Abstract
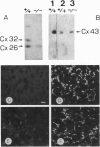
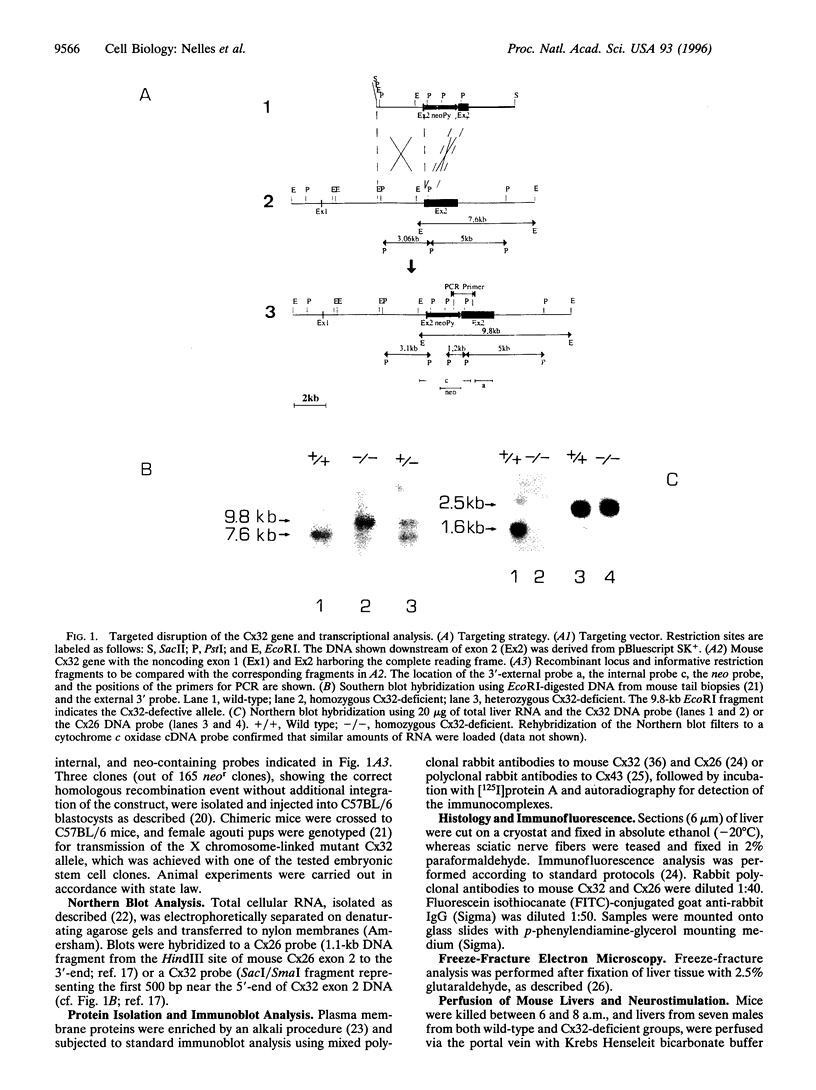
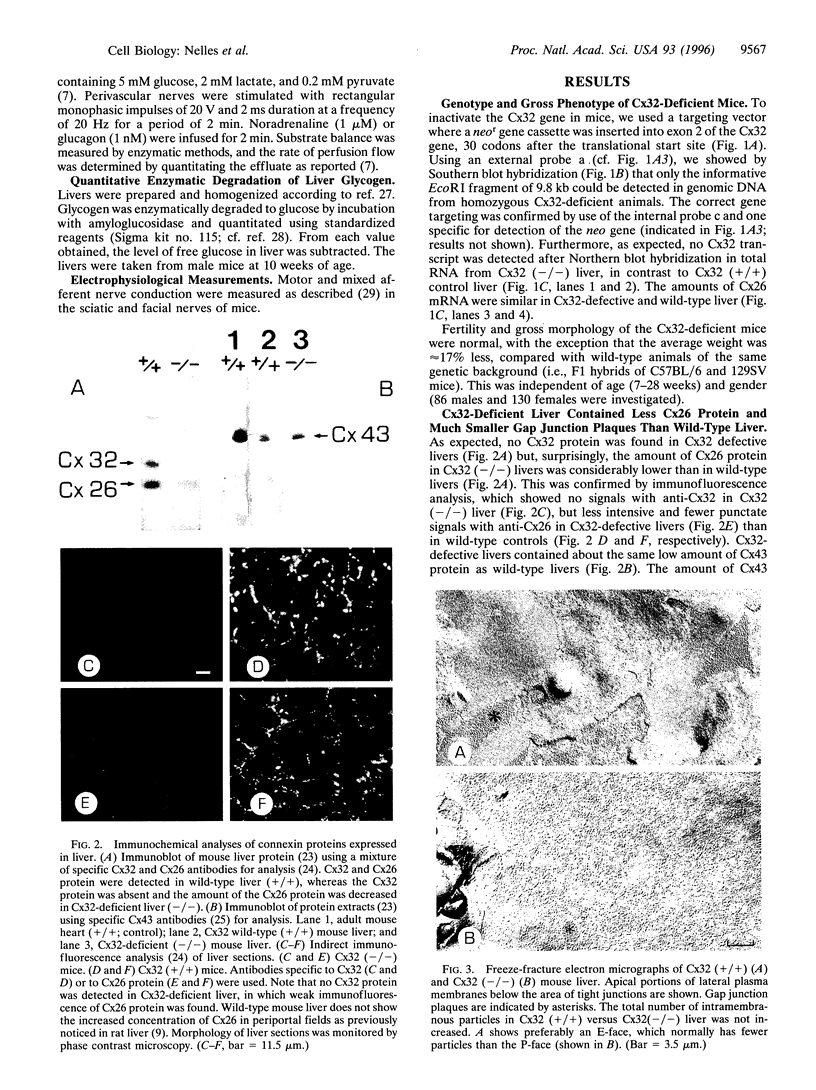
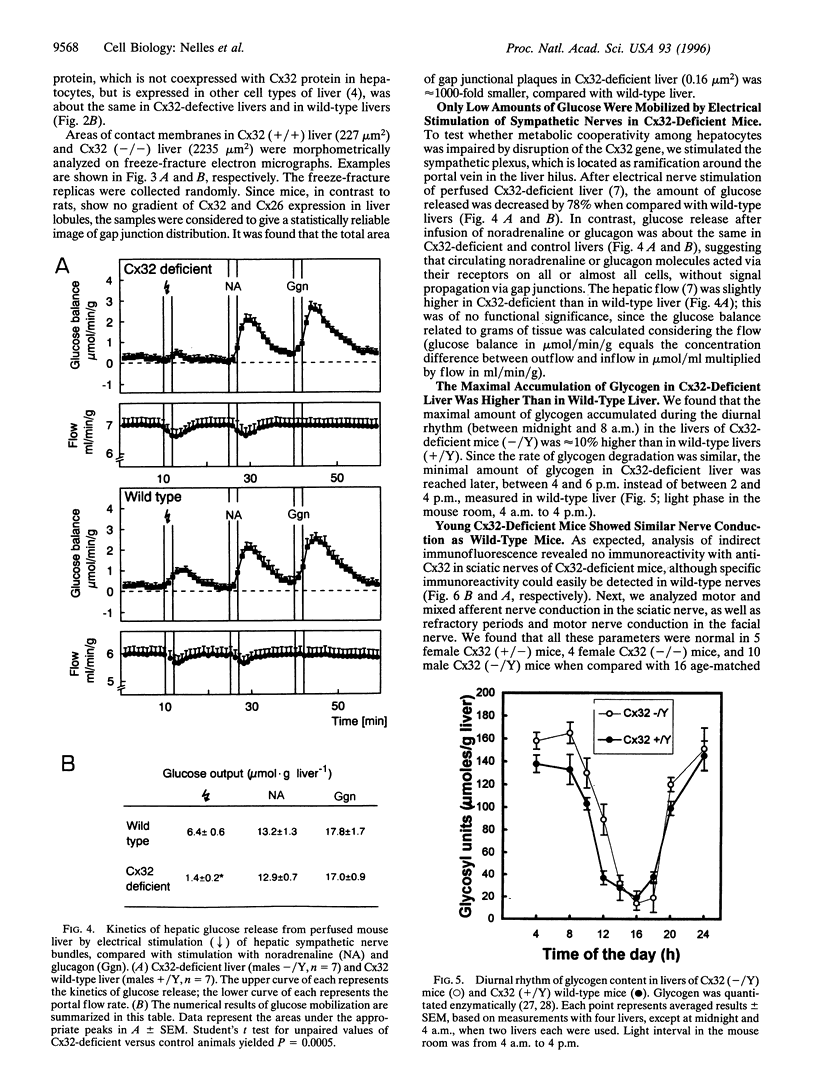
The gap junctional protein connexin32 is expressed in hepatocytes, exocrine pancreatic cells, Schwann cells, and other cell types. We have inactivated the connexin32 gene by homologous recombination in the mouse genome and have generated homozygous connexin32-deficient mice that were viable and fertile but weighed on the average approximately 17% less than wild-type controls. Electrical stimulation of sympathetic nerves in connexin32-deficient liver triggered a 78% lower amount of glucose mobilization from glycogen stores, when compared with wild-type liver. Thus, connexin32-containing gap junctions are essential in mouse liver for maximal intercellular propagation of the noradrenaline signal from the periportal (upstream) area, where it is received from sympathetic nerve endings, to perivenous (downstream) hepatocytes. In connexin32-defective liver, the amount of connexin26 protein expressed was found to be lower than in wild-type liver, and the total area of gap junction plaques was approximately 1000-fold smaller than in wild-type liver. In contrast to patients with connexin32 defects suffering from X chromosome-linked Charcot-Marie-Tooth disease (CMTX) due to demyelination in Schwann cells of peripheral nerves, connexin32-deficient mice did not show neurological abnormalities when analyzed at 3 months of age. It is possible, however, that they may develop neurodegenerative symptoms at older age.
Full text
PDF
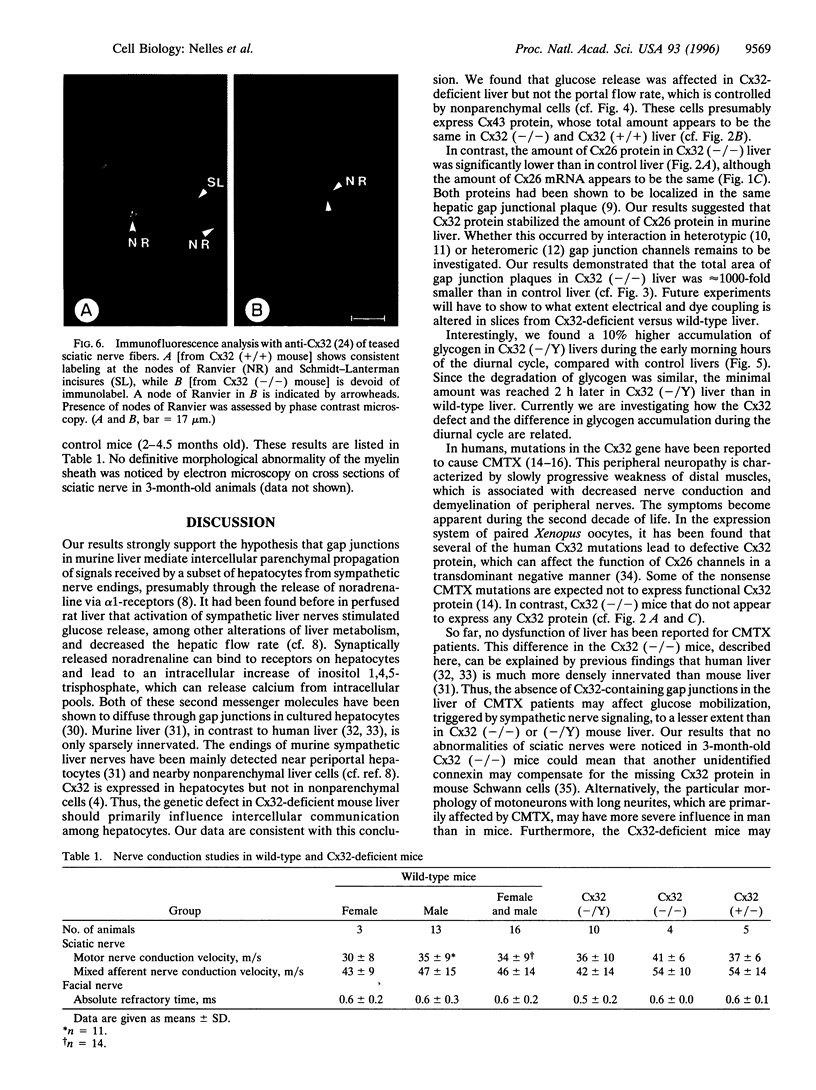

