Abstract
Background & Aims
Clostridium difficile infection (CDI) can cause life-threatening complications. Severe complicated CDI is characterized by hypotension, shock, sepsis, ileus, megacolon, and colon perforation. We created a model to identify clinical factors associated with severe complicated CDI.
Methods
We analyzed data from 1446 inpatient cases of CDI (48.6% female, median age 62.5 y, range 0.1–103.7 y) at the Mayo Clinic from June 28, 2007 through June 25, 2010. Patients with severe complicated CDI (n=487) were identified as those who required admission to the intensive-care unit (ICU) or colectomy, or died, within 30 days of CDI diagnosis. Logistic regression models were used to identify variables that were independently associated with the occurrence of severe complicated CDI in 2 cohorts. One cohort comprised all hospitalized patients; the other comprised a subset of these inpatients who were residents of Olmsted County, MN, to assess the association of comorbid conditions with the development of severe complicated infection in a population-based cohort. The linear combinations of variables identified using logistic regression models provided scores to predict the risk of developing severe-complicated CDI.
Results
In a multivariable model that included all inpatients, increasing age, leukocyte count >15×109/L, increase in serum level of creatinine >1.5-fold from baseline, and use of proton pump inhibitors or narcotic medications were independently associated with severe complicated CDI. In the secondary analysis, which included only patients from Olmsted County, comorbid conditions were not significantly associated with severe complicated CDI.
Conclusion
Older age, high numbers of leukocytes in blood samples, an increased serum level of creatinine, gastric acid suppression, and use of narcotic medications were independently associated with development of severe complicated CDI in hospitalized patients. Early aggressive monitoring and intervention could improve outcomes.
Keywords: database analysis, antibiotic resistant bacteria, PPI use, risk factor
INTRODUCTION
Clostridium difficile is the leading cause of infectious diarrhea and may be associated with severe complications and mortality. The incidence of Clostridium difficile infection (CDI) in the hospital setting has increased significantly over the past 15 years.1 Recent work has also shown a growing incidence of CDI in the outpatient setting in patients who lack established risk factors including hospitalization and antibiotic exposure.2 The increased incidence of CDI may be associated with the emergence of a highly virulent strain combined with increased antibiotic use. Also, there has been an increase in the severity of the disease with associated complications and mortality. For instance, the mortality associated with CDI increased fourfold, from 5.7 to 23.7 per million, in the United States from 1999 to 2004.3
Severe CDI is defined by the Infectious Disease Society of America/Society for Healthcare Epidemiology of America (IDSA/SHEA) as peripheral leukocytosis ≥15×109/L or an increase in serum creatinine ≥1.5 times above baseline. However, the criteria to define severe infection are based on expert opinion and have not yet been extensively validated. Severe-complicated infection is defined by hypotension, shock, and sepsis, all of which likely require intensive care unit (ICU) level of care; ileus, megacolon, and perforation, often necessitating colectomy; or death.4
Predicting the severity of CDI is important since treatment strategies are stratified based on disease severity.4 Specifically, oral vancomycin is indicated for severe CDI, with addition of intravenous metronidazole for severe-complicated infection.4 Predictors of severity may serve as markers of the risk of progression to complicated disease and therefore signal a need for close clinical follow up and/or more aggressive treatment.
Several studies have assessed predictors of severe CDI,5, 6 including older age, nursing home residence, antibiotic and antiperistaltic medication use, renal insufficiency, peripheral leukocytosis, hypoalbuminemia, physical findings, number of bowel movements, fever (temperature greater than 38°C), and computed tomography (CT) findings.5, 7–14 However, abnormal CT findings (i.e. colonic wall thickening, colonic dilatation, or ascites5) may not be available in every patient with CDI, and physical examination findings or number of bowel movements may not be objective or consistently measured variables. Therefore, we sought to formulate an objective, severity prediction model to predict severe-complicated CDI that is readily applicable in the clinical setting.
