Abstract
Background
This study was done to determine the immunogenicity of a single dose of hepatitis A vaccine in children, providing needed clinical data on the flexibility of booster administration.
Methods
Participants had received one dose of inactivated hepatitis A vaccine (Avaxim™ 80 U Pediatric) at 12–23 months of age or two doses of the same vaccine at 12 and 18 months of age prior to enrolment. Anti-hepatitis A antibody concentrations were measured at the first, second, and third year after vaccination. Suspected cases of hepatitis A in participant families were assessed and family socioeconomic data were collected.
Results
A series of 546 participants were enrolled. Of 467 (85.5%) participants completing 3 years of follow-up, 365 had received a single vaccine dose and 94 had received two vaccine doses. Seropositivity (anti-HAV ≥ 10 mIU/mL) at 3 years was 99.7% after one dose and 100% after two doses. At one year, geometric mean concentrations were higher after two doses (1433.9 mIU/mL, 95% confidence interval [CI] 1108–1855) than one (209.7 mIU/mL, 95% CI 190.6–230.6). Geometric mean concentrations decreased in both groups during the study, but remained well above 10 mIU/mL through the third year. The geometric mean of 3-year to one-year anti-hepatitis A concentration ratios was 0.74 (95% CI 0.70–0.79) following one dose and 0.57 (95% CI 0.47–0.70) following two doses. The greatest decrease in geometric mean concentrations occurred during the third year, ie, 21.2% in the one-dose group and 40.8% in the two-dose group. Six participants became seronegative during follow-up and responded strongly to a booster dose. Anti-hepatitis A concentrations increased in 135 children (34.9%) in the second year and 50 (13.7%) in the third year; none lived in a family with a case of hepatitis A. Three confirmed cases of hepatitis A occurred in family members. Participants belonged to a middle-income, urban/suburban population with good sanitation facilities and water supplies.
Conclusion
A single dose of hepatitis A vaccine at 12–23 months of age resulted in hepatitis A seropositivity in all but one vaccinee after 3 years. Increased anti-hepatitis A serum concentrations suggested exposure to wild-type hepatitis A virus in this middle-class socioeconomic environment. Continuing surveillance is required to confirm the effectiveness of a single-dose hepatitis A vaccination; however, the results of the first three years are encouraging.
Keywords: hepatitis A vaccine, single dose, antibody persistence, immunization programs
Introduction
Hepatitis A virus (HAV) infection is a frequent cause of viral hepatitis and is preventable by vaccination. The prevalence of HAV infection is primarily determined by socioeconomic conditions and by environmental factors, such as the quality of drinking water and sanitation systems. The World Health Organization classifies HAV endemicity as high, intermediate, or low, depending on the prevalence of anti-HAV (IgG) antibodies in the sera of specific age groups.1
Improved hygiene and sanitation result in an overall reduction in HAV circulation and decrease of endemicity from high to intermediate and from intermediate to low. Evidence of decreasing HAV seroprevalence in younger age groups has been reported in Argentina for more than a decade.2 Argentina is considered to be a region of intermediate HAV endemicity3 because of socioeconomic differences, but populations that are highly susceptible may live side by side with populations in which HAV is widely circulating. As a consequence, many individuals escape early childhood infection only to be infected later in life when they are more likely to develop more severe clinically significant HAV disease. In 1997, González et al4 reported low endemicity (13%) in Buenos Aires, with zones of intermediate endemicity in localities like Rosario (46.5%), and others of high endemicity such as Tucumán (81.4%).