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrio L. C., Suchyna T., Bargiello T., Xu L. X., Roginski R. S., Bennett M. V., Nicholson B. J. Gap junctions formed by connexins 26 and 32 alone and in combination are differently affected by applied voltage. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8410–8414. doi: 10.1073/pnas.88.19.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. V., Barrio L. C., Bargiello T. A., Spray D. C., Hertzberg E., Sáez J. C. Gap junctions: new tools, new answers, new questions. Neuron. 1991 Mar;6(3):305–320. doi: 10.1016/0896-6273(91)90241-q. [DOI] [PubMed] [Google Scholar]
- Bergoffen J., Scherer S. S., Wang S., Scott M. O., Bone L. J., Paul D. L., Chen K., Lensch M. W., Chance P. F., Fischbeck K. H. Connexin mutations in X-linked Charcot-Marie-Tooth disease. Science. 1993 Dec 24;262(5142):2039–2042. doi: 10.1126/science.8266101. [DOI] [PubMed] [Google Scholar]
- Beyer E. C. Gap junctions. Int Rev Cytol. 1993;137C:1–37. [PubMed] [Google Scholar]
- Bruzzone R., White T. W., Scherer S. S., Fischbeck K. H., Paul D. L. Null mutations of connexin32 in patients with X-linked Charcot-Marie-Tooth disease. Neuron. 1994 Nov;13(5):1253–1260. doi: 10.1016/0896-6273(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Carroll J. J., Smith N., Babson A. L. A colorimetric serum glucose determination using hexokinase and glucose-6-phosphate dehydrogenase. Biochem Med. 1970 Sep;4(2):171–180. doi: 10.1016/0006-2944(70)90093-1. [DOI] [PubMed] [Google Scholar]
- Chandross K. J., Chanson M., Spray D. C., Kessler J. A. Transforming growth factor-beta 1 and forskolin modulate gap junctional communication and cellular phenotype of cultured Schwann cells. J Neurosci. 1995 Jan;15(1 Pt 1):262–273. doi: 10.1523/JNEUROSCI.15-01-00262.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dermietzel R. Junctions in the central nervous system of the cat. I. Membrane fusion in central myelin. Cell Tissue Res. 1974 May 8;148(4):565–576. doi: 10.1007/BF00221940. [DOI] [PubMed] [Google Scholar]