METHODS
The microbiology laboratory database and patient medical records were queried to identify all inpatient cases of CDI at our institution between June 28, 2007 and June 25, 2010. CDI cases were based on C. difficile polymerase chain reaction (PCR) assay positivity and compatible clinical symptoms. Only patients whose first C. difficile PCR assay was positive were included in this analysis; those with subsequent positive tests obtained after a previous negative C. difficile PCR assay were excluded. We did not include any subsequent positive PCR test, as multiple positive PCR tests could have represented recurrent episodes of CDI. Patients with a positive C. difficile PCR but without clinical symptoms were excluded. The microbiology laboratory had transitioned to a PCR based assay for the detection of C. difficile in June 2007.15
Severe-complicated CDI was defined as the need for ICU admission, colectomy, or death within 30 days of CDI diagnosis. The electronic medical records were abstracted for patient demographics, weighted Charlson Comorbidity index16, fever >38°C, maximum peripheral leukocyte count, serum albumin, change in serum creatinine (compared to baseline over the past year), and serum lactate, all measured within 7 days of CDI diagnosis. These variables were obtained prior to ICU admission, colectomy, or death. Charlson co-morbidity index was studied only in Olmsted County patients as we were not confident we could accurately identify all comorbidities in our referral population. We also abstracted information on medication use, which included antibiotics (divided into two periods, 90 days before diagnosis, and within 30 days after diagnosis), narcotics (opiate derivatives), histamine-2 receptor blockers, proton pump inhibitors (PPI), and antimotility drugs (all recorded between 7 days before and 30 days after diagnosis). Histamine-2 blockers and PPIs were analyzed together as gastric acid suppression medications. Peripheral leukocytosis was dichotomized as greater than or less than 15×109/L. Antimotility agents included loperamide, prochlorperazine, diphenoxylate/atropine, and bismuth subsalicylate. Antibacterial use was studied 30 days after CDI diagnosis to assess whether or not ongoing use predicts severe-complicated disease. We chose to include antibiotic use after diagnosis in a prognostic model since in many cases a clinician knows that a patient with CDI will need ongoing antibiotics after the diagnosis is made. Vancomycin and metronidazole were excluded from the list of antibacterials that were analyzed as risk factors.
Statistical analysis
Descriptive statistics for demographics and outcomes are reported as median (range) or frequency (percent). Univariate and multiple variable logistic regression models examined a set of clinical and demographic variables based on previous assessments in the literature. These models were used to assess the association of the candidate variables with complicated CDI. Serum albumin and lactate were excluded from the multivariable model due to missing data, as more than 50% of patients lacked these laboratory results.
Two multivariable models were obtained based on clinical judgment and formal statistical assessment (at an alpha level of 0.05). The choice of variables to include in these final multivariable models was based on the amount of missing data. The first model was based on the cohort of all (local and referred) inpatients. Due to the lack of accurate and complete information on comorbidities in our referral population, a second model was restricted to residents of Olmsted County to enable the assessment of the association of co-morbidities with the risk of developing severe-complicated CDI. Only those patients with complete data were included in the analysis of individual variables. The multivariable model in all inpatients provided a score based on the linear weighted combination of the variables retained in that model. This score was then used to illustrate the predicted probabilities (i.e. risk) of developing severe-complicated CDI given several specific combinations of the variables retained in the model. The score was also used to generate receiver operating characteristic (ROC) curves illustrating the sensitivity and specificity of this score for predicting severe-complicated CDI. This work was approved by the institutional review boards of Mayo Clinic and Olmsted Medical Center.
RESULTS
A total of 1446 inpatients were identified during the study. The cohort included 702 females (48.5%) and had a median age of 62.5 years (range 0.1–103.7). A total of 487 patients (33.7%) had a severe-complicated infection. The frequencies of ICU admission, colectomy, and death within 30 days of diagnosis were 26.7%, 2.7%, and 8.9%, respectively. All patients either developed a defined complication within 30 days or had greater than 30 days of follow up.
Elevated peripheral leukocyte count, increasing serum creatinine, increasing age, low serum albumin, and elevated serum lactate were associated with severe-complicated CDI on univariate analysis (Table 1). The following medications were associated with severe-complicated CDI on univariate analysis: narcotics and H2 blockers or PPIs (Table 1). Use of antimotility agents was not significantly associated with severe-complicated CDI. Antibiotic use was specifically divided into 90 days prior to CDI diagnosis (n=374 [25.9%]) and within 30 days after CDI (n=230, [15.9%]). A total of 614 patients (42.5%) had antibiotic use both prior to and after CDI diagnosis. Both prior and concomitant antibiotic exposure was associated with severe complicated CDI in univariate analyses (Table 2).
Table 1.