In Argentina, the annual incidence of HAV disease estimated from the number of reported cases during 1995–2004 ranged from 70.5 to 173.8 cases per 100,000 people.5 Outbreaks of HAV infection occurred during 2003–2004, which increased the incidence from 70.5 cases per 100,000 people in 2002 to 139 and 172.7 cases per 100,000 people in 2003 and 2004, respectively.5,6 A year later, in 2005, a single dose of HAV vaccine for children 12 months of age was added to the national schedule.5 In the private market, which represented approximately 12% of the birth cohort, children could already receive two doses of the vaccine at 12 and 18 months of age.7 Overall, vaccine coverage in 2006 was estimated at 95% for the single dose. The National Notifiable Diseases Surveillance System in Argentina reported that HAV incidence rates decreased sharply after initiating the vaccination program. In 2007 it was 10.2 per 100,000, representing a decrease of 88% compared with the average incidence between 1998 and 2001. The incidence of HAV in all age groups has continued to decline through 2010, the last year for which national surveillance data were available.8 Reductions were reported in all age groups, even though only children in the second year of life were vaccinated, showing a marked herd effect.5
The rationale for a single vaccine dose immunization was partly based on an hypothesis that one dose would be sufficient to prime the immune system and provide immediate protection against infection. A single dose of HAV vaccine is known to control outbreaks of infection and to protect individuals living in regions at risk of repeated outbreaks.9–11 Exposure to wild-type HAV circulating in the environment would be expected to lead to a protective response in children who had received a single dose of vaccine, providing long-term protection. The effectiveness of this strategy needs to be confirmed.12
This study was designed to determine the persistence of antibodies to HAV in a group of children who had previously received one dose of the study vaccine, adding needed clinical data to confirm flexible booster dosing. Secondary objectives were to investigate a possible relationship between any confirmed HAV cases in family members and serum anti-HAV concentrations of the vaccinated study participant, and to describe the socioeconomic conditions of the participant families. We report here interim results from the first 3 of 5 years of planned follow-up.
Materials and methods
Study design
This single-center, prospective, cohort study assessed HAV antibody persistence following a single dose of inactivated HAV vaccine. The local independent ethics committee and institutional review board (Hospital Pediátrico Humberto J Notti) approved the protocol and written information was provided to the participants’ parents/guardians prior to the start of the study. The study was conducted in accordance with the Edinburgh revision of the Declaration of Helsinki, International Conference on Harmonization, Good Clinical Practice, and applicable national and local requirements. The Ministry of Health of Mendoza province and the Central Hospital of Mendoza also approved the protocol and study documentation. Before enrolment, written informed consent was obtained from the participant’s parent or legal guardian with an independent witness present.
Eligible participants had received (prior to enrolment in this study) one dose of HAV vaccine between June and December 2007 at 12–23 months of age following the national immunization schedule (Group A) or a two-dose primary series vaccination at 12 and 18 months of age from a private physician (Group B). The inactivated HAV vaccine administered in the national schedule in Argentina is Avaxim™ 80U Pediatric (Sanofi Pasteur, Lyon, France), which is prepared from formaldehyde-inactivated GBM strain HAV. Each 0.5 mL dose contains 80 HAV antigen units adsorbed to aluminum hydroxide (0.15 mg), 2-phenoxyethanol (2.5 μL), formaldehyde (12.5 μg), and water for injection.
Children were excluded if they had any health problems that, in the judgment of the investigator, could interfere with their immune response to the vaccine, as documented in their medical records or identified during the medical examination conducted at the start of the study. Included children were assigned a sequential enrolment number by the investigator, taken from a list supplied by the study sponsor. Study participants were enrolled between August 2008 and April 2009 (visit 1). Successive annual visits were scheduled for each participant on the annual anniversary of their HAV vaccination for a planned follow-up of 5 years (visits 2–5). Study completion is scheduled for December 2012. This article reports interim results obtained after a 3-year follow-up that ended in December 2010.
Serology
A 3 mL blood sample was obtained at each study visit for determination of anti-HAV concentrations. Participants found to have an anti-HAV IgG antibody concentration < 10 mIU/mL at a scheduled annual visit received a second (booster) dose of HAV vaccine and were continued in the study as Group C. An additional blood sample was collected 10 days after administration of the booster to measure the immune response, (ie, anti-HAV concentration).
Serum specimens were tested at BARC laboratories in Ghent, Belgium (under the supervision of the Sanofi Pasteur Global Clinical Immunology Department) using a commercial enzyme-linked immunosorbent assay kit (HAV 2.0 quantitative AXSYM HAVAB microparticle enzyme immunoassay technology, Abbott Laboratories, Abbott Park, IL) that permits the quantitative measurement of anti-HAV antibodies. Quantitative anti-HAV levels were expressed as geometric mean concentrations (in mIU/mL), as determined by comparison with a serial dilution of World Health Organization reference serum. The lower limit of quantification of 5 mIU/mL was assigned to all samples with no detectable antibodies. Anti-HAV seropositivity was defined as a serum anti-HAV concentration ≥ 10 mIU/mL.