- Elfgang C., Eckert R., Lichtenberg-Fraté H., Butterweck A., Traub O., Klein R. A., Hülser D. F., Willecke K. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J Cell Biol. 1995 May;129(3):805–817. doi: 10.1083/jcb.129.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairweather N., Bell C., Cochrane S., Chelly J., Wang S., Mostacciuolo M. L., Monaco A. P., Haites N. E. Mutations in the connexin 32 gene in X-linked dominant Charcot-Marie-Tooth disease (CMTX1) Hum Mol Genet. 1994 Jan;3(1):29–34. doi: 10.1093/hmg/3.1.29. [DOI] [PubMed] [Google Scholar]
- Forssmann W. G., Ito S. Hepatocyte innervation in primates. J Cell Biol. 1977 Jul;74(1):299–313. doi: 10.1083/jcb.74.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardemann A., Püschel G. P., Jungermann K. Nervous control of liver metabolism and hemodynamics. Eur J Biochem. 1992 Jul 15;207(2):399–411. doi: 10.1111/j.1432-1033.1992.tb17063.x. [DOI] [PubMed] [Google Scholar]
- Hennemann H., Kozjek G., Dahl E., Nicholson B., Willecke K. Molecular cloning of mouse connexins26 and -32: similar genomic organization but distinct promoter sequences of two gap junction genes. Eur J Cell Biol. 1992 Jun;58(1):81–89. [PubMed] [Google Scholar]
- Hertzberg E. L. A detergent-independent procedure for the isolation of gap junctions from rat liver. J Biol Chem. 1984 Aug 10;259(15):9936–9943. [PubMed] [Google Scholar]
- Ionasescu V., Searby C., Ionasescu R. Point mutations of the connexin32 (GJB1) gene in X-linked dominant Charcot-Marie-Tooth neuropathy. Hum Mol Genet. 1994 Feb;3(2):355–358. doi: 10.1093/hmg/3.2.355. [DOI] [PubMed] [Google Scholar]