Univariate logistic regression model used to predict severe-complicated C. difficile infection in hospitalized patients (n=1446).
| Variable | Odds Ratio | 95% Confidence Interval | p-value |
|---|---|---|---|
| Female gender | 0.9 | 0.7–1.1 | 0.4 |
|
| |||
| Age per 10 years1 | 1.1 | 1.0–1.2 | <0.0001 |
|
| |||
| Weighted Charlson Comorbidity index2 | |||
|
| |||
| =0 | 1.0(reference) | 0.0008 | |
| =1 | 5.7 | 1.8–17.6 | 0.0023 |
| >1 | 6.0 | 2.3–15.4 | 0.0002 |
|
| |||
| Peripheral WBC count 3 | 2.7 | 2.1–3.4 | <0.0001 |
|
| |||
| Serum creatinine increase 4 | 2.1 | 1.6–2.6 | <0.0001 |
|
| |||
| Serum albumin5 | 0.6 | 0.4–0.8 | <0.0001 |
|
| |||
| Fever6 | 1.6 | 1.1–2.3 | 0.01 |
|
| |||
| Serum lactate7 | 1.5 | 1.3–1.7 | <0.0001 |
|
| |||
| Narcotic use8 | 2.5 | 1.9–3.2 | <0.0001 |
|
| |||
| H2 blockers or PPI use8 | 2.7 | 2.0–3.6 | <0.0001 |
|
| |||
| Antimotility use8 | 1.0 | 0.7–1.5 | 0.79 |
Age was treated as a continuous variable and studied in 10 year intervals
Limited to Olmsted County, MN patients (n=535)
Peripheral white blood cell (WBC) count was dichotomized as ≥15 × 109/L or <15 × 109/L
Increase in serum creatinine was dichotomized as ≥1.5 fold or <1.5 fold compared to baseline
Serum albumin was treated as a continuous variable
Temperature was dichotomized as ≥38.3 degrees Celsius or <38.3 degrees
Serum lactate was treated as a continuous variable
Medication use was examined 7 days prior to and 30 days after C. difficile infection diagnosis
Table 2.
Univariate analysis of antibiotic use as a predictor of severe-complicated C. difficile infection (CDI) in hospitalized patients (n=1446). Antibiotic use was divided into two time periods: 90 days prior to CDI diagnosis and 30 days after CDI diagnosis.
| Time period of antibiotic use | Odds Ratio | 95% Confidence Interval | p-value |
|---|---|---|---|
| 30 days after CDI diagnosis | 2.5 | 1.6–3.8 | <0.001 |
| 90 days prior to CDI diagnosis | 1.7 | 1.2–2.5 | 0.007 |
| 90 days prior to and 30 days after CDI diagnosis | 2.4 | 1.7–3.4 | <0.001 |
| No antibiotic use | 1.0 | Reference |
There were 252 patients with missing baseline creatinine values, and these patients were excluded from the multivariable analysis, with 1194 (82.6%) of 1446 patients included in the final multiple variable model for all inpatients. Severe-complicated infection occurred in 37.3% of these excluded patients, similar to the 32.9% (393/1194) with severe-complicated infection among those included in this analysis. Age was associated with inclusion in the model (older subjects being more likely to have complete creatinine data).
In the multivariable model for all inpatients, increasing age, elevated peripheral leukocyte count, increasing serum creatinine, H2 blocker/PPI use, and narcotic use were independent predictors of severe-complicated CDI, but ongoing antibiotic exposure was not a predictor of severe complicated CDI (Table 3). The parameter estimates from this model were used to calculate a score (weighted linear combination) to predict the risk of developing severe-complicated CDI. Each variable other than age was dichotomized (0=no, 1=yes) based on current guidelines:
Table 3.
Multivariable logistic regression to predict severe-complicated C. difficile infection in hospitalized patients (n=1446).
