Abstract
Halichondrin B is a large polyether macrolide found in a rare Japanese sponge, Halichondria okadai and has been shown to have anticancer activity. Eribulin mesylate is a completely synthetic analog of halichondrin B with a unique mechanism of action relative to other antimicrotubule agents. This new agent has demonstrated activity in preclinical studies, and it is being developed for the treatment of different tumor types. Eribulin has been approved by the United States Food and Drug Administration and the European Medicines Agency as late-line therapy for metastatic breast cancer patients previously treated with an anthracycline and a taxane. It has demonstrated superiority over other treatments in overall survival (OS) (hazard ratio: 0.81, P = 0.041), leading to its regulatory approbation for clinical practice use. Median OS for the eribulin-treated group was 13.1 months versus 10.6 months in the physician’s treatment-of-choice group. Eribulin demonstrated a manageable toxicity profile. Most common adverse events associated with treatment were mild neutropenia and fatigue, mainly of grade 1 or 2. In contrast to other antimicrotubule agents, eribulin has a relatively low incidence of peripheral neuropathy and alopecia. Eribulin has been extensively studied in breast cancer and is currently being developed for treatment of other cancer types. Eribulin has demonstrated activity in Phase II trials in non-small cell lung cancer, pancreatic cancer, urothelial tract cancer, and sarcomas. Further studies in these cancers are ongoing. This article reviews pharmacology, mechanism of action, pharmacokinetics and efficacy of eribulin in breast cancer and other neoplasms.
Keywords: halichondrin B, eribulin, antimicrotubule, metastatic breast cancer
Introduction to microtubules as targets for chemotherapy
Microtubules are important cytoskeletal components formed by the polymerization of α – and β-tubulin heterodimers. These heterodimers are organized into an elongated tube, with polymerization and depolymerization occurring at the polarized end (α extreme), in a process called dynamic instability. Aside from structuring the cell, microtubules form the mitotic spindle, which is essential for cell division during mitosis. This role makes microtubules an important target for cancer treatment. Suppression of microtubule assembly during mitosis causes G2/M phase cell-cycle arrest, leading to apoptosis.1 The mitotic spindle checkpoint is activated by the presence of unattached kinetochores, which blocks the G2/M transition and thus prevents abnormal chromosome segregation. Cell-cycle arrest then induces Bcl-2 phosphorylation, leading to caspase activation and subsequent apoptosis.23
A long list of chemical drugs, such as taxanes, vinca alkaloids, and epothilones, bind tubulin and affect microtubule dynamic instability. The main limitations of the existing antimicrotubule agents are lack of tolerance and resistance development. Therefore, more research is needed to identify novel agents with improved efficacy that are better tolerated and able to evade drug resistance mechanisms.
Mechanism of action, pharmacology, and pharmacokinetics (PK) of halichondrins with antitumoral activity
Mechanism of action
Halichondrin B is a large polyether macrolide found in a rare Japanese sponge, Halichondria okadai.4,5 In 1986, halichondrin B was shown to have remarkable in vitro and in vivo anticancer activity.5,6 Subsequent studies7–9 confirmed the in vitro activity of halichondrin B and identified a tubulin-based antimitotic mechanism. Halichondrin B binds tubulin near the vinca site, thereby altering depolymerization. Although the location of the vinca domain has been difficult to identify, it appears to be adjacent to the exchangeable guanosine triphosphate binding site at the plus end interface of β-tubulin.10,11 Halichondrin B inhibits the formation of an intra-chain crosslink between two sulfhydryl groups in β-tubulin, whereas conventional anti-microtubule agents such as taxanes, epothilones, and vinca alkaloids inhibit both growth and shortening of microtubules. The unique mechanism of action of the halichondrins secondary to a specific conformational effect acting on β-tubulin suppresses microtubule growth with no effect on microtubule shortening, sequestering tubulin into nonfunctional aggregates. This leads to formation of dysfunctional mitotic spindles that cannot pass the metaphase/anaphase checkpoint.12
Despite the promising anticancer activity of halichondrin B, clinical development has been limited by the availability of the natural product. Therefore, scientists at the Eisai Research Institute produced a range of halichondrin B variants that are bioactive and structurally more stable than the natural parent compound.13 Of all the analogs produced, eribulin mesylate appears to be the most promising.
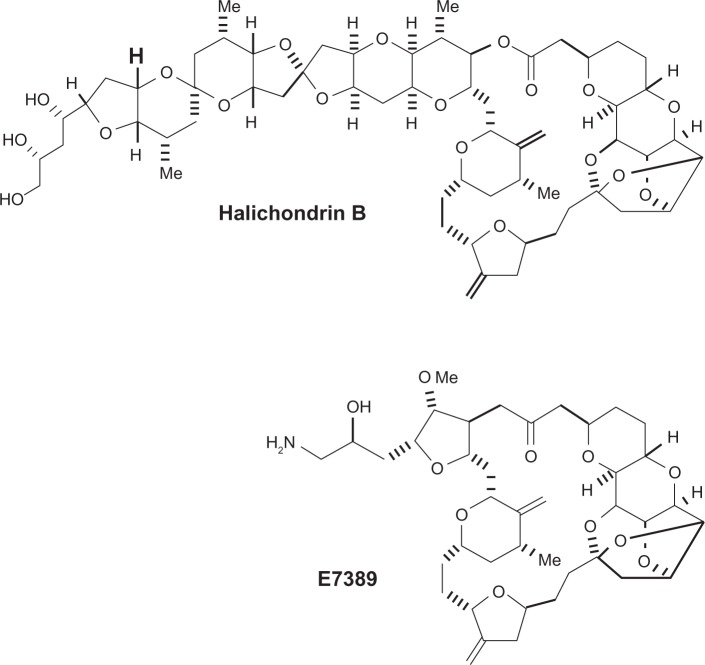
Eribulin mesylate (E7389, Eisai Research Institute, Andover, MA) is a synthetic analog of the macrolide halichondrin B. It is a structurally simplified derivative containing the biologically active macrocyclic lactone C1–C38 of the parent compound and consequently has the same potency (Figure 1). Like halichondrin B, eribulin inhibits tubulin polymerization by binding the β-tubulin subunit. This activity explains its ability to overcome taxane resistance conferred by β-tubulin mutations.14 In breast cancer cell lines, a significant correlation has been demonstrated between tubulin expression levels and sensitivity to eribulin. This observation suggests that higher levels of tubulin may be more responsive to eribulin.15 Surprisingly though, high expression of tubulin seems to confer resistance to some anti-microtubule agents such as paclitaxel and vinorelbine.16
Figure 1.
Molecular structure of halichondrin B and eribulin mesylate (E7389). Kuznetsov et al. Cancer Res. 2004.
Pharmacology
The eribulin structure consists of a simplified macrocyclic ketone in which the C1-lactone-ester of halichondrin B is replaced by ketone. Furthermore, the entire C39–C54 polyether side chain is removed, C31-methyl is replaced by methoxy and the tri-cyclic C29–C38 system is replaced by a single five-member ring.
