a recent fMRI study that explored whether learned music sequences or improvisation are associated with different patterns of brain activation in professional jazz musicians [Limb and Braun, 2008]. Although the study focused on music expertise and there may be some difficulties in generalizing the findings to nonprofessional musicians or other tasks, it showed that improvisation was associated with a consistent pattern of deactivation in dorsolateral prefrontal and lateral orbitofrontal regions bilaterally and increased activation in right and left medial prefrontal (frontopolar) cortex.
A potentially useful distinction that can be drawn between tasks associated with PFC involvement and tasks that appear to benefit from its disengagement is the extent to which a given task is close‐ended (i.e., it has either one or a finite number of possible responses, e.g., anagrams) or open‐ended (i.e., it has an infinite number of possible responses, e.g., the Alternative Uses Task). Depending on the characterization of a task on this dimension, there might be a tradeoff between prefrontal and posterior cortical regions such that close‐ended tasks may require PFC involvement and the disengagement of posterior regions whereas open‐ended tasks may gain from posterior cortical involvement and the disengagement of prefrontal regions [Dietrich, 2004; Limb and Braun, 2008]. To explore this prediction, we administered to one group of participants a close‐ended task (i.e., generating the typical, common use in response to pictures of everyday objects) and to another group of participants an open‐ended task (i.e., generating an atypical, uncommon use in response to pictures of everyday objects), in a randomized fMRI paradigm to examine whether these tasks would lead to different types of conceptual retrieval strategies (see Fig. 1A for trial timing and composition). We reasoned that generation of the common use of an object would benefit from the selection of a single object feature (i.e., function) among a set of task‐irrelevant features (e.g., shape, size, material), a process that has been linked to prefrontal activation in prior research [Ebisch et al., 2007] and may involve cognitive control mechanisms akin to those implicated in interference resolution [e.g., Badre and Wagner, 2007; Jonides and Nee, 2006; Thompson‐Schill et al., 2005]. In contrast, selective attention to a single feature would not be an optimal strategy when the participant cannot be certain to which features to attend while generating an uncommon use; therefore, performance of this open‐ended task might activate posterior regions associated with the representation and retrieval of object properties (e.g., fusiform gyrus and lateral occipital complex) within a distributed conceptual network [Thompson‐Schill et al., 2006; Tyler and Moss, 2001].
Figure 1.
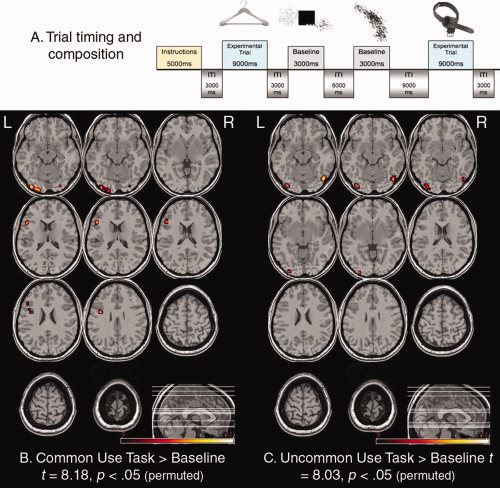
(A) Example trials and their duration. Participants in the first condition (n = 12) generated aloud the common use for each object; participants in the second condition (n = 12) generated aloud an uncommon use for each object; both participant groups also performed a baseline task in which they verified aloud whether a square box was superimposed on top of abstract black and white images; ms = milliseconds; ITI = intertrial interval. The events were jittered with the inclusion of additional null trials, which extended the ISI from 3,000 ms to either 6,000, 9,000, or 12,000 ms. (B) Results of the random effects analysis for the Common Use task relative to the perceptual baseline; (C) Results of the random effects analysis for the Uncommon Use task relative to the perceptual baseline; L = left hemisphere, R = right hemisphere.
To ensure that participants followed the instructions and were not inadvertently switching tasks during the session, we opted for a between‐subjects design relative to a within‐subjects design; our rationale was that a between‐subjects design would allow for the most direct examination of the experimental hypothesis because participants in each condition would be completely naïve to the task instructions of the other condition. We predicted that there would be a tradeoff between activity in prefrontal and posterior brain regions under these two distinct circumstances, such that common use generation (a close‐ended task) would be associated with increased prefrontal activity, whereas uncommon use generation (an open‐ended task) would be associated with increased activity in posterior regions associated with object processing.
MATERIALS AND METHODS
Participants
Twenty‐four (N = 24) right‐handed, native English speakers (mean age = 23.04, 10 males) participated. All subjects provided informed consent and were paid $40 for their time. The study was approved by the University of Pennsylvania's Institutional Review Board.