- Iwai M., Miyashita T., Shimazu T. Inhibition of glucose production during hepatic nerve stimulation in regenerating rat liver perfused in situ. Possible involvement of gap junctions in the action of sympathetic nerves. Eur J Biochem. 1991 Aug 15;200(1):69–74. doi: 10.1111/j.1432-1033.1991.tb21049.x. [DOI] [PubMed] [Google Scholar]
- Keppler D., Decker K. Studies on the mechanism of galactosamine-1-phosphate and its inhibition of UDP-glucose pyrophosphorylase. Eur J Biochem. 1969 Sep;10(2):219–225. doi: 10.1111/j.1432-1033.1969.tb00677.x. [DOI] [PubMed] [Google Scholar]
- Laird P. W., Zijderveld A., Linders K., Rudnicki M. A., Jaenisch R., Berns A. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 1991 Aug 11;19(15):4293–4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E., Bestor T. H., Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992 Jun 12;69(6):915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- Montag D., Giese K. P., Bartsch U., Martini R., Lang Y., Blüthmann H., Karthigasan J., Kirschner D. A., Wintergerst E. S., Nave K. A. Mice deficient for the myelin-associated glycoprotein show subtle abnormalities in myelin. Neuron. 1994 Jul;13(1):229–246. doi: 10.1016/0896-6273(94)90472-3. [DOI] [PubMed] [Google Scholar]
- Nicholson B., Dermietzel R., Teplow D., Traub O., Willecke K., Revel J. P. Two homologous protein components of hepatic gap junctions. Nature. 1987 Oct 22;329(6141):732–734. doi: 10.1038/329732a0. [DOI] [PubMed] [Google Scholar]
- Paul D. L. New functions for gap junctions. Curr Opin Cell Biol. 1995 Oct;7(5):665–672. doi: 10.1016/0955-0674(95)80108-1. [DOI] [PubMed] [Google Scholar]
- Reaume A. G., de Sousa P. A., Kulkarni S., Langille B. L., Zhu D., Davies T. C., Juneja S. C., Kidder G. M., Rossant J. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995 Mar 24;267(5205):1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- Seseke F. G., Gardemann A., Jungermann K. Signal propagation via gap junctions, a key step in the regulation of liver metabolism by the sympathetic hepatic nerves. FEBS Lett. 1992 Apr 27;301(3):265–270. doi: 10.1016/0014-5793(92)80254-e. [DOI] [PubMed] [Google Scholar]