Eribulin, which is prepared in an aqueous solution, has a short infusion time, does not require premedication with steroids or antihistamines, and does not need protection from light. These properties of eribulin mesylate make it superior to other approved microtubule-targeting agents.17
Pharmacokinetics
Several Phase I studies have been conducted to study the PK of eribulin. In the first Phase I study, eribulin was administered as a 1–2 minute bolus 3 weeks out of 4, starting at 0.125 mg/m2/week. A total of 40 patients with refractory or advanced solid tumors were included. The maximum tolerated dose (MTD) was 1.4 mg/m2/week. PK analysis demonstrated tri-phasic elimination and a prolonged terminal half-life of 36–48 hours. At the MTD, plasma levels of eribulin were above concentrations required for in vitro cytotoxicity for more than 1 week.18,19
In another study, 32 patients were treated with eribulin mesylate via a 1-hour intravenous (IV) infusion on days 1, 8, and 15 of a 28-day cycle at a dose ranging from 0.25 to 1.40 mg/m2. The MTD was 1 mg/m2 and was limited by neutropenia and fatigue. PK analysis indicated the dose range studied was linear, and the half-life was 38.7 hours. The plasma concentration–time profile showed a rapid distribution phase with a mean distribution half-life of 0.43 hours. Minimal urinary excretion was seen (5%–6%).20
Eribulin PK was also studied in 21 patients receiving eribulin via a 1-hour IV infusion every 21 days. Doses ranged from 0.25 to 4.00 mg/m2, and showed linear kinetics and a terminal half-life of 2 days. Rapid extensive volume of distribution and slow elimination with a minimum renal clearance (7% of the drug excreted unchanged in the urine) were observed. The MTD was 2 mg/m2 due to neutropenia as the dose limiting toxicity at higher doses.21 A Phase I Japanese trial in patients with refractory solid tumors established a similar recommended dose of eribulin as 1.4 mg/m2 administered for 5 minutes on days 1 and 8 of a 21-day cycle.22
In a Phase I study conducted on patients with liver impairment (Child A and B), eribulin was generally safe and well tolerated. Hepatic damage decreased clearance and prolonged the elimination half-life. Eribulin exposure increased with decreasing hepatic function,23 requiring dose adjustments. The dose used in patients with mild hepatic impairment (Child A) was 1.1 mg/m2, and in patients with moderate dysfunction (Child B) it was 0.7 mg/m2. Because elimination is primarily hepatic, the effect of CYP3A4 (the main metabolic enzyme) inhibitors, such as ketoconazole, on eribulin PK was evaluated in another trial with 12 patients. The mean dose, when normalized using area under the curve values, was similar when eribulin was administered with or without ketoconazole. The observation suggests that eribulin can be safely co-administered with CYP3A4 inhibitors.24
A Phase II study evaluated eribulin (1.4 mg/m2 IV bolus on days 1 and 8 of a 21-day cycle) PK in heavily pretreated, locally advanced, or metastatic breast cancer (MBC) patients. A three-compartment model described eribulin PK and, similar to other studies, distribution was rapid with slow elimination. There was appreciable interpatient PK variability, and only a small fraction could be explained by alterations in liver or renal function.25
Efficacy studies of eribulin in breast cancer and other neoplasms
Phase II studies in breast cancer
To the authors’ knowledge, three Phase II studies with eribulin mesylate used as a single agent have been conducted in patients with heavily pretreated breast cancer. All included patients pretreated with taxanes.
An open-label, single-arm, Phase II study was performed to evaluate the efficacy and tolerability of eribulin in patients with MBC who had previously received at least an anthracycline and a taxane. The trial enrolled 103 patients to receive a 2–5 minute IV infusion of eribulin mesylate at a dose of 1.4 mg/m2 on days 1, 8, and 15 of a 28-day cycle. However, after a tolerability assessment showed a high number of patients with grade 3–4 neutropenia on day 15, the trial was amended, and a second group of 33 patients received eribulin mesylate 1.4 mg/m2 on days 1 and 8 of a 21-day cycle. Patients were heavily pretreated, having received a median of four prior chemotherapy regimens (range 1–11 prior regimens). The median age was 55 years, and more than half of the patients (54%) had an Eastern Cooperative Oncology Group (ECOG) performance status of 1. Both the 28-day (n = 70) and 21-day (n = 33) treatment arms were well balanced with respect to baseline demographic and disease characteristics. Patients in the 28-day cohort received a median of 2.5 cycles of therapy, compared with a median of 4 cycles in the 21-day cohort. Of the 70 patients in the 28-day cohort, 44 (63%) experienced dose interruptions, delays, reductions, or omissions in cycle 1, primarily due to neutropenia. However, in the 21-day cohort, neutropenia was observed in only six (18%) out of 33 patients. The primary endpoint was objective response rate (ORR). Sixteen out of the 103 patients did not fulfill the entry criteria after being reviewed independently. Of the 87 patients (84%) who met the key inclusion criteria, eribulin achieved an overall ORR of 11.5% (all partial responses [PRs]) (95% confidence interval [CI]: 5.7–20.1). The median duration of response (DOR) was 5.6 months, the median progression-free survival (PFS) was 2.6 months, and the median OS was 9 months. The most common treatment-related adverse events (AE) were neutropenia, fatigue, alopecia, nausea, and anemia. The incidence of grade 3 or 4 neutropenia was 64% in both cohorts, but the incidence of febrile neutropenia was low, as it occurred in only 4% of patients (three in the 28-day cohort and one in the 21-day cohort). Only five patients experienced grade 3 peripheral neuropathy (four in the 28-day cohort), and none of them had grade 4. Although the two schedules were not tested concurrently, the 21-day schedule appeared to be associated with a more favorable tolerability profile than the 28-day schedule.26
A second open-label, single-arm Phase II study was performed to evaluate the activity and safety of eribulin in patients (n = 291) with locally advanced or MBC previously treated with an anthracycline, taxane, and capecitabine. Patients received eribulin mesylate 1.4 mg/m2 on days 1 and 8 of a 21-day cycle. The median prior number of chemotherapeutic regimens was 4, the median age was 56 years, 63% of patients had an ECOG score of 1 or 2, and 21% of the patients had triple-negative tumors. The median number of cycles received per patient was four. Of the 291 patients, 269 patients finally met key inclusion criteria once reviewed by an independent review committee. The primary endpoint, ORR, was 9.3% (95% CI: 6.1–13.4) (all PRs). Stable-disease (SD) was seen in 46.5% of patients, and the clinical benefit rate (CBR; complete response [CR]: + PR + SD > 6 months) was 17.1%. The median DOR was 4.1 months, the median PFS was 2.6 months, and the median OS was 10.4 months. The most frequent grade 3 or 4 treatment-related AEs were neutropenia (54%; febrile neutropenia 5.5%), leukopenia (14%), and asthenia/fatigue (10%). Grade 3 neurotoxicity occurred in 6.9% of patients.27
The last single-arm Phase II study was conducted in 81 anthracycline and taxane-pretreated Japanese patients with locally advanced or MBC. Eribulin was administered at a dose of 1.4 mg/m2 on days 1 and 8 of a 21-day cycle. The primary efficacy endpoint was ORR. The median number of previous chemotherapy regimens was three. The median age was 54 years, and 27.5% of patients had triple-negative tumors. The median number of eribulin treatment cycles was 5. ORR was 21.3% (all PRs) (95% CI: 12.9–31.8). The SD rate was 37.5%, and the CBR was 27.5%. The median DOR was 3.9 months, the median PFS was 3.7 months, and the median OS was 11 months. The most frequent treatment-related grade 3 or 4 toxicities were neutropenia (95.1%; febrile neutropenia 13.6%), leukopenia (74.1%), and lymphopenia (12.3%). Grade 3 peripheral neuropathy occurred in 3.7% of patients (no grade 4).28 These data are summarized in Table 1.
