Quorum sensing is a cell-to-cell communication system used by pathogenic bacteria to control expression of virulence factors (1–6). In Pseudomonas aeruginosa, quorum-sensing mutants show reduced virulence (1–3, 7, 8), and, in a recent issue of PNAS, Chun et al. (9) reported that human respiratory epithelia have the capacity to inactivate a P. aeruginosa quorum-sensing signal. This capacity appears to be enzymatic in nature, and it functions in some but not all mammalian cells. This finding opens a new area of research and indicates that humans have evolved mechanisms to interfere with a quorum-sensing pathway.
Quorum-Sensing Signal Molecules
P. aeruginosa produces two quorum-sensing signal molecules, N-(3-oxodode-canoyl)-l-homoserine lactone (3OC12-HSL) and N-butanoyl-l-homoserine lactone (C4-HSL), that regulate production of virulence factors and biofilm formation. These compounds are produced extracellularly and, at sufficient concentrations, can induce transcription of a battery of virulence genes. In well studied strains, quorum sensing is hierarchical: threshold levels of 3OC12-HSL are required to activate C4-HSL production (10, 11). Considerable attention has been directed at developing anti-quorum-sensing agents as possible infection control therapeutics (12). This approach has been particularly enticing in the case of P. aeruginosa because this bacterium often causes incurable chronic infections, as occur in the lungs of people who have the genetic disease cystic fibrosis.
Disruption of Quorum Sensing
Recent investigations have shown that some bacteria have the ability to disrupt quorum sensing. A soil bacterium (Bacillus sp.) produces an enzyme coded by the aiiA gene that hydrolyzes the lactone ring of acyl-homoserine lactones. One might imagine that the role of the aiiA-encoded enzyme is to interfere with acyl-homoserine lactone signaling by other bacterial species with which the Bacillus competes in nature. Identification of the aiiA gene led to experiments with recombinant plants that can degrade acyl-homoserine lactones. These plants show resistance to quorum-sensing-dependent bacterial infection (13–17). This finding suggests that there could be an enzyme in mammals that inactivates acyl-homoserine lactones, and that such an enzyme might play a role in their innate defenses against molecules involved in quorum sensing.
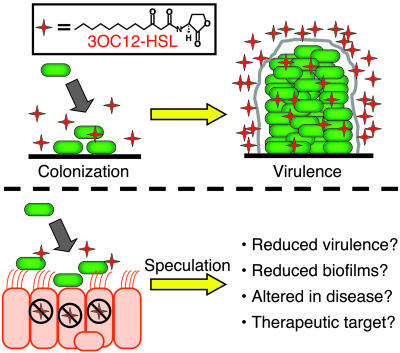
The idea that quorum-sensing signals might be intercepted by the host is not new. In fact, there is a body of literature describing the effects of P. aeruginosa 3OC12-HSL quorum-sensing signal on mammalian cytokine production and inflammation, as reviewed by Chun et al. (9). Unfortunately, these reports sometimes contradict each other, and it has been difficult to draw conclusions about specific ways in which the host responds to 3OC12-HSL and about whether the response is beneficial or detrimental to the host. Based on the findings of Chun et al., one might speculate that in some experiments 3OC12-HSL was being degraded rapidly whereas in others it persisted for longer periods of time. The authors of the previous publications would have had no reason to suspect that the signal was being inactivated by the mammalian cells in their experiments. It has even been suggested that, in addition to serving as an inducer of virulence factors, the signal itself is a virulence factor (18) (Fig. 1).
Fig. 1.
(Upper) A model for quorum-sensing control of the development of a chronic P. aeruginosa biofilm infection. (Lower) Speculation about how cellular inactivation of the quorum-sensing signal 3OC12-HSL might affect virulence and function as a therapeutic target.
A Host Defense Against Quorum Sensing
In the collaboration of Chun et al. (9), a group of quorum-sensing microbiologists teamed with a group of epithelial cell biologists to perform simple but elegant experiments that show human respiratory epithelia degrade 3OC12-HSL but not C4-HSL. Although the authors did not suggest it, one could speculate that inactivation of 3OC12-HSL is a specific host defense mechanism that targets the top of the P. aeruginosa quorum-sensing cascade (recall that 3OC12-HSL is required to activate the C4-HSL system). Chun et al. report that the apparent enzyme shows specificity for certain acylhomoserine lactones, but it is not limited to 3OC12-HSL inactivation, as it also inactivates C6-HSL, for example. They also show that not all mammalian cells lines inactivate 3OC12-HSL rapidly. The data are limited but consistent with the idea that cells derived from human epithelia tissue exposed to pathogens have the best ability to inactivate the quorum-sensing signal.