Materials
Seventy‐two black‐and‐white photographs of everyday objects, divided in three blocks of 24 items, were used as experimental stimuli. They were selected from a larger set of 220 items based on pilot data from a different group of participants (N = 62, mean age = 20.14, 28 males) who reported how easy it was to generate usual and unusual uses for each object (on a 1‐ to 7‐point Likert‐like scale). Objects with high name agreement (>75%) and ease‐of‐use‐generation rating (>5) were selected for the experiment. Seventy‐two scrambled black‐and‐white images, divided in three blocks of 24 items, were used as stimuli in a nonconceptual baseline task; these images were constructed after the application of Adobe® Photoshop distortion filters on a different set of object photographs. A black, 1 in. × 1 in. box was superimposed on the center of half of the baseline images. Within each block, the order of stimulus presentation was randomized.
Procedure
Study design.
Participants were randomly assigned to one of two conditions. In each of three blocks of trials, participants performed one of the two experimental tasks in addition to the perceptual baseline task (see Fig. 1A). For the Common Use condition, participants reported (aloud) the most typical or commonly encountered use for each object; for the Uncommon Use condition, they generated a novel use for the object, one they had not seen or attempted before that would be plausible, yet, which would deviate significantly from the object's common use. Participants were informed that the tasks had no right or wrong answers and that they should feel free to produce any response they judged fit. They were instructed to respond as quickly as possible and to remain silent if unable to generate a response. For the perceptual baseline task, subjects were asked to say aloud “yes” if the black box was superimposed on the scrambled image and “no” if it was not. This perceptual task was chosen to serve as the baseline over other tasks requiring overt verbal responses because it minimizes the involvement of any processes that might have been implicated in the experimental task, namely the activation of any lexical or semantic information.
Each 9‐min block comprised 92 trials: 24 experimental trials [lasting 9,000 ms, followed by a 3,000‐ms intertrial interval (ITI)], 28 baseline trials (lasting 3,000 ms, followed by a 3,000‐ms ITI), and 40 null events (lasting 3,000 ms; see Fig. 1A for trial timing and composition). The onset times of the events were jittered by pseudorandomizing the trial types within each block using Optseq2 (http://surfer.nmr.mgh.harvard.edu/optseq); the first and last six trials within each block were null events. The task instructions were presented at the beginning of each block; a prompt also appeared above each trial item (i.e., “Common Use,” “Uncommon Use,” or “Box?”). Before the experiment, all subjects completed a 5‐min training session to familiarize themselves with the experimental procedures.
Stimulus presentation.
Stimuli were presented using E‐prime software (Psychology Software Tools) on a PC computer connected to an Epson 8100 3‐LCD projector (Epson America, Long Beach, CA), housed in a custom RF shield box. Subjects' overt responses were obtained using Litemic™ 3140 fiber optical Dual‐Channel Noise Canceling Microphone System for MRI Communication (Or‐Yehuda, Israel, http://www.optoacoustics.com) and were transcribed using a MacBook Pro laptop computer (Apple Computer, Cupertino, CA).
Image acquisition and preprocessing.
Following acquisition of sagittal and axial T1‐weighted localizer images, echo‐planar fMRI was performed in 42 contiguous 3‐mm axial slices (TR = 3,000 ms, TE = 30 ms, 64 × 64 pixels in a 19.2‐cm field of view, voxel size = 3 mm × 3 mm × 3 mm), using a 3.0‐T Siemens Trio system (Malvern, PA) and a USA instruments (Aurora, OH) four‐channel head coil. A 3‐D prospective acquisition correction was performed online during data acquisition, which allowed for real‐time linear and rotational motor detection and correction (Siemens‐Medical, 2004). Offline data processing was performed using VoxBo software (http://www.voxbo.com). After image reconstruction, the data were sinc interpolated in time to correct for the staggered fMRI acquisition sequence. Data were corrected for motion with a six‐parameter, least squares, rigid body realignment routine using the first functional image as a reference and spike artifacts were removed. Data were normalized in SPM2 to a standardized Montréal Neurological Institute (MNI) space and smoothed with a 3‐voxel isoptropic FWHM Gaussian kernel. Normalization maintained 3‐mm isotropic voxels and used fourth degree B‐spline interpolation. The average power spectrum across voxels and across scans was obtained, and (square root of) the power spectrum was fit with a 1/f frequency function. This model of intrinsic noise was used for the regression analysis using the general linear model (GLM) to inform the estimation of intrinsic temporal autocorrelation. Consistent with previous findings [Heim et al., 2006; Kan and Thompson‐Schill, 2004], overt responding did not lead to excessive motion artifacts (exclusion criterion was a 2‐mm displacement).
Image analysis.
All analyses were event‐related. Whole‐brain analyses were performed on each subject's data using the GLM as implemented in VoxBo [Worsley and Friston, 1995]. The model included covariates modeling the task conditions, a subject‐specific estimate of the intrinsic temporal autocorrelation, and sine and cosine regressors for frequencies below those of the task. Task covariates were boxcar waveforms convolved with an estimate of the blood oxygenation level–dependent (BOLD) hemodynamic transfer function empirically derived from the ROIs in separate groups of subjects [Aguire et al., 1998].