Table 1.
Phase II trials with eribulin mesylate in metastatic breast cancer patients
| Trial | N | ORR (%) | CBR (%) | Median DOR (months) | Median PFS (months) | Median OS (months) | 6-month PFS (%) | 6-month OS (%) | Grade 3/4 toxicities |
|---|---|---|---|---|---|---|---|---|---|
| Vahdat et al26 | 87 | 11.5 | 17.2 | 5.6 | 2.6 | 9 | 25.9 | 67.8 | Neutropenia, leukopenia, fatigue |
| Cortes et al27 | 269 | 9.3 | 17.1 | 4.1 | 2.6 | 10.4 | 12.4 | 72.3 | Neutropenia, leukopenia, fatigue |
| Iwata et al28 | 81 | 21.3 | 27.5 | 3.9 | 3.7 | 11 | 20.1 | 72.3 | Neutropenia, leukopenia, febrile neutropenia |
Abbreviations: ORR, overall objective response rate; CBR, clinical benefit rate; DOR, duration of response; PFS, progression-free survival; OS, overall survival.
Phase III study in breast cancer
The encouraging results observed in Phase II studies led to the design of two randomized Phase III trials.
The first of these trials, named EMBRACE (Eisai Metastatic Breast Cancer Study Assessing Physician’s Choice Versus E7389; NCT00388726; E305), was an open-label, multicenter, randomized, controlled, Phase III study in heavily pretreated MBC patients. All of the patients were previously treated with at least two prior chemotherapies, including an anthracycline and a taxane (unless contraindicated), and OS was compared between eribulin-treated patients and patients receiving current standard of care options.29
Patients were randomized (2:1) to receive eribulin 1.4 mg/m2 in a 2–5 minute IV bolus on days 1 and 8 of a 21-day cycle or treatment of physician’s choice (TPC). TPC included any single-agent chemotherapy, hormonal or biological treatment approved for the treatment of cancer, radiotherapy, or symptomatic treatment alone. Randomization was stratified by geography, previous capecitabine treatment, and human epidermal growth factor receptor 2 (HER2) status. The primary endpoint of the trial was OS. Secondary endpoints were PFS, ORR and DOR, and safety assessment.
A total of 762 patients were randomly allocated to treatment groups (508 eribulin, 254 TPC). The majority of TPC patients received chemotherapy (96%), essentially vinorelbine (25%), gemcitabine (19%), and capecitabine (18%). The remaining 4% of patients received hormonal therapy, and no patient received supportive care alone.
The median age of patients was 55 years. Ninety-one percent of patients had an ECOG score of 0 or 1, 16% were HER2-positive, and 19% were triple-negative. The median number of prior chemotherapy regimens was four, and 73% of the patients had received prior capecitabine. The most common metastatic sites were bone and liver, and 51% of patients had metastatic disease involving at least three organs.
The study met its primary endpoint, finding a significant increase in OS for eribulin-treated patients compared with TPC-treated patients (hazard ratio [HR]: 0.81, 95% CI: 0.66–0.99; P = 0.041). The median OS was 13.1 months (95% CI: 11.8–14.3) in patients receiving eribulin and 10.6 months (95% CI: 9.3–12.5) in patients receiving TPC. The median PFS was 3.7 months (95% CI: 3.3–3.9) with eribulin and 2.2 months (2.1–3.4) with TPC (HR: 0.87; 95% CI: 0.71–1.05; P = 0.137) in the independent review. ORR was 12% with eribulin (including three CRs) and 5% with TPC (P = 0.002). The median DOR for eribulin was 4.2 months (95% CI: 3.8–5.0) and for TPC was 6.7 months (6.7–7.0) (P = 0.159).
Serious adverse events occurred in 25% of patients on eribulin and in 26% of patients on TPC. Adverse events leading to therapy discontinuation occurred in 13% of eribulin-treated patients and 15% of TPC-treated patients. The most common adverse events in eribulin-treated patients were neutropenia, asthenia/fatigue, alopecia, and peripheral neuropathy. Neutropenia was observed in 52% of patients receiving eribulin (45% grade 3 or 4; with only 5% febrile neutropenia) and in 30% (21% grade 3 or 4) of patients receiving TPC. Asthenia was reported in 54% of patients receiving eribulin and in 40% of patients receiving TPC. Alopecia was observed in 45% of the patients in the eribulin arm and in 10% of patients in the TPC arm. Peripheral neuropathy was recorded in 35% of patients on eribulin (8% grade 3 and <1% grade 4) (Table 2). These data led to the regulatory approval of eribulin mesylate in the USA and Europe for patients who have received at least two chemotherapeutic regimens for the treatment of MBC, with prior treatment including an anthracycline and a taxane.
Table 2.