This report opens up a new area of investigation. The enzyme involved in acyl-homoserine lactone inactivation needs to be identified and characterized. The product of the reaction is yet to be determined. Given the knowledge that under some conditions 3OC12-HSL will not persist long in the presence of human cells, the nature of the cellular response to this signal should be revisited. Of course, the big question is whether the ability of epithelial cells to inactivate 3OC12-HSL confers any host protection against P. aeruginosa infections? If the answer to this question is yes, then this new research area might lead to a novel therapeutic approach to blocking or managing certain bacterial infections, an approach that targets the production of this newly discovered acyl-homoserine lactone inactivator.
See companion article on page 3587 in issue 10 of volume 101.
References
- 1.Smith, R. S. & Iglewski, B. H. (2003) Curr. Opin. Microbiol. 6, 56–60. [DOI] [PubMed] [Google Scholar]
- 2.De Kievit, T. R. & Iglewski, B. H. (2000) Infect. Immun. 68, 4839–4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donabedian, H. (2003) J. Infect. 46, 207–214. [DOI] [PubMed] [Google Scholar]
- 4.Davies, D. G., Parsek, M. R., Pearson, J. P., Iglewski, B. H., Costerton, J. W. & Greenberg, E. P. (1998) Science 280, 295–298. [DOI] [PubMed] [Google Scholar]
- 5.Schuster, M., Lostroh, C. P., Ogi, T. & Greenberg, E. P. (2003) J. Bacteriol. 185, 2066–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner, V. E., Bushnell, D., Passador, L., Brooks, A. I. & Iglewski, B. H. (2003) J. Bacteriol. 185, 2080–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuqua, C. & Greenberg, E. P. (2002) Nat. Rev. Mol. Cell Biol. 3, 685–695. [DOI] [PubMed] [Google Scholar]
- 8.Whitehead, N. A., Barnard, A. M., Slater, H., Simpson, N. J. & Salmond, G. P. (2001) FEMS Microbiol. Rev. 25, 365–404. [DOI] [PubMed] [Google Scholar]
- 9.Chun, C. K., Ozer, E. A., Welsh, M. J., Zabner, J. & Greenberg, E. P. (2004) Proc. Natl. Acad. Sci. USA 101, 3587–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Latifi, A., Foglino, M., Tanaka, K., Williams, P. & Lazdunski, A. (1996) Mol. Microbiol. 21, 1137–1146. [DOI] [PubMed] [Google Scholar]
- 11.Pesci, E. C. & Iglewski, B. H. (1997) Trends Microbiol. 5, 132–134. [DOI] [PubMed] [Google Scholar]
- 12.Smith, R. S. & Iglewski, B. H. (2003) J. Clin. Invest. 112, 1460–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong, Y. H., Wang, L. H., Xu, J. L., Zhang, H. B., Zhang, X. F. & Zhang, L. H. (2001) Nature 411, 813–817. [DOI] [PubMed] [Google Scholar]
- 14.Dong, Y. H., Xu, J. L., Li, X. Z. & Zhang, L. H. (2000) Proc. Natl. Acad. Sci. USA 97, 3526–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin, Y. H., Xu, J. L., Hu, J., Wang, L. H., Ong, S. L., Leadbetter, J. R. & Zhang, L. H. (2003) Mol. Microbiol. 47, 849–860. [DOI] [PubMed] [Google Scholar]
- 16.Zhang, H. B., Wang, L. H. & Zhang, L. H. (2002) Proc. Natl. Acad. Sci. USA 99, 4638–4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leadbetter, J. R. & Greenberg, E. P. (2000) J. Bacteriol. 182, 6921–6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith, R. S., Harris, S. G., Phipps, R. P. & Iglewski, B. H. (2002) J. Bacteriol. 184, 1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]