Statistical analysis
All children attending each study visit and with evaluable sera were included in the analysis. The statistical analysis was descriptive, and no hypothesis was tested. Seropositivity rates, ie, percentages of participants with antibody concentration ≥ 10 mIU/mL, were calculated with their 95% confidence intervals (CI) using the exact binomial method. Geometric mean concentrations and enrolment annual follow-up geometric mean concentration ratios (geometric means of individual anti-HAV concentration ratios) were calculated with 95% CI using the normal approximation. Socioeconomic data were collected annually using questionnaires, including environmental conditions, household characteristics, education, and employment status of parents/guardians.
Results
Disposition of participants
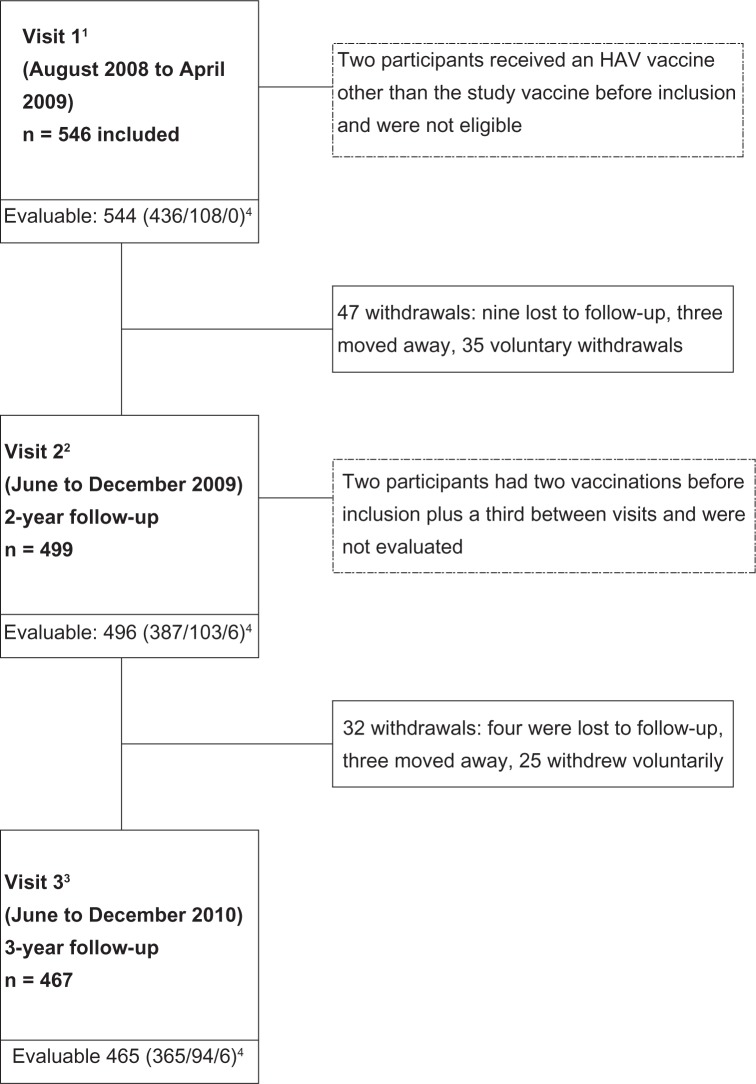
A total of 546 participants were enrolled, of whom 467 (85.5%) completed three years of follow-up after the first HAV vaccination (visit 3). The number of male (50.4%) and female (49.6%) participants was similar. The mean age at enrolment was 28.5 ± 2.4 months. A total of 47 children (8.6%) discontinued between the first and second visit; 32 (6.4%) discontinued between the second and third visit (Figure 1). Voluntary withdrawal was the main reason leading to study discontinuation. Seven children (1.3%) received a dose of vaccine during the first year, but one of them had already been given two doses before inclusion, and was therefore excluded from the analyses. No participant received a vaccine dose between visits 2 and 3. Three years after the first HAV vaccination, there were 365 participants included in Group A, 94 in Group B, and six in Group C, ie, those who had been given a booster after study inclusion (Figure 1).
Figure 1.
Subject disposition.