Adverse events with an incidence >10% in eribulin treatment group and treatment of physician’s choice in EMBRACE Phase III trial in metastatic breast cancer
| Eribulin (n = 503)
|
TPC (n = 247)
|
|||||
|---|---|---|---|---|---|---|
| All grades | Grade 3 | Grade 4 | All grades | Grade 3 | Grade 4 | |
| Hematological | ||||||
| Neutropenia† | 260 (52%) | 106 (21%) | 121 (24%) | 73 (30%) | 35 (14%) | 17 (7%) |
| Leucopenia | 116 (23%) | 59 (12%) | 11 (2%) | 28 (11%) | 12 (5%) | 2 (1%) |
| Anemia | 94 (19%) | 9 (2%) | 1 (<1%) | 56 (23%) | 8 (3%) | 1 (<1%) |
| Non-hematological | ||||||
| Asthenial/fatigue | 270 (54%) | 41 (8%) | 3 (1%) | 98 (40%) | 25 (10%) | 0 |
| Alopecia | 224 (45%) | – | – | 24 (10%) | – | – |
| Peripheral neuropathy‡ | 174 (35%) | 39 (8%) | 2 (<1%) | 40 (16%) | 5 (2%) | 0 |
| Nausea | 174 (35%) | 6 (1%) | 0 | 70 (28%) | 6 (2%) | 0 |
| Constipation | 124 (25%) | 3 (1%) | 0 | 51 (21%) | 2 (1%) | 0 |
| Arthralgia/myalgia | 109 (22%) | 2 (<1%) | 0 | 29 (12%) | 3 (1%) | 0 |
| Weight loss | 107 (21%) | 3 (1%) | 0 | 35 (14%) | 1 (<1%) | 0 |
| Pyrexia | 105 (21%) | 1 (<1%) | 0 | 31 (13%) | 1 (<1%) | 0 |
| Anorexia | 98 (19%) | 2 (<1%) | 0 | 32 (13%) | 3 (1%) | 0 |
| Headache | 97 (19%) | 2 (<1%) | 0 | 29 (12%) | 0 | 1 (<1%) |
| Diarrhea | 92 (18%) | 0 | 0 | 45 (18%) | 0 | 0 |
| Vomiting | 91 (18%) | 4 (1%) | 1 (<1%) | 44 (18%) | 3 (1%) | 0 |
| Back pain | 79 (16%) | 3 (1%) | 1 (<1%) | 18 (7%) | 3 (1%) | 1 (<1%) |
| Dyspnea | 79 (16%) | 18 (4%) | 0 | 31 (13%) | 6 (2%) | 1 (<1%) |
| Cough | 72 (14%) | 0 | 0 | 21 (9%) | 0 | 0 |
| Bone pain | 60 (12%) | 9 (2%) | 0 | 23 (9%) | 4 (2%) | 0 |
| Pain in extremity | 57 (11%) | 5 (1%) | 0 | 25 (10%) | 3 (1%) | 0 |
| Mucosal inflammation | 43 (9%) | 7 (1%) | 0 | 25 (10%) | 5 (2%) | 0 |
| Palmar-plantar erythrodysaesthesia | 7 (1%) | 2 (<1%) | 0 | 34 (14%) | 9 (4%) | 0 |
Abbreviation: EMBRACE, Eisai Metastatic Breast Cancer Study Assessing Physician’s Choice Versus E7389 (NCT00388726; E 305). With permission: Cortes et al. Lancet. 2011.
A second open-label, multicenter, randomized, Phase III study with eribulin was recently completed and the results will be available soon. The primary objective of this study was to compare eribulin and capecitabine in terms of OS and PFS. Secondary objectives included assessments of response data, DOR, 1-, 2-, and 3-year survival, quality of life, and safety. Patients had to have locally advanced or MBC with ≤2 previous chemotherapeutic regimens for MBC, including an anthracycline and a taxane. A total of 1102 patients were randomized (1:1) to receive either eribulin or capecitabine.30
Phase II studies in other cancers
Non-small cell lung cancer (NSCLC)
Two Phase II trials have been conducted in patients with advanced NSCLC. The first one was an open-label, single arm, Phase II study for patients with prior progression to a platinum-based doublet chemotherapy and an ECOG performance status of 0 or 1. Eribulin was administered on a 28-day schedule, with a 1.4 mg/m2 bolus infusion on days 1, 8, and 15. The 28-day schedule was later changed to a 21-day schedule due to neutropenia occurrence on day 15, similar to what has been observed in patients with breast cancer. Patients were enrolled in two strata based on prior taxane exposure. A total of 103 patients (83 with prior taxane therapy and 20 taxane naïve) were treated, with 77 patients on a 28-day schedule and 26 patients on a 21-day schedule. The median age was 65 years, and the median number of prior therapies was two. The primary endpoint was ORR, confirmed by independent radiologic review. A median number of three cycles (range 1–15) was administered. The ORR (all PRs) was 9.7%, with 10.8% PRs in the taxane pretreated cohort, and 5% PR in the taxane naïve cohort. Overall disease control rate (PR + SD) was 55.3%. The median DOR was 5.8 months, the median PFS was 3.4 months, and the median OS was 9.4 months. The most common drug-related AEs were neutropenia (54%; 49% grade 3/4), fatigue (49%; 11% grade 3, no grade 4), nausea (38%; 1% grade 3, no grade 4), alopecia (32%), anemia (29%, 4% grade 3/4), and peripheral neuropathy (23%; 2% grade 3, no grade 4). The study concluded that eribulin administered on days 1 and 8 of a 21-day cycle was active and well tolerated as second- or later-line treatment in patients with NSCLC.31
A second Phase II trial with eribulin in NSCLC was conducted in platinum-based therapy and taxane-pretreated patients, with no more than two prior regimens in the metastatic setting and ECOG of 2 or below. Patients were classified by taxane-sensitivity status as taxane sensitive (TS; progression >90 days after taxanes) or taxane resistant (TR; progression during or <90 days after taxanes). Forty-one patients were administered 1.4 mg/m2 IV eribulin over 1–2 minutes on days 1 and 8 of a 21-day schedule. There were three (15%) objective responses in 20 TS patients, and no responses were observed in 21 TR patients. The SD rates were 60% and 24% in the TS and TR cohorts, respectively. The median PFS was 6.3 months in the TS subgroup (95% CI: 2.5–8.6) and 1.2 months in the TR subgroup (95% CI: 1.1–4.1). Grade 3 or 4 toxicities were predominantly hematological (46% of patients; including one episode of febrile neutropenia), although nonhematological toxicities were also observed (20% patients; including fatigue, dehydration, nausea, constipation, and one grade 3 neuropathy). Eribulin was well tolerated and showed promising activity in the TS cohort, which was expanded to include another 25 patients. The results of this new cohort of patients are awaited.32 Other trials using eribulin in combination with pemetrexed, erlotinib, and carboplatin, are currently ongoing in NSCLC patients.33–35
Head and neck cancer
A multi-center Phase II trial was conducted to evaluate eribulin in patients with metastatic or recurrent squamous cell carcinoma of the head and neck. Forty eligible patients who had not received prior chemotherapy for metastatic or recurrent disease and with an ECOG score of 0–1 were enrolled. The median age of patients was 61.2 years. The primary endpoint was ORR, and secondary objectives were PFS, OS, and safety. Thirty-three patients (83%) had metastatic disease. The primary tumor sites included: 38% oropharynx, 30% lip/oral cavity, 15% larynx, 10% hypopharynx, 5% other/unknown, and 3% nasopharynx. Patients received a 1.4 mg/m2 dose of eribulin mesylate on days 1 and 8 of a 21-day cycle. Two confirmed PRs were observed, for an ORR of 5% (95% CI: 1%–17%). The median PFS was 3 months (95% CI: 1–3), and the median OS was 7 months (95% CI: 5–10). The more common grade 3 and 4 toxicities were lymphopenia (15%), leukocytopenia (13%), neutropenia (10%), hyponatremia (5%), fatigue (5%), diarrhea (5%), and dyspnea (5%), with one treatment-related death due to pulmonary hemorrhage. Thus, although eribulin was well tolerated, it did not show any significant efficacy in terms of ORR or PFS, and was not recommended for future development in refractory or metastatic head and neck cancer.36
Prostate cancer
In a first Phase II study evaluating eribulin activity in metastatic castration-resistant prostate cancer (CRPC) patients, eribulin was administered as 1.4 mg/m2 IV bolus infusion on days 1 and 8 of a 21-day cycle. Patients were eligible if they had bilateral orchiectomy or failure of primary hormonal therapy. The primary efficacy endpoint was prostate-specific antigen (PSA) response rate, defined as two consecutive ≥50% reductions in PSA levels from baseline. Secondary endpoints were duration of PSA response and ORR. A total of 108 patients were enrolled, and the median age was 71 years. The median number of treatment cycles received was four (range 1–25). Patients were stratified by prior taxane treatment status. In the taxane pretreated cohort, 50 patients received eribulin, but only 47 could be evaluated as per protocol. Although no patients had an objective response, 8.7% of patients had ≥50% reduction in PSA levels. The 6-month PFS and 12-month OS were 12.6% and 57.2%, respectively. Treatment related grade 3 and 4 toxicities were neutropenia (40%), leukopenia (16%), fatigue (8%), and peripheral neuropathy (6%, no grade 4). In the taxane-naïve cohort, 58 patients were treated. The ORR was 8.8%, and 22.8% of patients had a ≥50% decrease in PSA levels. The 6-month PFS and 12-month OS were 19.6% and 73.5%, respectively. Treatment-related grade 3 and 4 toxicities were neutropenia (22.4%), leukopenia (8.6%), and fatigue (6.9%).37
In a second multicenter trial (ECOG 5805), 119 patients (116 eligible) with metastatic CRPC were treated with eribulin at the same dose and on the same schedule. The primary endpoint was PSA response rate. The median age was 70 years and the median number of treatment cycles received was 4 (range 1–20+). Patients were stratified by prior treatment: chemonaïve, prior-taxanes only, or two prior cytotoxic chemotherapies.
