Abstract
While clinical studies have established that antigen-loaded DC vaccines are safe and promising therapy for tumors 1, their clinical efficacy remains to be established. The method described below, prepared in accordance with Good Manufacturing Process (GMP) guidelines, is an optimization of the most common ex vivo preparation method for generating large numbers of DCs for clinical studies 2.
Our method utilizes the synthetic TLR 3 agonist Polyinosinic-Polycytidylic Acid-poly-L-lysine Carboxymethylcellulose (Poly-ICLC) to stimulate the DCs. Our previous study established that Poly-ICLC is the most potent individual maturation stimulus for human DCs as assessed by an upregulation of CD83 and CD86, induction of interleukin-12 (IL-12), tumor necrosis factor (TNF), interferon gamma-induced protein 10 (IP-10), interleukmin 1 (IL-1), and type I interferons (IFN), and minimal interleukin 10 (IL-10) production.
DCs are differentiated from frozen peripheral blood mononuclear cells (PBMCs) obtained by leukapheresis. PBMCs are isolated by Ficoll gradient centrifugation and frozen in aliquots. On Day 1, PBMCs are thawed and plated onto tissue culture flasks to select for monocytes which adhere to the plastic surface after 1-2 hr incubation at 37 °C in the tissue culture incubator. After incubation, the lymphocytes are washed off and the adherent monocytes are cultured for 5 days in the presence of interleukin-4 (IL-4) and granulocyte macrophage-colony stimulating factor (GM-CSF) to differentiate to immature DCs. On Day 6, immature DCs are pulsed with the keyhole limpet hemocyanin (KLH) protein which serves as a control for the quality of the vaccine and may boost the immunogenicity of the vaccine 3. The DCs are stimulated to mature, loaded with peptide antigens, and incubated overnight. On Day 7, the cells are washed, and frozen in 1 ml aliquots containing 4 - 20 x 106 cells using a controlled-rate freezer. Lot release testing for the batches of DCs is performed and must meet minimum specifications before they are injected into patients.
Keywords: Cancer Biology, Issue 78, Medicine, Immunology, Molecular Biology, Cellular Biology, Biomedical Engineering, Anatomy, Physiology, Dendritic Cells, Immunotherapy, dendritic cell, immunotherapy, vaccine, cell, isolation, flow cytometry, cell culture, clinical techniques
Protocol
1. Isolation and Cryopreservation of PBMCs 4
Aseptically spike one of the access ports in the leukapheresis bag using a plasma transfer set. Using a 60 ml syringe, transfer the leukapheresis obtained from patients into a sterile 500 ml bottle.
Adjust the volume of the leukapheresis to 2x its original volume using room temperature RPMI. Mix thoroughly.
Gently mix the bottle of Ficoll-Paque PLUS. Add 12 ml Ficoll-Paque PLUS into sterile 50 ml conical tube.
Gently layer 30 ml of the diluted leukapheresis product to each sterile 50 ml conical tube containing Ficoll. Be careful not to disturb the interface between the Ficoll and cell suspension.
Repeat steps 1.3 and 1.4 until all of the leukapheresis has been layered.
Centrifuge the 50 ml conical tubes at 1,000 × g for 20 min at room temperature with no brake.
Carefully harvest the cloudy layer of PBMCs from each tube and transfer to new sterile 50 ml tubes.
Add RPMI to each tube to a final volume of 50 ml. Mix gently by inversion.
Centrifuge the cells at 500 × g for 10 min at 4 °C with full brake.
Remove the supernatants from each tube. Resuspend the cell pellets from each tube and add RPMI to a final volume of 50 ml. Mix gently by inversion.
Centrifuge the cells at 500 × g for 6 min at 4 °C.
Remove the supernatants from each tube. Resuspend and pool together the cell suspensions into one 50 ml conical tube.
Bring the volume of the pooled PBMCs up to 50 ml with more RPMI. Mix gently by inversion.
Centrifuge the cells at 300 × g for 6 min at 4 °C.