| Variable | Odds Ratio | 95% Confidence Interval | p-value |
|---|---|---|---|
| Peripheral WBC count1 | 2.2 | 1.7–2.9 | <0.0001 |
| Serum creatinine rise2 | 1.6 | 1.3–2.1 | <0.0001 |
| Age, per 10 years3 | 1.1 | 1.0–1.1 | 0.0029 |
| Narcotic use4 | 2.1 | 1.5–3.0 | <0.0001 |
| H2Blocker/PPI4 | 1.8 | 1.3–2.6 | 0.0002 |
Peripheral white blood cell (WBC) count was dichotomized in ≥15 × 109/L and <15 × 109/L
Increase in serum creatinine 1.5 fold or more from baseline was compared to an increase <1.5-fold from baseline
Age was treated as a continuous variable and studied in 10 year intervals
Medication use was examined 7 days prior to and 30 days after C. difficile infection diagnosis
This score was then used to predict the risk of severe-complicated CDI using the following equation:
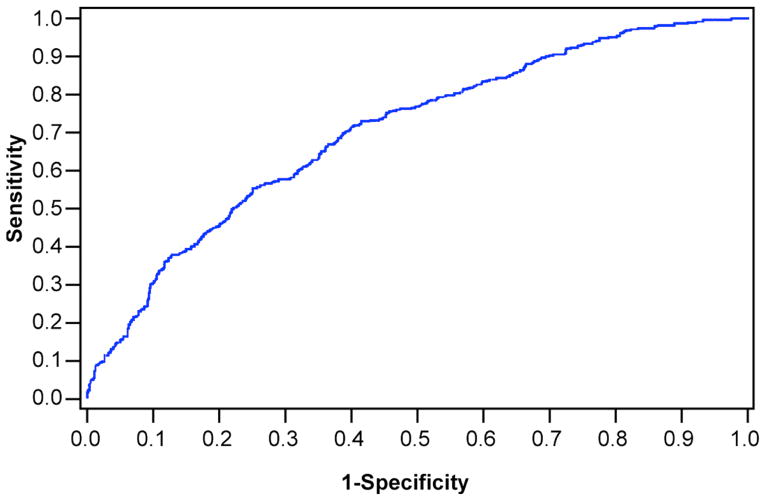
The receiver-operator curve for this model is shown in Figure 1. The corresponding sensitivities and specificities for various cut-off scores of this model in predicting the risk of severe-complicated CDI are shown in Table 4. For instance, a model output of −1.10 predicts a 25% probability of developing severe complicated CDI with a sensitivity of 80% and specificity of 46%.
Figure 1.
Receiver operator characteristic curve (ROC) using the inpatient prediction model to predict severe-complicated Clostridium difficile infection. Area under the curve is 0.706.
Table 4.
Sensitivities and specificities for various cut-off scores of the complete inpatient prediction model for severe-complicated Clostridium difficile infection
| Model output | Probability of severe CDI | Sensitivity | Specificity |
|---|---|---|---|
| −1.6 | 0.1 | 94% | 22% |
| −1.4 | 0.1 | 90% | 31% |
| −1.2 | 0.2 | 85% | 36% |
| −1.1 | 0.2 | 80% | 46% |
| −0.8 | 0.3 | 70% | 61% |
| −0.4 | 0.3 | 53% | 76% |
C-statistic = 0.706
An analysis of Olmsted County hospitalized patients indicated that the weighted Charlson Comorbidity index (categorized as no comorbidities [0, reference level], any 1 comorbidity, and >1 comorbidity) was significant on univariate analysis, however was not significantly associated with severe-complicated CDI after controlling for other variables in the model. Compared to patients with no comorbidities (Charlson index =0), those with a Charlson index =1 (OR 2.2, 95% CI [0.6–8.4]) or a Charlson index>1 (OR 1.5, 95% CI [0.5–4.2]) were not more likely to develop severe-complicated CDI (p= 0.46).
DISCUSSION
In this study, peripheral leukocyte ≥15×109/L, serum creatinine rise ≥ 1.5 fold compared to baseline, increasing age, narcotic use, and acid suppression were independent predictors of severe-complicated CDI in hospitalized patients. In the population-based model, comorbid conditions (as measured by the Charlson Comorbidity index) were not independent predictors of severe-complicated infection after controlling for these significant variables. Our findings validate expert guideline recommendations that advocate the use of an elevated peripheral leukocyte count and change in serum creatinine to predict severe CDI.4 In addition, our work was able to combine weighted variables to create a clinically applicable severity prediction score; this model predicted the risk of severe-complicated infection with an area under the ROC curve of 0.706.
On univariate analysis, serum albumin and lactate were associated with the development of severe-complicated CDI, but were not included in the multivariable model due to high rates of missing data. It is conceivable that the model could have better predicted the risk of severe-complicated CDI if these variables were included. Fever was not prognostic of severe-complicated CDI on univariate analysis, and therefore was not included in the multivariable model. Interestingly, ongoing antibiotic use was a significant predictor on univariate analysis, but was not independently prognostic of severe-complicated CDI in the multivariable model.