In the chemonaïve arm (41 patients), 24% had a ≥50% PSA reduction (90% CI: 13.9–37.9), 15% had SD for more than 9 weeks, and 8% showed response. The median duration of PSA response was 7.1 months, and the median OS was not reached. In patients previously treated with taxanes (n = 51), 10% had a ≥50% PSA reduction (90% CI: 4.0–19.5), 20% had SD for more than 9 weeks, and 3% showed an objective response. The median duration of PSA response was 3.6 months, and the median OS was 11.4 months. In patients who had previously undergone two cytotoxic chemotherapeutic regimens (n = 51), 4% had a ≥50% PSA reduction (90% CI: 0.2–18.3), 29% had SD for more than 9 weeks, and 8% responded. The median duration of PSA response could not be evaluated, and the median OS was 13.7 months. Treatment-related grade 3 or 4 toxicities in patients that were taxane naïve, previously taxane-treated, and previously treated with two chemotherapeutic regimens were neutropenia (52%, 50%, 68%, respectively), leukopenia (33%, 44%, 52%, respectively), fatigue (17%, 8%, 12%, respectively), and sensory neuropathy (14%, 16%, 4%, respectively). Although eribulin showed some activity in taxane-naïve metastatic CRPC, the overall response rate did not reach predetermined levels (40%), so the development of eribulin as a treatment for CPRC was discontinued.38
Ovarian cancer
A multicenter Phase II study in recurrent epithelial ovarian, fallopian tube, or peritoneal cancer was conducted based on the activity of eribulin in a human ovarian xenograft model. Eribulin was administered as 1.4 mg/m2 infusion over 15 minutes on days 1 and 8 of a 21-day cycle. To be eligible for the study, patients had to have epithelial ovarian cancer (or fallopian tube or peritoneal cancer) with measurable disease, a progression-free interval of less than 6 months since the last platinum-based therapy, and undergone two or fewer prior cytotoxic treatments. The primary endpoint was ORR. Thirty-six patients were enrolled (35 evaluable patients), with a median age of 61 years. The median platinum-free interval was 3 months (range 0.1–5.9). All patients received platinum-taxane as first-line treatment. ORR was 5.7% (two PRs, with DOR of 84 days and 128 days) and SD was reported in 46% of patients. A greater than 50% decrease in CA125 was observed in 3 of 31 CA125-evaluable patients. The median PFS was 2 months, and the treatment-related grade 3 or 4 toxicities were neutropenia (44%) and leukopenia (31%).39
Another Phase II trial was conducted to determine the activity of eribulin in women with platinum-sensitive recurrent ovarian cancer. Patients had to have recurrent platinum-sensitive (progression-free interval >6 months from last platinum-based therapy) epithelial ovarian, fallopian tube, or primary peritoneal cancer, measurable disease and should have been previously treated with two or fewer cytotoxic regimens. Thirty-seven patients were enrolled, and all were evaluated for response. The median age was 60 years, and the median platinum-free interval was 10 months (range 6.5–45.5). PR was achieved in seven patients (19%) and SD in 20 patients (54%). CA125 response was observed in 11 of 31 patients (35% of patients with CA125 evaluable disease). The median PFS was 4.1 months (95% CI: 2.8–5.8). Grade 3 or 4 treatment-related AEs included neutropenia (54%), leukopenia (3%), fatigue (5.4%), and alteration of liver enzymes (5.4%). Eribulin showed significant activity in platinum-sensitive epithelial ovarian cancer patients.40 Pancreatic cancer
An open-label, second-line, single-arm, Phase II trial was conducted in patients with pancreatic cancer. Eligibility criteria included confirmed locally advanced, unresectable or metastatic pancreatic adenocarcinoma with measurable disease and evidence of disease progression after gemcitabine therapy. The primary endpoint of the study was ORR. Patients received 1.4 mg/m2 IV eribulin on days 1 and 8 of a 21-day cycle. Fifteen patients were enrolled, 14 of them received treatment, and 12 were able to be evaluated for response. The median age was 61 years, and each patient received a median of two cycles of treatment (range 1–15). There was no CR or PR, but SD was reported in 42% (5/12) of the patients. Of these five patients, three had SD for 12 cycles or greater. Grade 3 and 4 treatment-related AEs included neutropenia (29%), fatigue (14%), peripheral neuropathy (7%), and thrombosis (7%). In conclusion, eribulin was well tolerated but it did not result in any objective responses in gemcitabine refractory pancreatic cancer. However, the large number of patients with prolonged stable disease when treated with eribulin suggests further studies of eribulin activity in pancreatic cancer are warranted.41
Sarcoma
The EORTC62052 trial assessed the efficacy and safety of eribulin mesylate in four independent strata of patients with soft tissue sarcoma: leiomyosarcoma (LMS), adipocytic (ADI), synovial (SYN), and other subtypes (OTH) of soft tissue sarcomas. Eligibility criteria included intermediate or high grade soft tissue sarcomas with documented progression and treatment with two or fewer previous chemotherapies (up to two single agents or one combination) for advanced disease. The dose of eribulin was 1.4 mg/m2 IV over 2–5 minutes on days 1 and 8 of a 3-week cycle. The primary endpoint was the progression-free rate at 12 weeks (PFR-12 weeks). The PFR-12 weeks was 32% (12/37 evaluable patients) in LMS, 45% (15/33) in ADI, 21% (4/19) in SYN, and 19% (5/26) in OTH. The median PFS in LMS was 3 months (95% CI: 2–5), and the median OS was 20 months (95% CI: 14–), with a 1-year survival rate of 70%. The PFS in ADI was 3 months (95% CI: 2–6), and the OS was 10 months (95% CI: 8–16), with a 1-year survival rate of 48%. The PFS in SYN was 3 months (95% CI: 2–3), and the OS was 11 months (95% CI: 7–15), with a 1-year survival rate of 36%. The PFS in OTH was 2 months (95% CI: 1–3), and the OS was 6 months (95% CI: 5–15), with a 1-year survival rate of 30%. Grade 3 or 4 drug-related AEs were neutropenia (50%), leukopenia (33%), anemia (9%), and fatigue (4%). Eribulin mesylate was well tolerated in pretreated patients with soft tissue sarcomas. Further studies are warranted in the LMS and ADI sarcoma subgroups, as the PFS-12 weeks reached predefined statistical requirements.42