Notes: 1Participants received one dose of HAV vaccine between June and December 2007; 22-year anniversary of HAV vaccination; 33-year anniversary of HAV vaccination; 4participants given one HAV vaccine dose before inclusion (Group A), participants given two doses before inclusion (Group B), participants given one dose before inclusion and a second dose during the study (Group C).
Abbreviation: HAV, hepatitis A virus.
Antibody persistence
In all groups, seropositivity rates (percentage of children with anti-HAV concentrations ≥ 10 mIU/mL) remained high at visit 3 three years after the first vaccination, and 99.7% in Group A and 100% in Groups B and C (Table 1). At visit 3, only one study participant (in Group A) was not seropositive. Only six study participants (Group C) had needed boosting between visits 1 and 2. After boosting, all six became seropositive, with a group geometric mean concentration of 551.3 mIU/mL (95% CI 130.3–23332.2) at visit 2. Geometric mean concentrations were high at year 1 and decreased in all three groups during the course of the study (Figure 2), but they remained well above the seropositivity threshold of 10 mIU/mL through the third year of follow-up. The greatest rate of decrease occurred during the third year, ie, 21.2%, 35.5%, and 40.8% in Groups A, B, and C, respectively. At each study visit, geometric mean concentrations were highest among participants who had received two vaccine doses before inclusion (Group B). The ratio of one-year to 3-year anti-HAV geometric mean concentrations was 0.74 (95% CI 0.70–0.79) in Group A and 0.57 (0.47–0.70) in Group B (Table 1).
Table 1.
Proportion of subjects with an anti-HAV titer ≥ 10 mIU/mL, geometric mean concentrations, and geometric mean of individual concentration ratios at years 1, 2, and 3 after primary vaccination
| Visit 1 % (95% CI) | Visit 2 (95% CI) | Visit 3 (95% CI) | ||||
|---|---|---|---|---|---|---|
| Group A: one dose of hepatitis A vaccine before enrolment | ||||||
| ≥10 mIU/mL | 98.6 (97.0–99.5) | 100.0 (99.1–100.0) | 99.7 (98.5–100.0) | |||
| GMC | 209.7 (190.6–230.6) | 216.0 (198.0–235.7) | 170.2 (155.7–186.0) | |||
| GMCR | – | 0.95 (0.90–1.00) | 0.74 (0.70–0.79) | |||
| Group B: two doses of hepatitis A vaccine before enrolment | ||||||
| ≥10 mIU/mL | 99.1 (94.9–100.0) | 100.0 (96.5–100.0) | 100.0 (96.2–100.0) | |||
| GMC | 1433.9 (1108.4–1855.1) | 1353.8 (1116.2–1641.9) | 872.9 (710.2–1073.0) | |||
| GMCR | – | 0.88 (0.72–1.07) | 0.57 (0.47–0.70) | |||
| Group C: one dose of hepatitis A vaccine before enrolment; one dose during follow-up | ||||||
| ≥mIU/mL | NA | 100.0 (54.1–100.0) | 100.0 (54.1–100.0) | |||
| GMC | NA | 551.3 (130.3–2332.2) | 326.5 (66.5–1603.6) | |||
Abbreviations: CI, confidence interval; GMC, geometric mean concentration; GMCR, visit 2:visit 1 and visit 3:visit 1; NA, not applicable.
Figure 2.
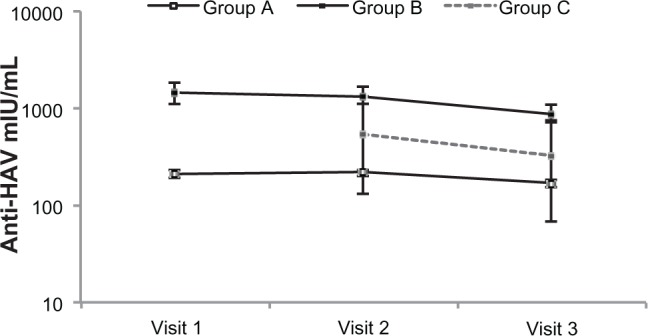
Anti-HAV geometric mean concentrations at years 1, 2, and 3 after primary vaccination.
Notes: Group A, participants given one HAV vaccine dose before inclusion (n = 365); Group B, participants given two doses before inclusion (n = 94); Group C, participants given one dose before inclusion and a second dose during the study (n = 6).