We included medication use after CDI diagnosis to assess the prognostic effect of continued exposure to these medications on the development of severe-complicated CDI. We analyzed ongoing antibiotic use in our prognostic model at the time of CDI diagnosis since in many cases a clinician will know at the time of CDI diagnosis that a patient will need ongoing antibiotics or narcotics, and if they had been prognostic of worse outcomes on multivariate analysis, that information could have been used to predict severity even at the time of diagnosis. We also decided to include ongoing use of antibiotics based on previous work, such by Mullane, et al., which revealed worse outcomes in patients with CDI who were exposed to concomitant antibiotics. Our study supports those findings based on univariate analysis but not multivariate analysis17.
Our weighted prediction for this model showed peripheral leukocytosis ≥15×109/L, use of narcotics and gastric acid suppression medications, and rising serum creatinine were the most important risk factors for predicting severe-complicated infection; age was less important. Based on our formula, variables with greater weight are more prognostic of the risk of developing severe-complicated CDI compared to those with less weight. For instance, leukocyte count is the most prognostic (weight=0.81) compared to age, which is least prognostic (weight=0.01). Since current guidelines indicate that treatment of CDI is stratified by severity, the higher an individual’s score in our model (e.g. WBC >15×109/L), the more justified a clinician would be in using vancomycin as initial treatment. Regarding the lack of association between comorbid conditions, based on a weighted Charlson Comorbidity index and the risk of severe CDI, other work, similarly found that comorbidities were not predictive of severe CDI in patients with inflammatory bowel disease complicated by CDI.18
A recent study showed that use of gastric acid suppressive medications and corticosteroids, older age, and inpatient admission for CDI were associated with complications.19 Also, older age and acid suppression were associated with increased mortality.19 The association between acid suppression and CDI was postulated to be secondary to the inability to inactivate the vegetative form of C. difficile in decreased acidic environments.19 However, these results are difficult to interpret as only 10% of the cohort in this study (n=47) had complicated CDI and the analysis was not adjusted for age or comorbidities.
The observation that narcotic use was independently prognostic of severe-complicated CDI is novel. Narcotic use could alter gut motility and thereby increase risk for complications such as megacolon and perforation. Also, patients with severe disease could require more narcotic medication for pain control. A similar observation has been made in patients with Crohn’s disease, where narcotic use was an independent predictor of serious infections.20
Recent work by Lungulescu, et al. defined a severe CDI prediction score at the time of hospital admission. Severe disease was associated with a peripheral white blood cell count greater than ≥20×109/L, low albumin, or a serum creatinine 1.5 fold greater than baseline.6 Limitations of this study included a small sample size (n=255, severe CDI, n=47), older study population compared to our study population (median age of patients with severe disease was 78.6 years and with non-severe disease 74 years), and a lack of assessment of antibiotic use as a risk factor. Also, our study assessed the current recommended cut-off for a peripheral white blood cell count (>15×109/L), which was prognostic of severe CDI.
Fugitani, et al. recently compared multiple CDI severity prediction models and found the Hines VA index to be the strongest predictor of severe CDI. The Hines VA model includes fever, low systolic pressure, peripheral leukocytosis, and abnormal findings on CT scan. However, this model may have been confounded by the fact that patients with severe CDI are more likely to undergo CT scanning than those with mild disease. We also found peripheral leukocytosis to be significant, but not fever. We chose not to study blood pressure, as this could be a transient finding easily corrected by fluid resuscitation. Rather, we chose to study ICU admission, which would require more severe or persistent hypotension, and thus is a more robust marker of severe infection. As opposed to our study, the Hines VA model did not assess medication use, serum albumin or lactate as predictors of severe complicated CDI.
There have been other recent studies that have proposed risk factors for the development of severe-complicated CDI.21, 22 One study of 365 patients from the University of Toronto found severe disease in 97 patients (26.6%), with repeat infection, confusion, hypotension, and elevated peripheral white blood cell count associated with severe complicated CDI.21 Protection was associated with early use of vancomycin and harm was associated with ongoing antibiotic use. We also showed that antibiotic use prior to and after CDI diagnosis predicted risk for severe-complicated CDI, but this variable was no longer significant on multivariable analysis. Confusion is a subjective finding, and hypotension can be transient as noted above, therefore we did not include these variables in our investigation.