Urothelial tract cancer (and renal insufficiency) After encouraging activity results were observed in a Phase I study of eribulin activity in patients with urothelial cancer and renal dysfunction,43 a multicenter Phase II trial is now being conducted. In the first part of this Phase II trial, patients were enrolled based on the following criteria: normal creatinine or calculated creatinine clearance ≥60 mL/min, presence of histologically or cytologically confirmed urothelial cancer, and no prior cytotoxic therapy for advanced disease (neo/adjuvant therapies were allowed). Eribulin 1.4 mg/m2 was given intravenously on days 1 and 8 of a 21-day cycle. The primary endpoint was ORR. A total of 40 patients entered the trial (37 evaluable patients). Their median age was 67 years, and 72.5% of the patients had received prior neo/adjuvant chemotherapy. The histological subtypes included transitional (35), adenocarcinoma (3), squamous (1), and small cell (1). ORR was 38% (one CR and 14 PR). Of the 15 responses, 13 were in transitional cell cancer patients (37% ORR). In patients with prior neo/adjuvant chemotherapies, the ORR was 34%. The median PFS was 3.9 months and the median OS was 9.4 months. Drug-related grade 3 and 4 toxicities included neutropenia (54%), hyponatremia (11%), hyperglycemia (8%), fatigue (3%), and sensory neuropathy (3%). A cohort of patients with creatinine clearance <40 mL/min in whom the 1.4 mg/m2 dose was shown to be tolerable is still being followed.44
Conclusions, place in therapy, future directions
Eribulin mesylate is a novel antimicrotubule drug that has been shown for the first time to improve survival in heavily pretreated MBC patients. This fact led to its approval by the United States Food and Drug Administration in November 2010 and the European Medicines Agency in March 2011 for treatment of patients with MBC that have previously been treated with anthracyclines and taxanes.
Eribulin has a manageable toxicity profile, with the most common adverse events being neutropenia, fatigue, nausea, and neuropathy, mainly grade 1 or 2. It does not require premedication and can be rapidly administered as a 1–5 minute IV bolus infusion, important advantages with respect to other chemotherapeutic agents. Eribulin is metabolized in the liver, which requires dose adjustments in patients with hepatic impairment, but it is well tolerated and safely used in patients with renal dysfunction.
Further studies combining eribulin with cytotoxics and other targeted agents are being conducted in breast cancer. The activity of eribulin in primary breast cancer is also under investigation. Phase II trials are currently underway to understand how eribulin acts in other contexts as well. For instance, eribulin is being studied in combination with trastuzumab in HER2-positive MBC45 and in the adjuvant setting following dose dense doxorubicin and cyclophosphamide.46 Eribulin is also being studied in the neoadjuvant setting in association with carboplatin in the triple-negative breast cancer population.47 Finally, eribulin is being tested as a first-line treatment for MBC,48 and in combination with capecitabine for pre-treated MBC.49
Although eribulin has not shown significant activity in prostate, ovarian, and head and neck cancers, it has shown promising activity in lung cancer, pancreatic cancer, and sarcoma. In fact, in lung cancer, trials of eribulin in combination with pemetrexed and erlotinib are ongoing, and a Phase III trial in patients with sarcoma50 is currently recruiting patients.
Preclinical studies to produce second generation structurally modified analogues of eribulin with efficacy in multidrug resistant tumors, oral bioavailability, and brain penetration are ongoing.51–53
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kuznetsov G, Towle MJ, Cheng H, et al. Induction of morphological and biochemical apoptosis following prolonged mitotic blockage by halichondrin B macrocyclic ketone analog E7389. Cancer Res. 2004;64:5760–5766. doi: 10.1158/0008-5472.CAN-04-1169. [DOI] [PubMed] [Google Scholar]
- 2.Masuda A, Maeno K, Nakagawa T, et al. Association between mitotic spindle checkpoint impairment and susceptibility to the induction of apoptosis by anti-microtubule agents in human lung cancers. Am J Pathol. 2003;163(3):1109–1116. doi: 10.1016/S0002-9440(10)63470-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang TH, Wang HS, Soon YK, et al. Paclitaxel-induced cell death: where the cell cycle and apoptosis come together. Cancer. 2000;88(11):2619–2628. doi: 10.1002/1097-0142(20000601)88:11<2619::aid-cncr26>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 4.Uemura D, Takahashi K, Yamamoto T, et al. Norhalichondrin A: an antitumor polyether macrolide from a marine sponge. J Am Chem Soc. 1985;107:4796–4798. [Google Scholar]
- 5.Hirata Y, Uemura D. Halichondrins: antitumor polyether macrolides from amarine sponge. Pure Appl Chem. 1986;58:701–710. [Google Scholar]
- 6.Towle MJ, Salvato KA, Budrow J, et al. In vitro and in vivo anticancer activities of synthetic macrocyclic ketone analogues of halichondrin B. Cancer Res. 2001;61:1013–1021. [PubMed] [Google Scholar]
- 7.Bai RL, Paull KD, Herald CL, Malspeis L, Pettit GR, Hamel E. Halichondrin B and homohalichondrin B, marine natural products binding in the vinca domain of tubulin. Discovery of tubulin-based mechanism of action by analysis of differential cytotoxicity data. J Biol Chem. 1991;266:15882–15889. [PubMed] [Google Scholar]
- 8.Bai RL, Cichacz ZA, Herald CL, Pettit GR, Hamel E. Spongistatin 1, a highly cytotoxic, sponge-derived, marine natural product that inhibits mitosis, microtubule assembly, and the binding of vinblastine to tubulin. Mol Pharmacol. 1993;44:757–766. [PubMed] [Google Scholar]
- 9.Hamel E. Natural products which interact with tubulin in the vinca domain: maytansine, rhizoxin, phomopsin A, dolastatins 10 and 15 and halichondrin B. Pharmacol Ther. 1992;55:31–51. doi: 10.1016/0163-7258(92)90028-x. [DOI] [PubMed] [Google Scholar]