Abbreviation: HAV, hepatitis A virus.
In Group A (single dose of HAV vaccine), the anti-HAV concentration increased in 135 children (34.9%) in the second year after vaccination (between visits 1 and 2) and in 50 children (13.7%) in the third year (between visits 2 and 3). None of these children lived in a family with a suspected case of HAV. No increase in anti-HAV concentration was observed for the child who lived in a family with a case of HAV. In Group B (two doses of HAV vaccine before the start of the study), anti-HAV concentration increased in 25 children (24.8%) between visits 1 and 2, and in seven children (7.4%) between visits 2 and 3. None of these children lived in a family with a suspected case of HAV. No increase in anti-HAV concentration was observed for the two children who lived in a family with a case of HAV.
Socioeconomic conditions
The majority of participants lived in a middle-income, urban population, with good sanitation facilities and water supply, which are associated with a limited natural exposure to HAV. Most children lived in urban (73.8%) or suburban (23.1%) areas, in houses with more than one bedroom (88.6%) and at least one bathroom (100%) inside the house (97.1%). Access to potable water (99.3%) and a public sewage network (92.7%) was very common, and most homes had floors made of ceramic tiles (88.8%). Few children changed their residence during the study period, so there were no notable changes in environmental conditions during the course of this analysis. At inclusion, most participants had only one (35.5%) or no (33.7%) siblings. They were usually cared for at home. The number and percentage of children cared for outside the home and the number of days each week of care outside the home per week increased during the study. At visit 3, the majority (63.3%) were cared for outside the home versus 25.3% at inclusion, and nearly all of them (94.9%) for 5 days a week. More than one-third of the fathers or legal guardians and half of the mothers had secondary or tertiary education level, and 96.9% of the fathers and 50.9% of the mothers maintained their employment status throughout the course of the study.
Discussion
This report presents 3-year interim results of a study to determine the long-term (5-year) immunogenicity of a single dose of HAV vaccine given at approximately 12 months of age. The study also looked for any relationships between serum anti-HAV concentrations of study participants and cases of HAV that were diagnosed among their family members. Finally, socioeconomic and environmental data were collected about the participants and their families.
The study design allowed evaluation of immunogenicity data from three distinct subpopulations. Thus, the seropositivity rates and anti-HAV geometric mean concentrations of participants who had received one dose of vaccine prior to the start of the study could be distinguished from those who had received two doses and from those who needed a second dose of vaccine after enrolment to maintain anti-HAV concentrations ≥ 10 mIU/mL. Seropositivity was high at inclusion and remained high. Only six participants (Group C) received an additional (booster) dose of vaccine according to the study protocol, and only one study participant (in Group A) was not seropositive after 3 years.
Currently, there is no clear definition of an HAV seroprotection threshold following vaccination. Vaccine-induced antibody levels are generally high, and few cases of HAV have been reported among vaccinated persons. Anti-HAV concentrations of 20 mIU/mL after administration of immunoglobulins are known to protect against HAV.13,14 However, in vitro studies have shown HAV antibody concentrations < 20 mIU/mL to be neutralizing.15 Because no absolute protective level has been defined, detectable concentrations of HAV antibodies are often considered to indicate seroprotection.14 The lower limit of the assay method used in this study was 5 mIU/mL; we have considered an antibody concentration ≥ 10 mIU/mL as seroprotective as well as seropositive.