Another study from Louisiana State University concluded that peripheral white blood cell count, platelet count, and serum albumin levels were not predictive of severe CDI in immunosuppressed patients.22 This conclusion should be interpreted with caution, however, since this was a small study with 29 patients, only 9 of whom had severe disease.22
The strength of our study lies in the large sample size, which afforded statistical power to assess multiple variables for their association with the development of severe-complicated CDI. On the other hand, limitations of our study included missing data in some patients (e.g. serum lactate, serum albumin, and temperature) that limited our ability to assess these variables in multiple variable models. Regardless, after excluding patients who were missing these variables, there were 1194 patients in the final model. Also, a small percentage of patients were diagnosed with CDI after ICU admission (9.8%), however we felt comfortable including them in the study as they may have proceeded to have other complications of CDI, i.e. colectomy or death. In these patients, ICU admission was not considered as indicative of severe complicated CDI. Finally, we did not study mode of acquisition (nosocomial versus community-acquired), recurrent CDI, or length of hospital stay as predictors of severe-complicated CDI.
In conclusion, the need to elucidate factors contributing to the risk of developing severe CDI is evidenced by the number of prediction models for severe-complicated CDI in the literature. The importance of identifying these factors is evidenced by the poorer outcomes of patients with severe disease, and the improvement in outcomes if patients with severe infection are treated with vancomycin as compared to metronidazole.11 We have shown that a peripheral leukocyte count ≥15×109/L, creatinine rise ≥1.5 fold from baseline, older age, and use of gastric acid suppression and narcotic medications independently predict severe-complicated CDI in hospitalized patients. The presence of co-morbidities did not predict severe-complicated CDI in our population-based cohort. Future studies should validate this model by determining whether early treatment directed by the severity score improves patient outcomes, and also prospectively assess whether the inclusion of serum albumin and lactate improve the operating characteristics of the model. Currently, aggressive monitoring and treatment of patients predicted to have severe-complicated CDI early in the hospital course appears warranted and may help minimize or prevent complications.
Acknowledgments
Grant support: Mayo Clinic, Division of Gastroenterology and Hepatology, Small Grants Program. This publication was made possible by the Mayo Clinic CTSA through grant number UL1 RR024150 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH).
Abbreviations
- CDI
Clostridium difficile infection
Footnotes
Conflicts of interest: None.
Writing assistance: None.
Author contribution: Study design, data collection, statistical analysis, and drafting the manuscript: Raina Shivashankar, Sahil Khanna, Darrell S. Pardi, and Patricia P. Kammer; drafting the manuscript: Larry M. Baddour; study design, statistical analysis, and drafting the manuscript: W. Scott Harmsen, and Alan R. Zinsmeister.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kelly CP, LaMont JT. Clostridium difficile--more difficult than ever. N Engl J Med. 2008;359:1932–40. doi: 10.1056/NEJMra0707500. [DOI] [PubMed] [Google Scholar]
- 2.Khanna S, Pardi DS, Aronson SL, et al. The Epidemiology of Community-Acquired Clostridium difficile Infection: A Population-Based Study. Am J Gastroenterol. 2011;107:89–95. doi: 10.1038/ajg.2011.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khanna S, Pardi DS. The growing incidence and severity of Clostridium difficile infection in inpatient and outpatient settings. Expert Rev Gastroenterol Hepatol. 2010;4:409–16. doi: 10.1586/egh.10.48. [DOI] [PubMed] [Google Scholar]
- 4.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431–55. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 5.Fujitani S, George WL, Murthy AR. Comparison of clinical severity score indices for Clostridium difficile infection. Infect Control Hosp Epidemiol. 2011;32:220–8. doi: 10.1086/658336. [DOI] [PubMed] [Google Scholar]
- 6.Lungulescu OA, Cao W, Gatskevich E, et al. CSI: a severity index for Clostridium difficile infection at the time of admission. J Hosp Infect. 2011;79:151–4. doi: 10.1016/j.jhin.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Rubin MS, Bodenstein LE, Kent KC. Severe Clostridium difficile colitis. Dis Colon Rectum. 1995;38:350–4. doi: 10.1007/BF02054220. [DOI] [PubMed] [Google Scholar]