- 10.Rai SS, Wolff J. Localization of the Vinblastine-binding site on β-tubulin. J Biol Chem. 1996;271:14707–14711. doi: 10.1074/jbc.271.25.14707. [DOI] [PubMed] [Google Scholar]
- 11.Zhou J, Giannakakou P. Targeting microtubules for cancer chemotherapy. Curr Med Chem Anticancer Agents. 2005;5:65–71. doi: 10.2174/1568011053352569. [DOI] [PubMed] [Google Scholar]
- 12.Jordan MA, Kamath K, Manna T, et al. The primary antimitotic mechanism of action of the synthetic halicondrin E7389 is suppression of microtubule growth. Mol Cancer Ther. 2005;4:1086–1095. doi: 10.1158/1535-7163.MCT-04-0345. [DOI] [PubMed] [Google Scholar]
- 13.Seletsky BM, Wang Y, Hawkins LD, et al. Structurally simplified macrolactone analogues of halichondrin B. Bioorg Med Chem Lett. 2004;14:5547–5550. doi: 10.1016/j.bmcl.2004.08.068. [DOI] [PubMed] [Google Scholar]
- 14.Alday PH, Correia JJ. Macromolecular interaction of halichondrin B analogues eribulin (E7389) and ER-076349 with tubulin by analytical ultracentrifugation. Biochemistry. 2009;48:7927–7938. doi: 10.1021/bi900776u. [DOI] [PubMed] [Google Scholar]
- 15.Agoulnik S, Kuznetsov G, Tendyke K, et al. Sensitivity to halichondrin analog E7389 and hemiasterlin analog E7974 correlates with βIII tubulin isotype expression in human breast cancer cell lines. J Clin Oncol. 2005;23(16S) abstr 2012. [Google Scholar]
- 16.Stengel C, Newman SP, Leese MP, Potter BV, Reed MJ, Purohit A. Class III beta-tubulin expression and in vitro resistance to microtubule targeting agents. Br J Cancer. 2010;102:316–324. doi: 10.1038/sj.bjc.6605489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisai Inc . Product information. Woodcliffe Lake, NJ, USA: [Google Scholar]
- 18.Synold TW, Morgan RJ, Newman EM, et al. A Phase I pharmacokinetic and target validation study of the novel anti-tubulin agent E7389: a California Cancer Consortium trial. 2005 ASCO Annual Meeting Proceedings. J Clin Oncol. 2005;23(16S):3036. [Google Scholar]
- 19.Synold TW, Lawrence J, Xi B, Colevas AD, Lewis MD, Doroshow JH. Human pharmacokinetics of E7389 (Halichondrin B analog), a novel anti-microtubule agent undergoing phase I investigation in the California Cancer Consortium (CCC) Proc Am Soc Clin Oncol. 2003;22 abstr 575. [Google Scholar]
- 20.Goel S, Mita AC, Mita M, et al. A Phase I study of Eribulin mesylate (E7389), a mechanistically novel inhibitor of microtubule dynamics, in patients with advanced solid malignancies. Clin Cancer Res. 2009;15:4207–4212. doi: 10.1158/1078-0432.CCR-08-2429. [DOI] [PubMed] [Google Scholar]
- 21.Tan AR, Rubin EH, Walton DC, et al. Phase I study of eribulin mesylate administered once every 21 days in patients with advanced solid tumors. Clin Cancer Res. 2009;15:4213–4219. doi: 10.1158/1078-0432.CCR-09-0360. [DOI] [PubMed] [Google Scholar]
- 22.Minami H, Mukohara T, Nagai S, Mukai H, Namiki M. A Phase I study of eribulin mesylate (E7389) in patients with refractory cancers. Eur J Cancer. 2008;6:140. doi: 10.1007/s10637-011-9741-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Witteveen P, Marchetti S, Mergui-Roelvink M, et al. Eribulin mesylate pharmacokinetics in patients with hepatic impairment. J Clin Oncol. 2010;28(15s) abst 2582. [Google Scholar]
- 24.Devriese L, Wanders J, Jenner A, et al. Eribulin mesylate pharmacokinetics in patients with solid tumors receiving repeated oral ketoconazole. EORTC-NCI-AACR Symposium. 2010;8:181. doi: 10.1007/s10637-012-9829-3. abst 574. [DOI] [PubMed] [Google Scholar]
- 25.Jansen M, Vernaz-Gris M, DesJardins C, et al. Population pharmacokinetics (PPK) of eribulin mesylate in patients with locally advanced or metastatic breast cancer (MBC) J Clin Oncol. 2009;27(15S) abst 2524. [Google Scholar]
- 26.Vahdat LT, Pruitt B, Fabian CJ, et al. Phase II study of eribulin mesylate, a halichondrin B analog, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2009;27:2954–2961. doi: 10.1200/JCO.2008.17.7618. [DOI] [PubMed] [Google Scholar]
- 27.Cortes J, Vahdat L, Blum JL, et al. Phase II study of the halichondrin B analog eribulin mesylate in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline, a taxane, and capecitabine. J Clin Oncol. 2010;28:3922–3928. doi: 10.1200/JCO.2009.25.8467. [DOI] [PubMed] [Google Scholar]
- 28.Iwata H, Aogi K, Masuda N, et al. Efficacy and safety of eribulin in Japanese patients with advanced breast cancer. J Clin Oncol. 2010;28 abst 1081. [Google Scholar]
- 29.Cortes J, O’Shaughnessy J, Loesch D, et al. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet. 2011;377:914–923. doi: 10.1016/S0140-6736(11)60070-6. [DOI] [PubMed] [Google Scholar]
- 30.Eisai Inc . ClinicalTrialsgov [Internet] Bethesda, MD: National Library of Medicine (US); Jun 13, 2006. E7389 versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with anthracyclines and taxanes. [last updated on August 2, 2010]. Available from: http://clinicaltrials.gov/ct2/show/NCT00337103 NLM identifier: NCT00337103. [Google Scholar]
- 31.Spira AI, Iannotti NO, Savin MA, et al. A Phase II study of eribulin mesylate E7389 in patients with advanced, previously treated non-small cell lung cancer. Clin Lung Cancer. 2011;13:31–38. doi: 10.1016/j.cllc.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Gitlitz BJ, Davies AM, Belani CP, et al. A Phase II study of the halichondrin B analog, E7389, in patients with advanced non-small cell lung cancer (NSCLC) previously treated with a taxane. A California Consortium/University of Pittsburgh/University of Chicago NCI/CTEP sponsored trial. J Clin Oncol. 2009;27(15s) abst 8056. [Google Scholar]
- 33.Eisai Inc. PharmaBio Development Inc . ClinicalTrialsgov [Internet] Bethesda, MD: National Library of Medicine (US); May 17, 2010. Eribulin mesylate administered in combination with pemetrexed versus pemetrexed alone as second line therapy in patients with stage IIIB or IV nonsquamous non-small cell lung cancer. [last updated on March 29, 2011]. Available from: http://clinicaltrials.gov/ct2/show/NCT01126736 NLM identifier: NCT01126736. [Google Scholar]