Remove the supernatant. Resuspend the cell pellet in 50 ml RPMI. Mix gently to ensure uniform cell suspension.
Count the number of cells and determine cell viability. Calculate the total number of viable cells.
Centrifuge the cells at 300 × g for 6 min at 4 °C. Prepare the freezing media. Freezing media consists of 10% Dimethyl sulfoxide (DMSO; Miltenyi Biotec) in human AB serum (Valley Biomedical).
Remove the supernatant. Resuspend the cells at a final concentration of 2 x 108 cells/ml in cold freezing media.
Make 1 ml aliquots in 1.8 ml cryovials.
Transfer the cryovials into the controlled-rate freezer and begin the freezing run, Program 1. At the end of the run, transfer the frozen cryovials immediately into the vapor phase of the liquid nitrogen freezer.
2. Day 0: Differentiation of Dendritic Cells from Monocytes
Add 30 ml of RPMI/1% autologous plasma to a sterile 50 ml conical tube.
Thaw aliquots of frozen PBMCs by gentle agitation in the 37 °C water bath. When completely thawed, transfer the contents of the vials to the sterile 50 ml conical tube. Mix thoroughly.
Centrifuge the cells for 6 min at 500 × g at room temperature. Remove the supernatant and resuspend the pelleted cells in a small volume of RPMI/1% autologous plasma. Then add more RPMI/1% autologous plasma to a final volume of 50 ml. Mix thoroughly.
Centrifuge the cells again for 6 min at 500 × g at room temperature. Aspirate the supernatant and resuspend the pellet in 50 ml RPMI/1% autologous plasma. Mix thoroughly.
Count the number of cells and determine cell viability. Calculate the total number of viable cells.
Plate 1.40 x 108 cells in 40 ml of RPMI/1% autologous plasma onto 225 cm2 EasyFlask. Repeat until all cells have been plated.
Incubate the EasyFlasks flat on its side for 1 - 2 hr at 37 °C in the tissue culture incubator containing 5% CO2 to allow the monocytes to adhere to the flask.
When the incubation is complete, remove the EasyFlask from the incubator and wash twice to remove the non-adherent cells using 30 ml of prewarmed RPMI.
After the second wash, add 40 ml of RPMI/1% autologous plasma with 400-1,000 IU/ml IL-4 and 100-1,000 IU/ml GM-CSF 5-8. For this protocol, we are using 400 IU/ml IL-4 and 100 IU/ml GM-CSF.
Incubate the EasyFlasks for 2 days at 37 °C in the tissue culture incubator containing 5% CO2.
3. Day 2: Feeding of Dendritic Cells with IL-4 and GM-CSF
Add 4 ml of RPMI/1% autologous plasma with 400-1,000 IU/ml IL-4 and 100-1,000 IU/ml GM-CSF to each EasyFlask. Mix by swirling and rocking on its side.
Incubate the EasyFlasks for 3 more days (until Day 5) at 37 °C in the tissue culture incubator containing 5% CO2.
4. Day 5: Harvesting of Immature Dendritic Cells
Harvest the cultures by vigorously swirling the flasks and by pipetting up and down to resuspend non-adherent and loosely adherent cells. Transfer the harvested cells to new 50 ml conical tubes.
Centrifuge the tubes at 500 × g for 6 min at room temperature. Remove the supernatants and resuspend the cell pellet in 50 ml RPMI/1% autologous plasma. Mix thoroughly.
Count the number of cells and determine cell viability. Calculate the total number of viable cells.
Centrifuge the tubes at 500 × g for 6 min at room temperature. Plate 1-2 x 106 cells per well in 3 ml RPMI/1% autologous plasma with 400 IU/ml IL-4 and 100 IU/ml GM-CSF in 6-well tissue culture plates.
Incubate the plates at 37 °C in the tissue culture incubator containing 5% CO2 overnight.
5. Day 6: Maturation and Antigen Loading of Dendritic Cells
Add 10 μg/ml KLH to 1/3 of the wells in the 6-well culture plates. Swirl the plates to mix. KLH is only added to 1/3 of the wells because KLH is only used for the first DC vaccine. Subsequent DC vaccines contain only the tumor antigen peptides.