ROIs were defined functionally by drawing on each subject's anatomy 27‐voxel spherical masks (radius = 1.83 voxels) around the local maxima identified in the group analysis of the main effect (i.e., for all subjects, regardless of condition, the experimental task versus perceptual baseline comparison, assessed at a permutation‐derived threshold of t > 5.41, P < 0.001). Analyses of variance (task‐type × ROI) were used to examine main effects and interactions across conditions. All voxels within each functionally defined ROI were included in analyses between each experimental condition and the perceptual baseline, and the magnitude of each contrast was estimated with a measure of percent signal change (i.e., beta values).
RESULTS
Overview
Each participant was randomly assigned to one of the two conditions and provided verbal responses to three blocks of 24 experimental trials, intermixed with 28 baseline trials and 40 null events (Fig. 1A). Participants' responses were recorded and transcribed for subsequent analysis. The trials for which a participant did not respond (<2%) were excluded from all analyses.
Behavioral Results
Two raters (blind to the study design) evaluated the novelty and plausibility of all transcribed responses on two 1–5 Likert‐like scales (inter‐rater reliability, Pearson's r = 0.87, P < 0.001 for novelty, and r = 0.89, P < 0.001 for plausibility). Participants' responses to the Uncommon Use task were judged to be more novel, t(11.32) = 17.94, P < 0.001, d = 7.33, and less plausible, t(13.37) = 20.56, P < 0.001, d = 8.40, than were responses to the Common Use task (degrees of freedom adjusted for unequal variances). These results confirm that participants were engaged in the tasks according to the experimental instructions. The participants' responses were further coded qualitatively for response type; this analysis is presented in a later section.
fMRI Results
Whole‐brain analyses of task effects.
The whole‐brain, random effects analysis for the Common Use task relative to the perceptual baseline (Fig. 1B) showed regions of significant activation at a permuted critical threshold of t = 8.18, P < 0.05, in the left inferior PFC, the fusiform gyrus bilaterally, and the right inferior occipital gyrus (Talairach coordinates for local maxima x = −50, y = 25, z = 19; x = −28, y = −72, z = −16; x = 26, y = −69, z = −16; and x = 32, y = −85, z = −4, respectively). For the Uncommon Use task relative to baseline (Fig. 1C), no prefrontal region exceeded the permuted critical threshold of t = 8.03, P < 0.05; however, there was significant activation in the left and right fusiform gyri, and the left inferior occipital gyrus (Talairach coordinates for local maxima x = −23, y = −61, z = −25; x = 39, y = −55, z = −16; and x = −29, y = −85, z = −1, respectively). For all whole‐brain analyses, permutation testing was used as the optimal way to control for multiple comparisons; permutation testing was implemented in VoxBo by performing 1,000 Monte Carlo permutation tests on the data for each comparison of interest [Nichols and Holmes, 2002].
Region of interest analyses of task interactions.
We employed a functional region of interest approach to directly compare these different activation patterns across tasks. Regions of interest (ROIs) were defined functionally by contrasting both experimental tasks across all subjects to the perceptual baseline task (Fig. 2A). At a critical threshold of t = 5.53, P < 0.05 (derived from a permutation analysis of the effect), we identified 16 independent contiguous clusters, eight anterior and eight posterior, that showed increased activation at the group level in the experimental tasks relative to baseline. In each subject, individual ROIs were then defined by locating the coordinates of these local maxima on each anatomical scan, using the subject's normalization parameters, and then drawing 27‐voxel spherical masks (radius = 1.83 voxels) around each point. Note that this procedure enabled us to identify main‐effect functional ROIs in this between‐subjects design (in which creating them on an individual basis is not possible).
Figure 2.
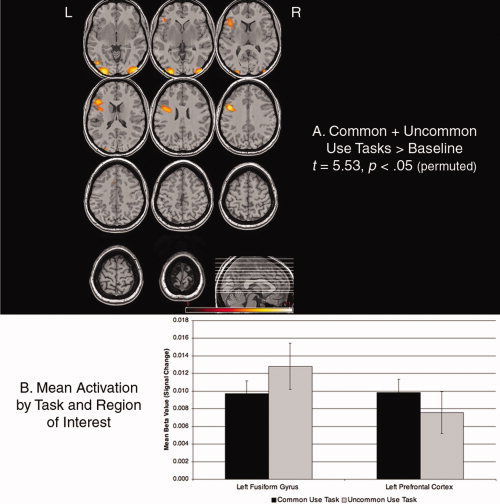
(A) Results of the random effects analysis for both experimental tasks relative to the perceptual baseline; this analysis was used for the identification of functional regions of interest (ROIs). (B) Mean blood oxygenation level–dependent (BOLD) signal change (beta value) by task (Common Use vs. Uncommon Use) and posterior and anterior ROIs of maximum activation (P < 0.04). Error bars indicate the standard error of the means. L = left hemisphere, R = right hemisphere.