- Stauffer K. A. The gap junction proteins beta 1-connexin (connexin-32) and beta 2-connexin (connexin-26) can form heteromeric hemichannels. J Biol Chem. 1995 Mar 24;270(12):6768–6772. [PubMed] [Google Scholar]
- Sáez J. C., Connor J. A., Spray D. C., Bennett M. V. Hepatocyte gap junctions are permeable to the second messenger, inositol 1,4,5-trisphosphate, and to calcium ions. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2708–2712. doi: 10.1073/pnas.86.8.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Traub O., Eckert R., Lichtenberg-Fraté H., Elfgang C., Bastide B., Scheidtmann K. H., Hülser D. F., Willecke K. Immunochemical and electrophysiological characterization of murine connexin40 and -43 in mouse tissues and transfected human cells. Eur J Cell Biol. 1994 Jun;64(1):101–112. [PubMed] [Google Scholar]
- Traub O., Look J., Dermietzel R., Brümmer F., Hülser D., Willecke K. Comparative characterization of the 21-kD and 26-kD gap junction proteins in murine liver and cultured hepatocytes. J Cell Biol. 1989 Mar;108(3):1039–1051. doi: 10.1083/jcb.108.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub O., Look J., Paul D., Willecke K. Cyclic adenosine monophosphate stimulates biosynthesis and phosphorylation of the 26 kDa gap junction protein in cultured mouse hepatocytes. Eur J Cell Biol. 1987 Feb;43(1):48–54. [PubMed] [Google Scholar]
- YAMADA E. SOME OBSERVATIONS ON THE NERVE TERMINAL ON THE LIVER PARENCHYMAL CELL OF THE MOUSE AS REVEALED BY ELECTRON MICROSCOPY. Okajimas Folia Anat Jpn. 1965 Jan;40:663–677. doi: 10.2535/ofaj1936.40.4-6_663. [DOI] [PubMed] [Google Scholar]