- 8.McEllistrem MC, Carman RJ, Gerding DN, et al. A hospital outbreak of Clostridium difficile disease associated with isolates carrying binary toxin genes. Clin Infect Dis: an official publication of the Infectious Diseases Society of America. 2005;40:265–72. doi: 10.1086/427113. [DOI] [PubMed] [Google Scholar]
- 9.Louie TJ, Peppe J, Watt CK, et al. Tolevamer, a novel nonantibiotic polymer, compared with vancomycin in the treatment of mild to moderately severe Clostridium difficile-associated diarrhea. Clin Infect Dis: an official publication of the Infectious Diseases Society of America. 2006;43:411–20. doi: 10.1086/506349. [DOI] [PubMed] [Google Scholar]
- 10.Belmares J, Gerding DN, Parada JP, et al. Outcome of metronidazole therapy for Clostridium difficile disease and correlation with a scoring system. J Infect. 2007;55:495–501. doi: 10.1016/j.jinf.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 11.Zar FA, Bakkanagari SR, Moorthi KM, et al. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis: an official publication of the Infectious Diseases Society of America. 2007;45:302–7. doi: 10.1086/519265. [DOI] [PubMed] [Google Scholar]
- 12.Gujja D, Friedenberg FK. Predictors of serious complications due to Clostridium difficile infection. Aliment Pharmacol Ther. 2009;29:635–42. doi: 10.1111/j.1365-2036.2008.03914.x. [DOI] [PubMed] [Google Scholar]
- 13.Louie T, Gerson M, Grimard D. Program and abstracts of the 47th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, DC: American Society for Microbiology; 2007. Results of a phase III trial comparing tolevamer, vancomycin and metronidazole in Clostridium difficile-associated diarrhea (CDAD) p. Abstract K 425a. [Google Scholar]
- 14.University of Pittsburgh Medical Center. [Date accessed: November 2011];Guide to Antimicrobial Chemotherapy. (4). 2008 http://www.residency.dom.pitt.edu/Hcorner/Documents/AntimicrobialGuide2008.
- 15.Khanna S, Pardi DS, Rosenblatt JE, et al. An evaluation of repeat stool testing for Clostridium difficile infection by polymerase chain reaction. J Clin Gastroenterol. 2012;46:846–9. doi: 10.1097/MCG.0b013e3182432273. [DOI] [PubMed] [Google Scholar]
- 16.Guzzo TJ, Dluzniewski P, Orosco R, et al. Prediction of mortality after radical prostatectomy by Charlson Comorbidity Index. Urology. 2010;76:553–557. doi: 10.1016/j.urology.2010.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mullane KM, Miller MA, Weiss K, et al. Efficacy of Fidaxomicin Versus Vancomycin as Therapy for Clostridium difficile Infection in Individuals Taking Concomitant Antibiotics for Other Concurrent Infections. Clin Infect Dis. 2011;53:440–447. doi: 10.1093/cid/cir404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ananthakrishnan AN, Guzman-Perez R, Gainer V, et al. Predictors of severe outcomes associated with Clostridium difficile infection in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2012;35:789–95. doi: 10.1111/j.1365-2036.2012.05022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrison RH, Hall NS, Said M, et al. Risk Factors Associated With Complications and Mortality in Patients With Clostridium difficile Infection. Clin Infect Dis: an official publication of the Infectious Diseases Society of America. 2011;53:1173–8. doi: 10.1093/cid/cir668. [DOI] [PubMed] [Google Scholar]
- 20.Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infections and mortality in association with therapies for Crohn’s disease: TREAT registry. Clin Gastroenterol Hepatol 2006. 2006;4:621–30. doi: 10.1016/j.cgh.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Manek K, Williams V, Callery S, et al. Reducing the risk of severe complications among patients with Clostridium difficile infection. Can J Gastroenterol. 2011;25:368–72. doi: 10.1155/2011/153020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pant C, Sferra TJ, Ondrade C, et al. Serum markers for severe Clostridium difficile infection in immunosuppressed hospitalized patients. J La State Med Soc. 2011;163:91–4. [PubMed] [Google Scholar]
- 23.Khanna S, Aronson SL, Kammer PP, et al. Gastric acid suppression and outcomes from Clostridium difficile infection: A population-based study. Mayo Clin Proc. 2012;87:636–42. doi: 10.1016/j.mayocp.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khanna S, Pardi DS, Aronson SL, et al. Outcomes in community acquired Clostridium difficile infection. Aliment Pharmacol Ther. 2012;35:613–8. doi: 10.1111/j.1365-2036.2011.04984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]