To contrast directly the activation patterns in frontal and posterior regions in a straightforward 2 (region) × 2 (condition) analysis of variance, we used these smaller ROIs to create two functional–anatomical ROIs in each subject as follows: The peak voxel (based on the group main effect) in frontal cortex (x = −52, y = 12, z = 24) was located in the left, inferior frontal gyrus; we aggregated data from all four spherical loci that fell within this anatomically defined gyrus [Duvernoy, 1991], into a single anterior ROI (108 voxels). (We will revisit the eight smaller ROIs, including those that fell outside of this anatomical boundary, below.) We repeated this procedure in posterior regions, based on the identification of a peak voxel in left fusiform gyrus (x = −27, y = −97, z = −20), to create a single posterior ROI (53 voxels). Within each of these two ROIs, we examined differences in activation in each subject between the experimental task and the perceptual baseline. A 2 × 2 analysis of variance showed a significant interaction in line with the experimental hypothesis, between ROI and task type (F[1,22] = 4.71, P = 0.04, η2 = 0.18; Fig. 2B). This interaction would suggest that the two tasks elicited dissociable patterns of activation in the left PFC and the left fusiform gyrus. Post hoc comparisons did not detect reliably higher activation for the Common Use task relative to the Uncommon Use task in the left PFC, or reliably higher activation for the Uncommon Use task relative to the Common Use task in the left fusiform gyrus (Ps > 0.10).
To explore the extent of these effects in all 16 functionally defined ROIs, we contrasted activation for the experimental task versus the perceptual baseline task for each subject in each condition. The eight anterior ROIs were localized in the left inferior, middle, and superior frontal gyri and the cingulate sulcus. There was one ROI (14 voxels) identified in the right inferior frontal gyrus; however, for the majority of participants, this region fell out of cortical boundaries and was not significantly different from a region of no activation. The eight posterior ROIs were localized in the left and right fusiform gyri, the right inferior occipital gyrus, and the left middle occipital gyrus (Fig. 3A,B). Consistent with our predictions, of the eight anterior ROIs, seven showed higher activation for the Common Use task than the Uncommon Use task (binomial P = 0.035). In contrast, of the eight posterior ROIs, seven showed the reverse pattern, namely higher activation for the Uncommon Use task than the Common Use task (binomial P = 0.035). We further note that the observed activation for the perceptual baseline task relative to null events was not significantly different between the two experimental conditions for any ROI (all Ps > 0.28), which confirms that the observed effects were task‐driven and not attributed to differences in baseline activation levels between the two participant groups.
Figure 3.
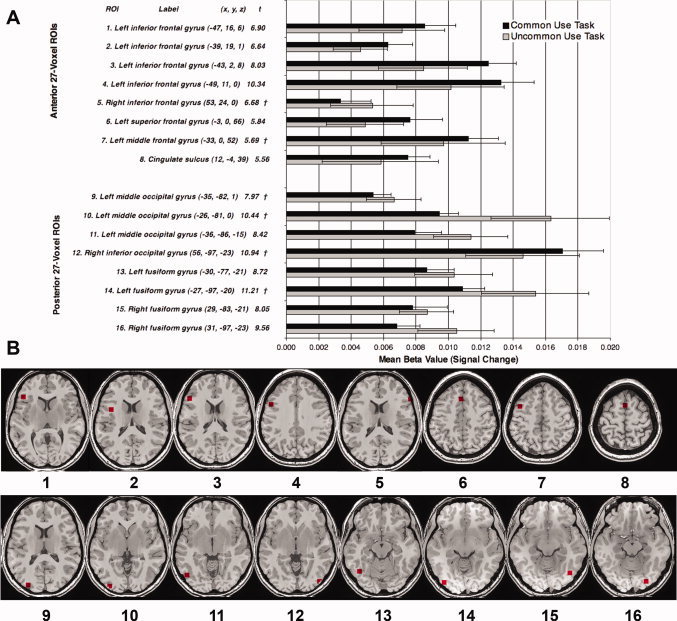
(A) Identification of ROIs by labels, Talairach coordinates (x y z) and t value (critical threshold t = 5.53, P < 0.05) and mean signal change by 27‐voxel region of interest (ROI) and task. †Because of the proximity of the local maxima to the edge of the image, the ROI did not extend fully in all directions and so contains fewer than 27 voxels (min = 14). (B) Identification of ROIs by position.
Qualitative Analysis of Uncommon Responses and Activation in Posterior Cortical Regions
Overview.
The ROI analysis revealed the involvement of posterior regions during the Uncommon Use task. We hypothesized that the nature of this activation might be related to participants' heightened attention to perceptual aspects of the objects' representation. To explore this hypothesis in more detail, we coded participants' responses qualitatively, using a categorization system that allows for the classification of object function on a continuum ranging from conceptually driven, top–down, contextually independent responses to perceptually driven, bottom–up, contextually bound responses. This categorization system was developed and standardized for the purposes of another study that included behavioral data from an independent group of subjects (N = 63; mean age = 21.12 years, 23 males) performing a version of the tasks employed in the present study (Chrysikou et al., Finding functions: Determining object use in open‐ended tasks depends on stimulus modality, submitted).