Geometric mean concentrations decreased in all groups, with the greatest change occurring in the third year. The group of participants who had received two doses of vaccine before inclusion had higher geometric mean concentrations at each study visit than those who had received a single dose. The overall percent decrease in geometric mean concentration was smaller in Group A, which had received only one dose of vaccine. The group differences are not likely to be clinically important because antibody concentrations in all groups far exceeded 10 mIU/mL. At this time, we cannot account for the between-group differences in geometric mean concentration rate of change, but it will be interesting to see what occurs in the final 2 years of follow-up. A previous 3-year follow-up of a two-dose primary series of HAV vaccination with the study vaccine in a country with intermediate endemicity, also found antibody persistence to be high, as well as evidence that some participants had been exposed to HAV. In that study, and in another with a 15-year follow-up, the greatest decrease in geometric mean concentration occurred in the first year and then slowed afterwards.16,17
The rationale for a single-dose vaccination against HAV is based in part on the expectation that the vaccine would prime the immune system, which would subsequently respond to or be boosted by exposure to wild-type HAV circulating in the environment. Hepatitis A is a reportable disease in Argentina, and surveillance confirms that decreases in HAV incidence have continued since routine mass vaccination of children began in 2005, with only 358 reported cases in 2010.5,8
Previous studies of long-term immunity produced by HAV vaccines have shown that a single priming dose can induce seroprotective antibody concentrations for at least 2 years, induce cellular immunity, and an immunologic memory response to booster vaccination some years later, thus providing protection even if serum antibody concentrations were not detectable.13,18–22 The six participants in this study who were seronegative, ie, had antibody concentrations < 10 mIU/mL during follow-up, had strong responses to a vaccine booster dose. The duration of protection provided by vaccination may thus be longer than suggested by the decline in antibody level. Underlying immune memory may provide protection for longer than the duration of detectable anti-HAV antibodies.23 Current reviews conclude that 2-dose and 3-dose vaccination regimens provide protection for 15–17 years.24,25 The World Health Organization position is open to adoption of one-dose HAV vaccination regimens if the immunologic evidence and reduction in disease burden support it.12 A single-dose regimen would have both public health and economic benefits.
Although person-to-person transmission of HAV is thought to occur most frequently among family members,26 no relationships were observed between the anti-HAV concentrations of the study participants and occurrence of HAV among their family members, and up to a third of participants had increases in anti-HAV concentration. It is not clear where exposure to HAV occurred, but increasing attendance at day care outside the home, as documented in our socioeconomic results, may have accounted for some of the environmental exposure.27 It is notable that although the study participants were drawn from middle-income urban and suburban areas, there was sufficient exposure to circulating HAV to result in increases in serum antibodies of some study participants and HAV infection in some family members. An analysis of the relation between seroprevalence, water quality, socioeconomic factors, and infection risk for HAV has recently been published.28
Quantitation of anti-HAV concentrations is highly dependent on the assay used. Indeed, some increase in individual antibody concentrations observed at the annual study visits may be attributable to assay variability. Varying sensitivity resulting from differences in assay design also affect the ability to detect low antibody concentrations.29,30 Thus persons who are anti-HAV negative by standard assays after vaccination might nevertheless have protective antibody concentrations.14,15 Use of a standard reference serum and assay modifications have increased the sensitivity of the assay used in this study.31 Differences in the populations evaluated and the laboratory techniques used make comparisons between studies difficult, but we believe that longitudinal studies such as this one can provide an accurate picture of long-term immunogenicity following HAV vaccination.
Conclusion
A single dose of HAV vaccine (Avaxim 80U Pediatric) at 12–23 months of age resulted in HAV seropositivity that lasted for at least 3 years in 364 of 365 (99.7%, 95% CI 98.5–100) of vaccinees in this interim analysis. Geometric mean concentrations were higher in children who had received a full vaccination series of two doses at 12 and 18 months of age, but anti-HAV concentrations in both groups at 3 years of follow-up remained far higher than the seropositivity threshold of 10 mIU/mL. Some participants had increased anti-HAV serum concentrations during the course of the study, suggesting exposure to wild-type HAV in this middle-income socioeconomic environment. Continuing surveillance is required to confirm the protective effectiveness of a single-dose public health strategy for HAV vaccination of children; however, the results of the first three years are encouraging.
Acknowledgments
We thank the children who participated in this study and their parents. We acknowledge the assistance of Mariela Linares, Ivana Lo Castro and Abelardo Manzur who collaborated in this study. We would like to thank Daniel Shouval (Hadassah-Hebrew University Hospital, Jerusalem, Israel) for critically reviewing the manuscript.
Footnotes
Disclosure
The authors thank Clement Weinberger, of The Stylus Medical Communications, for assistance with preparation of the manuscript on behalf of Sanofi Pasteur in accordance with the European Medical Writers Association guidelines.
GH and AR are employees of Sanofi Pasteur. CE, LB, and HC have received honoraria from Sanofi Pasteur for conducting this clinical study, which was done with the financial support of Sanofi Pasteur.