DCs can be stimulated to mature using different stimuli. The most common maturation stimuli that has been used in clinical studies has been a cocktail of cytokines (IL-1 β, TNFα, and IL-6) and prostaglandin E2 (PGE2) 9,10. More recently, the use of Toll-like receptor (TLR) agonists have gained popularity 11,12. In this protocol, add 2 mg/ml Polyinosinic-Polycytidylic Acid-poly-L-lysine Carboxymethylcellulose (Poly-ICLC) to the wells and mix well. Poly-ICLC is the clinical-grade formulation of Poly-IC that is stabilized with poly-L-*-lysine and carboxymethylcellulose (manufactured by Oncovir, Inc.).
Add 100 μg/ml long peptide antigens (NY-ESO-1 and/or Melan-A/MART-1) to their designated wells, each well receiving only a single peptide to avoid cross-competition 13.
Incubate the plates at 37 °C in the tissue culture incubator containing 5% CO2 O/N.
6. Day 7: Cryopreservation of Dendritic Cells
Prepare DC Freezing Solution using autologous plasma and 10% DMSO. Centrifuge the DC Freezing Solution at 2,000 × g for 20 min at 4 °C to remove any particulate material. Transfer supernatant to a new labeled 15 ml conical tube. Store at 4 °C until use.
When the overnight incubation is finished, harvest and pool all the wells containing DCs pulsed with the peptides into 50 ml conical tubes.
Centrifuge the tubes at 500 × g for 6 min. Remove the supernatants. Resuspend the cell pellet in 5 ml of RPMI/1% autologous plasma. Bring the total volume up to 50 ml with RPMI/1% autologous plasma. Mix thoroughly.
Count the number of cells and determine cell viability. Calculate total number of viable cells.
Centrifuge the tubes at 500 × g for 6 min at room temp. Remove the supernatants and resuspend each pellet in 5 ml sterile 0.9% NaCl, USP and transfer to new 15 ml conical tubes. Bring each cell suspension to 14 ml with more sterile 0.9% NaCl, USP. Cap and mix by inversion.
Repeat previous step twice. After the last centrifugation, remove the supernatants, getting as close as possible to the cell pellets without disturbing the pellets.
Resuspend the cell pellets in DC Freezing Solution at 4 - 20 x 106 cells/ml and aliquot 1 ml to each 1.8 ml CryoTube vial.
Use the controlled-rate freezer, Program 1, to freeze down the aliquots of DC vaccines and transfer immediately into the vapor phase of a liquid nitrogen freezer for long-term storage.
7. Lot Release Testing
Each batch of DC vaccine must be evaluated and meet specific lot release criteria (Table 1) before it is released for injection into patients in the study, typically 5 to 6 weeks after vaccine preparation.
Viability: Evaluate viability of the DC vaccine using an automated cell counter. The minimum acceptable viability specification is >70%.
Identity: Evaluate the identity of the mature DCs (CD11c+CD14-CD83+) by flow cytometry. The percentage of CD11c+ cells should be >50%. The percentage of CD11c+ and CD14+ cells should be <30% and the percentage of CD11c+, CD14-, and CD83+ cells should be >50%.
Sterility: Test the DC vaccine for the presence of anaerobic and aerobic bacteria and fungus by direct culture and Gram stains. Evaluate for the presence of mycoplasma using a direct culture system and DNA fluorochrome Staining Assay with Indicator Cell Line. For these tests, the minimum specification set is that no growth is observed from all the cultures.
Endotoxin: Evaluate the endotoxin content using the Kinetic-QCL kinetic chromogenic LAL assay and it must be < 50 EU/ml to meet the minimum criteria.
Function: Evaluate DC function in a mixed lymphocyte reaction by incubation with allogeneic T cells. The presence of proliferation compared to unstimulated DCs is reported (Figure 3).