The coding scheme includes four categories: Category 1 was used to describe functions that were typical of the object's function or used the object in the same way but in a different context (e.g., chair: to sit on/to sit on when on the beach); Category 2 was used to describe functions that substituted the object for another tool based on shared abstract properties (i.e., properties not visible or available without prior knowledge of what the object is; e.g., hairdryer: to blow leaves); Category 3 was used to describe functions that substituted the object for another tool based on shared perceptual properties (i.e., properties visible or available without prior knowledge of the object's identity; e.g., tennis racket: to use as a snow shoe); finally, Category 4 was used to describe the generation of a new function for the object or deconstruction of the object to allow for a new function based on its perceptual properties (i.e., properties visible or available without prior knowledge of the object's identity; e.g., chair: to use as firewood).
Rating procedure.
Three independent raters, blind to the participants' condition, were trained on the use of the coding system and coded all responses on the abovementioned scale (i.e., with scores ranging from 1 to 4). Inter‐rater reliability between rater pairs was examined by means of the Kappa statistic and was considered substantial (Kappa coefficient, on average, 0.89, P < 0.001). Any differences among the raters were resolved in conference. The ratings across raters (after consensus) were used for subsequent analyses.
Individual differences analysis.
For each participant in the Uncommon Use condition, we calculated the median score on this four‐point scale and also the percent of “perceptual” responses (i.e., Categories 3 and 4); although raters were blind to subject condition when coding responses, there was no variation across responses for participants in the Common Use condition, unsurprisingly, so their data were not subjected to further analysis. We then examined the relationship between these measures of response type for the Uncommon Use condition and the degree of activation in the eight anterior and in the eight posterior ROIs, across subjects. Regarding the anterior regions, correlations between activity in the eight frontal ROIs and the extent to which responses in the task were categorized as perceptually based did not reach significance (all Ps > 0.17). In contrast, median scores of participants' responses on the coding system during the Uncommon Use task correlated positively with activity in the left middle occipital gyrus ROI (Pearson's r = 0.65, P = 0.02). The correlation between percent of perceptual responses and activity in this region also followed the same trend (Pearson's r = 0.57, P = 0.06, Fig. 4). In other words, the more participants' responses were categorized as perceptually based, the higher the activity in middle occipital cortex. These findings further support the hypothesis that the observed activation in this region reflects increased attention to perceptual attributes of the objects' representations during the Uncommon Use task; however, this pattern should be interpreted with some caution because the significance levels were not corrected for multiple comparisons, and we had no a priori reason to believe they would only be present in this one ROI.
Figure 4.
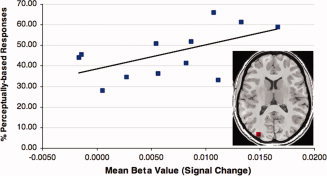
Relationship between signal change in the left middle occipital gyrus and percent of perceptually based responses for the Uncommon Use task on the qualitative analysis measure (Pearson's r = 0.54, P = 0.06; for median scores, Pearson's r = 0.65, P = 0.02).
DISCUSSION
Much is known about the role of the PFC in close‐ended tasks (Thompson‐Schill et al., 2006). Here, we report two novel results regarding the brain states supporting performance on open‐ended tasks (Dietrich, 2004) that point to a complex interplay between anterior and posterior brain systems: (a) When searching for an uncommon use for a visually depicted object, posterior cortical areas involved in high‐level visual processes are recruited, perhaps to a degree associated with the kind of use that is generated; and (b) generating an uncommon use for an object does not necessarily recruit PFC systems associated with performance on close‐ended tasks. In particular, our fMRI and behavioral findings revealed a tradeoff between anterior frontal and posterior occipitotemporal regions according to task demands: the PFC was engaged in a close‐ended task (i.e. Common Use task) that requires the controlled retrieval of a specific aspect of knowledge for objects, but was disengaged, in contrast, from an open‐ended task (i.e., Uncommon Use task) during which the search in conceptual space is spontaneous; for this task there was, instead, significant involvement of the left lateral occipitotemporal cortex.
We note that due to the constraints of collecting verbal responses in the fMRI environment, obtaining reliable voice‐onset reaction times from the fMRI participants was not possible. On the other hand, whenever fMRI activation patterns (which reflect BOLD signal integrated over time) are compared between two tasks, the possibility that effects are confounded with response time looms large. In this case, the presence of a double dissociation between brain regions reassures us that the entire pattern of effects cannot be explained by task difficulty. In particular, we highlight that the pattern observed in frontal regions—namely, greater activation for the Common Use task than the Uncommon Use task—runs counter to the alternative explanation that open‐ended tasks are simply easier than closed‐ended tasks and thus do not recruit prefrontal regions to the same degree: Behavioral data from an independent group of subjects (N = 24, mean age = 20.46, 13 males), who completed the identical experiment out of the scanner, indicate that the Common Use task is easier than the Uncommon Use task (see Table I). That is, PFC was active during the Common Use task and was associated with the shorter latencies in the behavioral study, but it was not significantly active for the Uncommon Use task that elicited the longer latencies in the behavioral study. While it remains possible that the pattern of activation in posterior regions is simply tracking time‐on‐task, the correlation across subjects between the types of responses given in the Uncommon Use task and the magnitude of activation in left occipital cortex somewhat reduces this possibility: in other research (Chrysikou et al., Finding functions: Determining object use in open‐ended tasks depends on stimulus modality, submitted), we have found that perceptual responses to the Uncommon Use task (which we find associated with greater activation) are made faster, not slower, than are conceptual responses, although this is clearly an area in need of further study. Although the difficulties with obtaining participant latencies is a limitation of the present fMRI experiment, future research should focus on the development and implementation of new techniques that will permit recording reliable voice‐onset reaction times from the fMRI environment.