- 34.Eisai Inc . ClinicalTrialsgov [Internet] Bethesda, MD: National Library of Medicine (US); Apr 12, 2010. Eribulin mesylate in combination with intermittent erlotinib in patients with previously treated, advanced non-small cell lung cancer. [last updated on December 8, 2010]. Available from: http://clinicaltrials.gov/ct2/show/NCT01104155 NLM identifier: NCT01104155. [Google Scholar]
- 35.Eisai Inc . ClinicalTrialsgov [Internet] Bethesda, MD: National Library of Medicine (US); Dec 21, 2005. A dose-finding study of E7389 in combination with carboplatin in patients with solid tumors. [last updated on May 3, 2011]. Available from: http://clinicaltrials.gov/ct2/show/NCT00268905 NLM identifier: NCT00268905. [Google Scholar]
- 36.Arnold SM, Moon J, Williamson SK, et al. Phase II evaluation of eribulin mesylate (E7389, NSC707389) in patients with metastatic or recurrent squamous cell carcinoma of the head and neck: Southwest Oncology Group trial S0618. Invest New Drugs. 2011;29:352–359. doi: 10.1007/s10637-009-9348-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Bono JS, Maroto P, Calvo E, et al. Phase II study of eribulin mesylate (E7389) in patients with metastatic castration-resistant prostate cancer (CRPC) stratified by prior taxane therapy. ASCO Genitourinary Cancer Symposium. 2009 doi: 10.1093/annonc/mdr380. abst 166. [DOI] [PubMed] [Google Scholar]
- 38.Stein MN, Chen Y, Hudes GR, et al. ECOG 5805: a Phase II study of eribulin mesylate (E7389) in patients with metastatic castration-resistant prostate cancer (CRPC) J Clin Oncol. 2010;28(15s) abst 4556. [Google Scholar]
- 39.Hensley ML, Kravetz SJ, Sima C, et al. Eribulin mesylate (halichondrin B analog E7389) in platinum-resistant epithelial ovarian cancer (PREOC): a CTEP-sponsored Phase II study. J Clin Oncol. 2009;27(15s) abst 5561. [Google Scholar]
- 40.Hensley ML, Kravetz SJ, Jia X, et al. Eribulin mesylate (halichondrin B analog E7389) in platinum-sensitivy ovarian cancer: a Phase II study, CTEP # 74. J Clin Oncol. 2011;29 doi: 10.1002/cncr.26569. abst 5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Renouf DJ, Tang PA, Major P, et al. A Phase II study of the halichondrin B analog eribulin mesylate in gemcitabine refractory advanced pancreatic cancer. Invest New Drugs. 2011 doi: 10.1007/s10637-011-9673-x. In press. [DOI] [PubMed] [Google Scholar]
- 42.Schoffski P, Ray-Coquard IL, Cioff A, et al. Activity of eribulin mesylate (E7389) in patients with soft tissue sarcoma (STS): Phase II studies of the European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group (EORTC 62052) J Clin Oncol. 2010;28(15s) abst 10031. [Google Scholar]
- 43.Synold TW, Tsao-Wei DD, Quinn DI, et al. Phase I and pharmacokinetic study of eribulin (E7389) in patients with renal dysfunction and advanced urothelial cancer (UC): a California Cancer Consortium Trial. J Clin Oncol. 2010;28(15s) abst 2527. [Google Scholar]
- 44.Quinn DI, Aparicio A, Tsao-Wei DD, et al. Phase II study of eribulin (E7389) in patients with advanced urothelial cancer (UC) – final report: a California Cancer Consortium-led NCI/CTEP-sponsored trial. J Clin Oncol. 2010;28(15s) abst 4539. [Google Scholar]
- 45.Eisai Inc . Clinical Trialsgov [Internet] Bethesda, MD: National Library of Medicine (US); Dec 31, 2010. Eribulin with trastuzumab as first-line therapy for locally recurrent or metastatic HER2 positive breast cancer. [last updated on March 30, 2011]. Available from: http://clinicaltrials.gov/ct2/show/NCT01269346 NLM identifier: NCT01269346. [Google Scholar]
- 46.Eisai Inc . ClinicalTrialsgov [Internet] Bethesda, MD: National Library of Medicine (US); Mar 22, 2011. Dose dense doxorubicin and cyclophosphamide followed by eribulin mesylate for the adjuvant treatment of early stage breast cancer. [last updated on December 13, 2011]. Available from: http://clinicaltrials.gov/ct2/show/NCT01328249 NLM identifier: NCT01328249. [Google Scholar]
- 47.Northwestern University. Eisai Inc . ClinicalTrialsgov [Internet] Bethesda, MD: National Library of Medicine (US); Jun 1, 2011. Carboplatin and eribulin mesylate in triple negative breast cancer patients. [last updated on June 10, 2011]. Available from: http://clinicaltrials.gov/ct2/show/NCT01372579 NLM identifier: NCT01372579. [Google Scholar]
- 48.Eisai Inc . ClinicalTrialsgov [Internet] Bethesda, MD: National Library of Medicine (US); Dec 28, 2010. A study of single-agent eribulin mesylate as first-line therapy for locally recurrent or metastatic Human epidermal growth factor receptor two (HER2) negative breast cancer. [last updated on April 28, 2011]. Available from: http://clinicaltrials.gov/ct2/show/NCT01268150 NLM identifier: NCT01268150. [Google Scholar]
- 49.Eisai Ltd. Eisai Inc . ClinicalTrialsgov [Internet] Bethesda, MD: National Library of Medicine (US); Mar 24, 2011. A Phase 1b/2, multicenter, randomized, open-label, dose-escalation and confirmation study of eribulin in combination with capecitabine. [last updated on March 24, 2011]. Available from: http://clinicaltrials.gov/ct2/show/NCT01323530 NLM identifier: NCT01323530. [Google Scholar]
- 50.Eisai Inc . ClinicalTrialsgov [Internet] Bethesda, MD: National Library of Medicine (US); Mar 31, 2011. Phase 3 study to compare the efficacy and safety of eribulin with dacarbazine in subjects with soft tissue sarcoma. [last updated on April 1, 2011]. Available from: http://clinicaltrials.gov/ct2/show/NCT01327885 NLM identifier: NCT01327885. [Google Scholar]
- 51.Narayan S, Carlson EM, Cheng H, et al. Novel second generation analogs of eribulin. Part I: Compounds containing a lipophilic C32 side chain overcome P-glycoprotein susceptibility. Bioorg Med Chem Lett. 2011;21:1630–1633. doi: 10.1016/j.bmcl.2011.01.111. [DOI] [PubMed] [Google Scholar]
- 52.Narayan S, Carlson EM, Cheng H, et al. Novel second generation analogs of eribulin. Part II: Orally available and active against resistant tumors in vivo. Bioorg Med Chem Lett. 2011;21:1634–1638. doi: 10.1016/j.bmcl.2011.01.097. [DOI] [PubMed] [Google Scholar]
- 53.Narayan S, Carlson EM, Cheng H, et al. Novel second generation analogs of eribulin. Part III: Blood-brain barrier permeability and in vivo activity in a brain tumor model. Bioorg Med Chem Lett. 2011;21:1639–1643. doi: 10.1016/j.bmcl.2011.01.096. [DOI] [PubMed] [Google Scholar]