References
- 1.World Health Organization Hepatitis A vaccines. Wkly Epidemiol Rec. 2000;75(5):38–44. [PubMed] [Google Scholar]
- 2.Tapia-Conyer R, Santos JI, Cavalcanti AM, et al. Hepatitis A in Latin America: a changing epidemiologic pattern. Am J Trop Med Hyg. 1999;61(5):825–829. doi: 10.4269/ajtmh.1999.61.825. [DOI] [PubMed] [Google Scholar]
- 3.Jacobsen KH, Wiersma ST. Hepatitis A virus seroprevalence by age and world region, 1990 and 2005. Vaccine. 2010;28(41):6653–6657. doi: 10.1016/j.vaccine.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez J, Fay O, Canero-Velasco MC, et al. Hepatitis A virus infection in children in Argentina: a pilot study. Acta Gastroenterol Latinoam. 1997;27(5):331–334. Spanish. [PubMed] [Google Scholar]
- 5.Vacchino MN. Incidence of hepatitis A in Argentina after vaccination. J Viral Hepat. 2008;15(Suppl 2):47–50. doi: 10.1111/j.1365-2893.2008.01029.x. [DOI] [PubMed] [Google Scholar]
- 6.Gentile A. The need for an evidence-based decision-making process with regard to control of hepatitis A. J Viral Hepat. 2008;15(Suppl 2):16–21. doi: 10.1111/j.1365-2893.2008.01023.x. [DOI] [PubMed] [Google Scholar]
- 7.Cervio G, Trentadue J, D’Agostino D, et al. Decline in HAV-associated fulminant hepatic failure and liver transplant in children in Argentina after the introduction of a universal hepatitis A vaccination program. Hepat Med. 2011;3:99–106. doi: 10.2147/HMER.S22309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ministerio de Salud de la Nación Boletín Epidemiológico Anual 2010[National Ministry of Health. Annual Epidemiological Bulletin 2010.] Available from: http://msal.gov.ar/htm/site/sala_situacion/PANELES/bep-anual-2010/BEPANUAL_2010.pdfAccessed June 7, 2012
- 9.D’Argenio P, Adamo B, Cirrincione R, Gallo G. The role of vaccine in controlling hepatitis A epidemics. Vaccine. 2003;21(19–20):2246–2249. doi: 10.1016/s0264-410x(03)00140-3. [DOI] [PubMed] [Google Scholar]
- 10.Selnikova O, Moisseeva A, Zadorozhnaja V, Dokachenko A, Kachur N, Gavrik S. Hepatitis A vaccination effectiveness during an outbreak in the Ukraine. Vaccine. 2008;26(25):3135–3137. doi: 10.1016/j.vaccine.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 11.Werzberger A, Mensch B, Kuter B, et al. A controlled trial of a formalin-inactivated hepatitis A vaccine in healthy children. N Engl J Med. 1992;327(7):453–457. doi: 10.1056/NEJM199208133270702. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization WHO position paper on hepatitis A vaccines – June 2012. Wkly Epidemiol Rec. 2012;87:261–276. [Google Scholar]
- 13.Hammitt LL, Bulkow L, Hennessy TW, et al. Persistence of antibody to hepatitis A virus 10 years after vaccination among children and adults. J Infect Dis. 2008;198(12):1776–1782. doi: 10.1086/593335. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55(RR-7):1–23. [PubMed] [Google Scholar]
- 15.Lemon SM, Binn LN. Serum neutralizing antibody response to hepatitis A virus. J Infect Dis. 1983;148(6):1033–1039. doi: 10.1093/infdis/148.6.1033. [DOI] [PubMed] [Google Scholar]
- 16.Dagan R, Greenberg D, Weber F. Immunogenicity of an inactivated hepatitis A pediatric vaccine: three-year post-booster follow-up. Vaccine. 2005;23(44):5144–5148. doi: 10.1016/j.vaccine.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 17.Van Damme P, Leroux-Roels G, Crasta P, Messier M, Jacquet J-M, Van Herck K. Antibody persistence and immune memory in adults, 15 years after a three-dose schedule of a combined hepatitis A and B vaccine. J Med Virol. 2012;84(1):11–17. doi: 10.1002/jmv.22264. [DOI] [PubMed] [Google Scholar]