Table I.
Mean BOLD signal change in left prefrontal and left fusiform regions from the fMRI experiment and mean median RTs from behavioral experiment by task
| Common | Uncommon | |
|---|---|---|
| Mean left PFC beta values (% BOLD signal change) from fMRI experimenta | 0.0099 (0.0015) | 0.0076 (0.0024) |
| Mean left fusiform gyrus beta values (% BOLD signal change) from fMRI experimenta | 0.0097 (0.0015) | 0.0128 (0.0026) |
| Mean median voice‐onset reaction times in milliseconds from behavioral experiment | 1,754.81 (66.20) | 4,321.07 (109.37) |
Values in parentheses represent standard errors.
BOLD, blood oxygenation level–dependent; PFC, prefrontal cortex; fMRI, functional magnetic resonance imaging; RT, reaction times.
Mean beta values (% BOLD signal change) for each condition relative to the perceptual baseline task.
We propose an interpretation of these results in the context of the role of the PFC as a dynamic filtering mechanism that selectively maintains task‐relevant information while gating task‐irrelevant information [Braver and Hannes, 2006; Dosenbach et al., 2008; Rowe et al., 2007; Shimamura, 2000]. Specifically, an object‐stimulus activates a distributed, interactive network of information that can be described as the object representation [Thompson‐Schill et al., 2006]; however, depending on the context and task goals, the pattern of activation can be biased by PFC signals, thus “sculpting” the resulting landscape [Frith, 2000]. This explanation has been offered to account for left PFC activation during various cognitive tasks (e.g., working‐memory, word‐association, and picture‐naming; see Badre and Wagner [ 2007], Jonides and Nee [ 2006], and Thompson‐Schill et al. [ 2005]). Consistent with previous work [e.g., Thompson‐Schill et al., 1999], during the Common Use task in the present study, the PFC sculpts the neural response by focusing attention on one aspect of the object's representation while suppressing others (e.g., the object's material, shape, size, and color are irrelevant when generating a common use).
In contrast, when generating an uncommon use, all of these usually unimportant aspects of the object representation become potentially task‐relevant. That is, due to the task's open‐ended nature, it is not immediately clear which aspect will be the most promising for an appropriate response. Hence, to explore successfully the conceptual space, a PFC‐mediated filtering of the input or biasing of the response by sculpting the representational landscape would be counterproductive. EEG studies have shown that successful performance at tasks of ideational fluency is associated with lower cortical arousal, particularly in prefrontal regions [Martindale and Hasenfus, 1978; Mölle et al., 1999]. According to these and other studies proposing a link between hypofrontality and creative thought [e.g., Heilman et al., 2003; Limb and Braun, 2008; Snyder et al., 2003], this state of diffuse (or distributed) attention to numerous aspects of the conceptual space should be particularly true when generating uncommon uses for objects; this was confirmed by the nonsignificant PFC involvement during the Uncommon Use task. Under this account, the increased lateral occipitotemporal activation during the Uncommon Use task likely reflects the heightened attention to visual aspects of the object representation that may be suppressed (i.e., filtered) during the Common Use task. For example, to recognize in a pinch that a baseball bat could be used as a rolling pin, aspects regarding the object's size and shape, which might not otherwise have been considered to generate the common use “hit a winning homerun,” suddenly become of paramount importance. Such an interpretation is further supported by our qualitative analysis, showing a positive relationship between left lateral occipital activation and perceptually driven responses. These results are consistent with distributed accounts of semantic memory [Thompson‐Schill et al., 2006; Tyler and Moss, 2001], according to which different components of an object are distributed in different brain regions based on experience and are dynamically selected depending on task demands (see also Hoenig et al. [ 2008]).
Regarding the laterality of these effects, previous work has shown the right PFC regions to be involved in remote semantic associations [Kounios et al., 2006, 2008]. We note that the right inferior frontal gyrus was the only region among our eight anterior ROIs that showed a trend of more activation during uncommon than common use generation. Although this finding is consistent with these earlier studies, we are cautious in its interpretation due to the small number of active voxels in this region—in addition to the fact that activation in this region did not significantly differ from a zero. With respect to patients with PFC deficits, it could be argued that their performance on this task might reveal the reverse pattern relative to that of normal subjects. Nevertheless, we note that despite the observed dissociations between anterior and posterior regions in this study, it would appear that some involvement of the PFC is necessary for performance on both tasks. Although we do not expect that patients would outperform normal participants on certain behavioral tasks, we do not exclude the possibility that they might provide different kinds of responses relative to normal subjects on measures such as the response categorization system we developed in this study. The present analysis does not provide details on the time course of PFC recruitment; future fMRI or EEG studies should examine whether a transient increase in PFC activity might be followed by a sustained decrease in activity in open‐ended tasks. Future work should also address whether the effects of flexible PFC involvement that we observed in this study in two independent groups can also be elicited in the same participants. This experiment is the first to demonstrate a dynamic tradeoff between prefrontal and posterior regions brought about by the close‐ or open‐ended task demands and provides a starting point from which to explore further the relationship between PFC function and the nature of flexible thought.