Representative Results
Between 10 - 20% of starting PBMCs differentiate into DCs at the end of the culture period. Mature DCs are CD11c+, CD14-, CD83+, CD40+, and CCR7+ (Figure 1). They express high levels of MHC class I and II molecules and the costimulatory molecules CD80 and CD86. Poly-ICLC also induced lower levels of PDL-1 as compared to other TLR agonists 14. Additionally, these Poly-IC-matured DCs secrete large amounts of IL-12 (Figure 2 and 15,16) and induce the proliferation of allogeneic T cells (Figure 3).
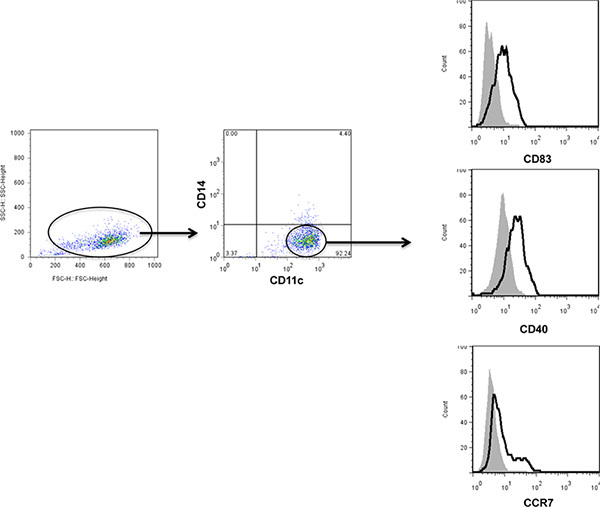 Figure 1. Representative phenotype of fully differentiated DCs matured with Poly-IC. All the DCs were gated on the forward- and side-scatter plot. DCs were identified as CD11c+ and CD14-. Maturation of DCs in response to Poly-ICLC (bold line) was evaluated based on the expression of CD83, CD40, and CCR7 and compared to unstimulated DCs (gray shade). Click here to view larger figure.
Figure 1. Representative phenotype of fully differentiated DCs matured with Poly-IC. All the DCs were gated on the forward- and side-scatter plot. DCs were identified as CD11c+ and CD14-. Maturation of DCs in response to Poly-ICLC (bold line) was evaluated based on the expression of CD83, CD40, and CCR7 and compared to unstimulated DCs (gray shade). Click here to view larger figure.
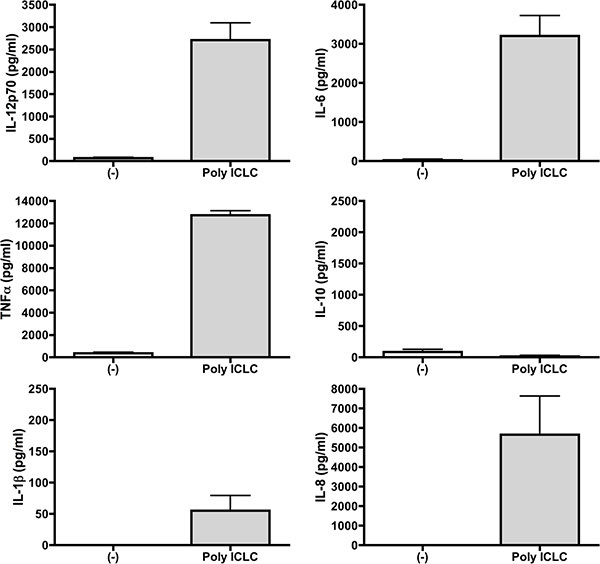 Figure 2. DCs matured with Poly-IC produce high levels of cytokines. Supernatants from DCs differentiated from 3 healthy donors were collected after overnight maturation of DCs with Poly-IC. Cytokines produced were measured by Cytokine Bead Array (CBA) Human Inflammatory Cytokines Kit (BD Biosciences) which measures IL-12p70, TNF, IL-10, IL-6, IL-1β, and IL-8.