- 18.Cederna JB, Klinzman D, Stapleton JT. Hepatitis A virus-specific humoral and cellular immune responses following immunization with a formalin inactivated hepatitis A vaccine. Vaccine. 1999;18(9–10):892–898. doi: 10.1016/s0264-410x(99)00342-4. [DOI] [PubMed] [Google Scholar]
- 19.Iwarson S, Lindh M, Widerstrom L. Excellent booster response 4–6 y after a single primary dose of an inactivated hepatitis A vaccine. Scand J Infect Dis. 2002;34(2):110–111. doi: 10.1080/00365540110077362. [DOI] [PubMed] [Google Scholar]
- 20.Orr N, Klement E, Gillis D, et al. Long-term immunity in young adults after a single dose of inactivated hepatitis A vaccines. Vaccine. 2006;24(20):4328–4332. doi: 10.1016/j.vaccine.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Van Herck K, Van Damme P, Lievens M, Stoffel M. Hepatitis A vaccine: indirect evidence of immune memory 12 years after the primary course. J Med Virol. 2004;72(2):194–196. doi: 10.1002/jmv.10574. [DOI] [PubMed] [Google Scholar]
- 22.Williams JL, Bruden DA, Cagle HH, et al. Hepatitis A vaccine: immunogenicity following administration of a delayed immunization schedule in infants, children and adults. Vaccine. 2003;21(23):3208–3211. doi: 10.1016/s0264-410x(03)00250-0. [DOI] [PubMed] [Google Scholar]
- 23.Van Damme P, Banatvala J, Fay O, et al. International Consensus Group on Hepatitis A Virus Immunity. Consensus Statement. Hepatitis A booster vaccination: is there a need? Lancet. 2003;362(9389):1065–1071. doi: 10.1016/S0140-6736(03)14418-2. [DOI] [PubMed] [Google Scholar]
- 24.Ott JJ, Irving G, Wiersma ST.Long-term protective effects of hepatitis A vaccines. A systematic review Available from: http://dx.doi.org/10.1016/j.bbr.2011.03.031Accessed June 7, 2012 [DOI] [PubMed]
- 25.Van Herck K, Crasta PD, Messier M, Hardt K, Van Damme P. Seventeen-year antibody persistence in adults primed with two doses of an inactivated hepatitis A vaccine. Hum Vaccin Immunother. 2012;8(3):323–327. doi: 10.4161/hv.18617. [DOI] [PubMed] [Google Scholar]
- 26.Victor JC, Surdina TY, Suleimenova SZ, Favorov MO, Bell BP, Monto AS. Person-to-person transmission of hepatitis A virus in an urban area of intermediate endemicity: implications for vaccination strategies. Am J Epidemiol. 2006;163(3):204–210. doi: 10.1093/aje/kwj029. [DOI] [PubMed] [Google Scholar]
- 27.Venczel LV, Desai MM, Vertz PD, et al. The role of child care in a community-wide outbreak of hepatitis A. Pediatrics. 2001;108(5):E78. doi: 10.1542/peds.108.5.e78. [DOI] [PubMed] [Google Scholar]
- 28.Jacobsen KH, Koopman JS. The effects of socioeconomic development on worldwide hepatitis A virus seroprevalence patterns. Int J Epidemiol. 2005;34(3):600–609. doi: 10.1093/ije/dyi062. [DOI] [PubMed] [Google Scholar]
- 29.Berger R, Just M, Althaus B. Time course of hepatitis A antibody production after active, passive and active/passive immunisation: the results are highly dependent on the antibody test system used. J Virol Methods. 1993;43(3):287–297. doi: 10.1016/0166-0934(93)90147-j. [DOI] [PubMed] [Google Scholar]
- 30.Wiedmann M, Boehm S, Schumacher W, Swysen C, Zauke M. Evaluation of three commercial assays for the detection of hepatitis A virus. Eur J Clin Microbiol Infect Dis. 2003;22(2):129–130. doi: 10.1007/s10096-002-0861-7. [DOI] [PubMed] [Google Scholar]
- 31.Miller WJ, Clark W, Hurni W, Kuter B, Schofield T, Nalin D. Sensitive assays for hepatitis A antibodies. J Med Virol. 1993;41(3):201–204. doi: 10.1002/jmv.1890410306. [DOI] [PubMed] [Google Scholar]