Acknowledgements
We thank the members of the Thompson‐Schill lab for suggestions on earlier versions of this manuscript.
REFERENCES
- Aguire GK, Zarahn E, D'Esposito M ( 1998): The variability of human BOLD hemodynamic responses. Neuroimage 8: 360–369. [DOI] [PubMed] [Google Scholar]
- Alexander JK, Hillier A, Smith RM, Tivarus ME, Beversdorf DO ( 2007): Beta‐adrenergic modulation of cognitive flexibility during stress. J Cogn Neurosci 19: 468–478. [DOI] [PubMed] [Google Scholar]
- Apperly IA, Samson D, Chiavarino C, Humphreys GW ( 2004): Frontal and temporo‐parietal lobe contributions to theory of mind: Neuropsychological evidence from a false belief task with reduced language and executive demands. J Cogn Neurosci 16: 1773–1784. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD ( 2007): Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia 45: 2883–1901. [DOI] [PubMed] [Google Scholar]
- Braver TS, Hannes R ( 2006): Functional neuroimaging of executive functions In: Cabeza R, Kingston A, editors. Handbook of Functional Neuroimaging of Cognition, 2nd ed. Cambridge, MA: MIT Press; pp 307–348. [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JDE ( 2002): Immature frontal lobe contributions to cognitive control in children: Evidence from fMRI. Neuron 33: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson I, Wendt PE, Risberg J ( 2000): On the neurobiology of creativity. Differences in frontal activity between high and low creative subjects. Neuropsychologia 38: 873–885. [DOI] [PubMed] [Google Scholar]
- Christensen PR, Guilford JP ( 1958): Creativity/Fluency Scales. Beverly Hills, CA: Sheridan Supply. [Google Scholar]
- Dietrich A ( 2004): The cognitive neuroscience of creativity. Psychol Bull Rev 11: 1011–1026. [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, Petersen SE ( 2008): A dual‐networks architecture of top–down control. Trends Cogn Sci 12: 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy H ( 1991): The Human Brain: Surface, Three Dimensional Anatomy, and MRI. New York: Springer. [Google Scholar]
- Ebisch SJ, Babiloni C, Del Gratta C, Ferretti A, Perrucci MG, Caulo M, Sitskoorn MM, Romani GL ( 2007): Human neural systems for conceptual knowledge of proper object use: A functional magnetic resonance imaging study. Cereb Cortex 17: 2744–2751. [DOI] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NUF, Church JA, Cohen JA, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL ( 2007): Development of distinct control networks through segregation and integration. Proc Natl Acad Sci USA 104: 13507–13512.17679691 [Google Scholar]
- Faust M, Lavidor M ( 2003): Semantically convergent and semantically divergent priming in the cerebral hemispheres: Lexical decision and semantic judgment. Cogn Brain Res 17: 585–597. [DOI] [PubMed] [Google Scholar]
- Finney GR, Heilman KM ( 2007): Artwork before and after onset of progressive nonfluent aphasia. Cogn Behav Neurol 20: 7–10. [DOI] [PubMed] [Google Scholar]
- Frith C ( 2000): The role of dorsolateral prefrontal cortex in the selection of action as revealed by functional imaging In: Monsell S, Driver J, editors. Control of Cognitive Processes. Cambridge, MA: The MIT Press; pp 549–565. [Google Scholar]
- Heilman KM, Nadeau SE, Beversdorf DO ( 2003): Creative innovation: Possible brain mechanisms. Neurocase 9: 369–379. [DOI] [PubMed] [Google Scholar]
- Heim S, Amunts K, Mohlberg H, Wilms M, Friederici AD ( 2006): Head motion during overt language production in functional magnetic resonance imaging. Neuroreport 17: 579–582. [DOI] [PubMed] [Google Scholar]
- Heonig K, Sim EJ, Bochev V, Herrnberger B, Kiefer M ( 2008): Conceptual flexibility in the human brain: Dynamic recruitment of semantic maps from visual, motor, and motion‐related areas. J Cogn Neurosci 20: 1–16. [DOI] [PubMed] [Google Scholar]
- Howard‐Jones PA, Blakemore SJ, Samuel EA, Summers IR, Claxton G ( 2005): Semantic divergence and creative story generation: An fMRI investigation. Cogn Brain Res 25: 240–250. [DOI] [PubMed] [Google Scholar]
- Jonides J, Nee DE ( 2006): Brain mechanisms of proactive interference in working memory. Neuroscience 139: 181–193. [DOI] [PubMed] [Google Scholar]
- Kan IP, Thompson‐Schill SL ( 2004): Effect of name agreement on prefrontal activity during overt and covert picture naming. Cogn Affect Behav Neurosci 4: 43–57. [DOI] [PubMed] [Google Scholar]