Figure 2. DCs matured with Poly-IC produce high levels of cytokines. Supernatants from DCs differentiated from 3 healthy donors were collected after overnight maturation of DCs with Poly-IC. Cytokines produced were measured by Cytokine Bead Array (CBA) Human Inflammatory Cytokines Kit (BD Biosciences) which measures IL-12p70, TNF, IL-10, IL-6, IL-1β, and IL-8.
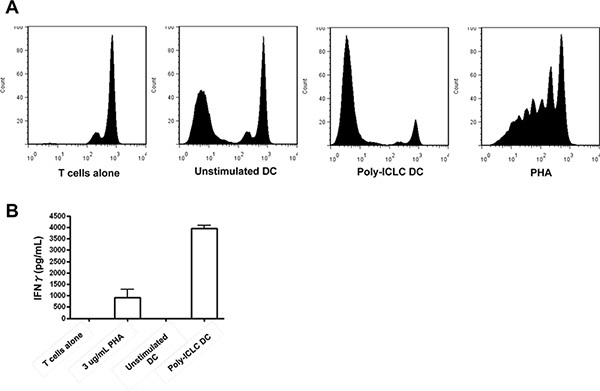 Figure 3. DCs matured with Poly-ICLC induce the proliferation of allogeneic T cells. Poly-ICLC-matured DCs from healthy donors were incubated 1:10 with Carboxyfluorescein Diacetate Succinimidyl Ester (CFSE)-labeled allogeneic T cells. After 6 days, proliferation (A) was evaluated by flow cytometry by gating on the CD3+ T cell population. X-axis shows the dilution of CFSE, as a measurement of proliferation, in the various co-culture conditions: T cells alone, unstimulated DC, Poly-ICLC stimulated DC, and phytohaemagglutinin (PHA)-stimulated T cells. Cytokine secretion (B) during the proliferation was evaluated from the supernatants in the cultures using the BD CBA Human Th1/Th2 Cytokine Kit II (BD Biosciences) which measures IFNγ, TNF, IL-10, IL-6, IL-4, and IL-2. Only IFNγ is shown as the other cytokines were not detected to measureable levels in the assay. Click here to view larger figure.
Figure 3. DCs matured with Poly-ICLC induce the proliferation of allogeneic T cells. Poly-ICLC-matured DCs from healthy donors were incubated 1:10 with Carboxyfluorescein Diacetate Succinimidyl Ester (CFSE)-labeled allogeneic T cells. After 6 days, proliferation (A) was evaluated by flow cytometry by gating on the CD3+ T cell population. X-axis shows the dilution of CFSE, as a measurement of proliferation, in the various co-culture conditions: T cells alone, unstimulated DC, Poly-ICLC stimulated DC, and phytohaemagglutinin (PHA)-stimulated T cells. Cytokine secretion (B) during the proliferation was evaluated from the supernatants in the cultures using the BD CBA Human Th1/Th2 Cytokine Kit II (BD Biosciences) which measures IFNγ, TNF, IL-10, IL-6, IL-4, and IL-2. Only IFNγ is shown as the other cytokines were not detected to measureable levels in the assay. Click here to view larger figure.
| Test | Method | Criteria |
| Viability | Guava Personal Cell Analysis with ViaCount Reagent | >70% |
| Identity | Flow Cytometry - % CD11c+ cells | > 50% |
| Flow Cytometry - % CD11c+CD14+ cells | < 30% | |
| Flow Cytometry - % CD11c+CD14-CD83+ cells | > 50% | |
| Sterility | Bacterial and Fungal Cultures | Negative |
| Mycoplasma: Direct Cell Culture | Negative | |
| Endotoxin | Kinetic Chromogenic LAL Assay | < 50 EU/ml |
| Function | Mixed Lymphocyte Reaction | Report Results |
Table 1. Lot Release Criteria.
Discussion
Phase I and II clinical trials of monocyte-derived DCs have shown that they induce immune responses in patients however clinical success has been limited 1. This may be partly due to the lack of consensus on how to generate the optimal DCs for tumor immunotherapeutic use. Although there are numerous ways to generate clinical-grade DCs, these methods vary in terms of the use of cytokines used to differentiate the monocytes, stimuli used to induce maturation, and methods of antigen loading. The formula for generating the optimal DCs still remains to be defined 2.
Recent in vitro studies have shown that the DCs matured with a cocktail of proinflammatory cytokines 10 which has been used in the majority of clinical trials, in particular the presence of PGE2, may induce the differentiation of regulatory T cells and Th2 responses 17, express IDO 18, and are deficient in IL-12p70 production 19. These effects significantly undermine the vaccine's ability to induce immune responses and therefore support the need to evaluate alternative methods of maturing DCs in order to optimize their effects in vivo.
The use of TLR agonists, in particular the TLR 3 agonist Poly-IC, to mature the DCs may improve the clinical efficacy of DCs. In vitro studies have shown that Poly-IC-matured DCs retained stable high expression of MHC molecules and CD83 and costimulatory molecules CD40, CD80, and CD86 14,15,20. Additionally, Poly-IC-matured DCs produced high levels of IL-12 14,16,20, an important cytokine for the generation of anti-tumor response 21, as well as other proinflammatory cytokines such as TNF-α, IL-6, IL-1β, IP-10, and type I IFNs 14. More importantly, as has been shown in murine tumor models, DCs pulsed with Human Papilloma Virus (HPV) antigens and matured with Poly-IC-primed cytotoxic T cell responses were capable of eradicating established HPV16-expressing tumors 22. As the maturation status of DCs and the presence of IL-12 appear to correlate with efficacy in clinical trials 23,24, the use of Poly-IC to mature DCs may be an important step towards attaining the goal of clinical success.
Disclosures
The authors have received financial support from the following: NIH (AI061684, AI071078, AI044628, and K08 AI084578 (Miller)), the Bill and Melinda Gates Foundation, Doris Duke Charitable Foundation, Cancer Research Institute, Alliance for Lupus Research, and the Emerald Foundation. Nina Bhardwaj is a coinventor on patents relating to the preparation and use of dendritic cells to manipulate immunity. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Acknowledgments
The authors would like to thank Andres Salazar (Oncovir, Inc.) for the gift of the Poly-ICLC.
References
- Lesterhuis WJ, et al. Dendritic cell vaccines in melanoma: from promise to proof. Crit. Rev. Oncol. Hematol. 2008;66:118–134. doi: 10.1016/j.critrevonc.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Sabado RL, Bhardwaj N. Directing dendritic cell immunotherapy towards successful cancer treatment. Immunotherapy. 2010;2:37–56. doi: 10.2217/imt.09.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher K. Keyhole limpet hemocyanin (KLH) conjugate vaccines as novel therapeutic tools in malignant disorders. J. Cancer. Res. Clin. Oncol. 2001;127(Suppl 2):1–2. doi: 10.1007/BF01470991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaatinen T, Laine J. Isolation of mononuclear cells from human cord blood by Ficoll-Paque density gradient. Curr. Protoc. Stem Cell Biol. 2007;Chapter 2:Unit 2A 1. doi: 10.1002/9780470151808.sc02a01s1. [DOI] [PubMed] [Google Scholar]
- Eichler H, et al. Multicenter study on in vitro characterization of dendritic cells. Cytotherapy. 2008;10:21–29. doi: 10.1080/14653240701744263. [DOI] [PubMed] [Google Scholar]
- Feuerstein B, et al. A method for the production of cryopreserved aliquots of antigen-preloaded, mature dendritic cells ready for clinical use. J. Immunol. Methods. 2000;245:15–29. doi: 10.1016/s0022-1759(00)00269-6. [DOI] [PubMed] [Google Scholar]
- O'Neill D, Bhardwaj N. Generation of autologous peptide- and protein-pulsed dendritic cells for patient-specific immunotherapy. Methods Mol. Med. 2005;109:97–112. [PubMed] [Google Scholar]