- Klahr D, Simon HA ( 1999): Studies of scientific discovery: Complementary approaches and convergent findings. Psychol Bull 125: 524–543. [Google Scholar]
- Kounios J, Frymiare JL, Boden EM, Fleck JI, Subramaniam K, Parrish TB, Jung‐Beeman M ( 2006): The prepared mind: Neural activity prior to problem presentation predicts solution by sudden insight. Psychol Sci 17: 882–890. [DOI] [PubMed] [Google Scholar]
- Kounios J, Fleck JI, Green DL, Payne L, Stevenson JL, Bowden EM, Jung‐Beeman M ( 2008): The origins of insight in resting‐state brain activity. Neuropsychologia 46: 281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limb CJ, Braun AR ( 2008): Neural substrates of spontaneous musical performance: An fMRI study of jazz improvisation. PLoS ONE 32: e1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martindale C, Hasenfus N ( 1978): EEG differences as a function of creativity, stage of the creative process, and effort to be original. Biol Psychol 6: 157–167. [DOI] [PubMed] [Google Scholar]
- Mölle M, Marshall L, Wolf B, Fehm HL, Born J ( 1999): EEG complexity and performance measures of creative thinking. Psychophysiology 36: 95–104. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP ( 2002): Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp 15: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reverberi, C , Toraldo, A , D'Agostini, S , Skrap, M ( 2005): Better without (later) frontal cortex? Insight problems solved by frontal patients. Brain 128: 2882–2890. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Sakai K, Lund TE, Ramsoy T, Christensen MS, Baare WFC, Paulson OB, Passingham RE ( 2007): Is the prefrontal cortex necessary for establishing cognitive sets? J Neurosci 27: 13303–13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumelhart DE, Ortony A ( 1977): The representation of knowledge in memory In: Anderson RC, Spiro RJ, Montague WE, editors. Schooling and the Acquisition of Knowledge. Hillsdale, NJ: Erlbaum; pp 99–135. [Google Scholar]
- Seeley WW, Matthews BR, Crawford RK, Gorno‐Tempini ML, Foti D, Mackenzie IR, Miller BL ( 2008): Unravelling Boléro: Progressive aphasia, transmodal creativity and the right posterior neocortex. Brain 131: 39–49. [DOI] [PubMed] [Google Scholar]
- Shimamura AP ( 2000): The role of the prefrontal cortex in dynamic filtering. Psychobiology 28: 207–218. [Google Scholar]
- Snyder A ( 2009): Explaining and inducing savant skills: Priviledged access to lower level, less‐processed information. Phil Trans R Soc Lond B Biol Sci 364: 1399–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder A, Mulcahy E, Taylor JL, Mitchell DJ, Sachdev P, Gandevia SC ( 2003): Savant‐like skills exposed in normal people by suppressing the left fronto‐temporal lobe. J Integr Neurosci 2: 149–158. [DOI] [PubMed] [Google Scholar]
- Snyder A, Bahramali H, Hawker T, Mitchell DJ ( 2006): Savant‐like numerosity skills revealed in normal people by magnetic pulses. Perception 35: 837–845. [DOI] [PubMed] [Google Scholar]
- Thompson‐Schill SL, D'Esposito M, Aguirre GK, Farah MJ ( 1997): Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proc Natl Acad Sci USA 94: 14792–14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson‐Schill SL, D'Esposito M, Kan IP ( 1999): Effects of repetition priming and competition on activity in left prefrontal cortex during word generation. Neuron 23: 513–522. [DOI] [PubMed] [Google Scholar]
- Thompson‐Schill SL, Bedny M, Goldberg RF ( 2005) The frontal lobes and the regulation of mental activity. Curr Opin Neurobiol 15: 219–224. [DOI] [PubMed] [Google Scholar]
- Thompson‐Schill SL, Kan IP, Oliver RT ( 2006): Functional neuroimaging of semantic memory In: Cabeza R, Kingston A, editors. Handbook of Functional Neuroimaging of Cognition, 2nd ed. Cambridge, MA: MIT Press; pp 149–190. [Google Scholar]
- Tyler LK, Moss HE ( 2001): Towards a distributed account of conceptual knowledge. Trends Cogn Sci 5: 244–252. [DOI] [PubMed] [Google Scholar]
- Weisberg RW ( 2006): Creativity: Understanding Innovation in Problem Solving, Science, Invention, and the Arts. Hoboken, NJ: Wiley. [Google Scholar]
- Wolford G, Miller MB, Gazzaniga M ( 2000): The left hemisphere's role in hypothesis formation. J Neurosci 20: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Friston K ( 1995): Analysis of fMRI time‐series revisited—Again. Neuroimage 2: 173–182. [DOI] [PubMed] [Google Scholar]