- de Vries IJ, et al. Phenotypical and functional characterization of clinical grade dendritic cells. J. Immunother. 2002;25:429–438. doi: 10.1097/00002371-200209000-00007. [DOI] [PubMed] [Google Scholar]
- Jonuleit H, et al. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur. J. Immunol. 1997;27:3135–3142. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- Lee AW, et al. A clinical grade cocktail of cytokines and PGE2 results in uniform maturation of human monocyte-derived dendritic cells: implications for immunotherapy. Vaccine. 2002;20(4):A8–A22. doi: 10.1016/s0264-410x(02)00382-1. [DOI] [PubMed] [Google Scholar]
- Bhardwaj N. Harnessing the immune system to treat cancer. J. Clin. Invest. 2007;117:1130–1136. doi: 10.1172/JCI32136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnjatic S, Sawhney NB, Bhardwaj N. Toll-like receptor agonists: are they good adjuvants? Cancer J. 2010;16:382–391. doi: 10.1097/PPO.0b013e3181eaca65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedl RM, Kappler JW, Marrack P. Epitope dominance, competition and T cell affinity maturation. Curr. Opin. Immunol. 2003;15:120–127. doi: 10.1016/s0952-7915(02)00009-2. [DOI] [PubMed] [Google Scholar]
- Bogunovic D, et al. TLR4 engagement during TLR3-induced proinflammatory signaling in dendritic cells promotes IL-10-mediated suppression of antitumor immunity. Cancer Research. 2011;71:5467–5476. doi: 10.1158/0008-5472.CAN-10-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdijk RM, et al. Polyriboinosinic polyribocytidylic acid (poly(I:C)) induces stable maturation of functionally active human dendritic cells. J. Immunol. 1999;163:57–61. [PubMed] [Google Scholar]
- Rouas R, et al. Poly(I:C) used for human dendritic cell maturation preserves their ability to secondarily secrete bioactive IL-12. Int. Immunol. 2004;16:767–773. doi: 10.1093/intimm/dxh077. [DOI] [PubMed] [Google Scholar]
- Jongmans W, Tiemessen DM, van Vlodrop IJ, Mulders PF, Oosterwijk E. Th1-polarizing capacity of clinical-grade dendritic cells is triggered by Ribomunyl but is compromised by PGE2: the importance of maturation cocktails. J. Immunother. 2005;28:480–487. doi: 10.1097/01.cji.0000171290.78495.66. [DOI] [PubMed] [Google Scholar]
- Krause P, et al. Prostaglandin E2 is a key factor for monocyte-derived dendritic cell maturation: enhanced T cell stimulatory capacity despite IDO. J. Leukoc. Biol. 2007;82:1106–1114. doi: 10.1189/jlb.0905519. [DOI] [PubMed] [Google Scholar]
- Morelli AE, Thomson AW. Dendritic cells under the spell of prostaglandins. Trends Immunol. 2003;24:108–111. doi: 10.1016/s1471-4906(03)00023-1. [DOI] [PubMed] [Google Scholar]
- Adams M, et al. Dendritic cell (DC) based therapy for cervical cancer: use of DC pulsed with tumour lysate and matured with a novel synthetic clinically non-toxic double stranded RNA analogue poly [I]:poly [C(12)U] (Ampligen R) Vaccine. 2003;21(12):787–790. doi: 10.1016/s0264-410x(02)00599-6. [DOI] [PubMed] [Google Scholar]
- Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:155–168. doi: 10.1016/s1359-6101(01)00032-6. [DOI] [PubMed] [Google Scholar]
- Mayordomo JI, et al. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat Med. 1995;1:1297–1302. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- Dhodapkar MV, et al. Rapid generation of broad T-cell immunity in humans after a single injection of mature dendritic cells. J. Clin. Invest. 1999;104:173–180. doi: 10.1172/JCI6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler-Thurner B, et al. Rapid induction of tumor-specific type 1 T helper cells in metastatic melanoma patients by vaccination with mature, cryopreserved, peptide-loaded monocyte-derived dendritic cells. J. Exp. Med. 2002;195:1279–1288. doi: 10.1084/jem.20012100. [DOI] [PMC free article] [PubMed] [Google Scholar]


