Abstract
Objective
To conduct a meta-analysis assessing the prevalence and trends of the abdominal aortic aneurysms (AAA) epidemic in general population.
Method
Studies that reported prevalence rates of AAA from the general population were identified through MEDLINE, EMBASE, Web of Science, and reference lists for the period between 1988 and 2013. Studies were included if they reported prevalence rates of AAA in general population from the community. In stratified analyses possible sources of bias, including areas difference, age, gender and diameter of aneurysms were examined. Publication bias was assessed with Egger's test method.
Results
56 studies were identified. The overall pooled prevalence of AAA was 4.8% (4.3%, 5.3%). Stratified analyses showed the following results, areas difference: America 2.2% (2.2%, 2.2%), Europe 2.5% (2.4%, 2.5%), Australia 6.7% (6.5%, 7.0%), Asia 0.5% (0.3%, 0.7%); gender difference: male 6.0% (5.3%, 6.7%), female 1.6% (1.2%, 1.9%); age difference: 55–64years 1.3% (1.2%, 1.5%), 65–74 years 2.8% (2.7%, 2.9%), 75–84 years1.2%(1.1%, 1.3%), ≥85years0.6% (0.4%, 0.7%); aortic diameters difference: 30–39 mm, 3.3% (2.8%, 3.9%), 40–49 mm,0.7% (0.4%,1.0%), ≥50 mm, 0.4% (0.3%, 0.5%). The prevalence of AAA has decreased in Europe from 1988 to 2013. Hypertension, smoking, coronary artery disease, dyslipidemia, respiratory disease, cerebrovascular disease, claudication and renal insufficiency were risk factors for AAA in Europe.
Conclusion
AAA is common in general population. The prevalence of AAA is higher in Australia than America and Europe. The pooled prevalence in western countries is higher than the Asia. Future research requires a larger database on the epidemiology of AAA in general population.
Introduction
Abdominal aortic aneurysm (AAA) is the pathologic local dilation of the abdominal aorta [1] and is defined as an aorta size more than 30 mm or a local dilation of abdominal aorta more than 50%, as compared to another site along the aorta. Epidemiological studies of AAA have shown an increased incidence worldwide, ranging from 4.2% to 11% per year. [2]–[5] Despite the evolution of our understanding and treatment of AAA in the past few decades, it continues to be a major threat to health because of grave outcome with 80% overall mortality in event of rupture. [6] Early identification of patients with AAA and offer of timely elective repair remain to be the most reliable strategy for prevention of death from ruptured AAA. The benefit of screening for AAA in elderly men had been proven by large-scale randomized studies that reported 50% reduction of AAA rupture and associated mortality. [7]–[9] However, specific information on the prevalence of AAA that would be useful for health services planning has been difficult to establish. To date, the epidemiological studies published have adopted different methodologies for case ascertainment and have demonstrated widely different prevalence that has varied between regions. Whether this variance reflects differences in biological substrates or the methodological approaches of each study has been difficult to determine. Thus far, no meta-analysis on the prevalence or trends of abdominal aortic aneurysms in general population exists. Accordingly, the aim of this study was to assess the prevalence rates of abdominal aortic aneurysms in the general population and to describe the secular trends in this prevalence from 1988 to 2013, and to examine potential moderator variables that may impact heterogeneity in prevalence rates.
Methods
This meta-analysis included cross-sectional studies, randomized controlled trials and prospective cohort studies which reported data involving the prevalence of patients with AAA. This study was conducted in accordance with the ‘preferred reporting items for systematic reviews and meta-analyses’ (Checklist S1) guidelines. No protocol exists for this meta-analysis.
Search Strategy
We assessed all English and Chinese publications that reported the prevalence of AAA among worldwide populations. We searched the electronic databases of MEDLINE, EMBASE, Web of Science for relevant papers published from 1988 through 2013. The search keywords were: abdominal aortic aneurysm, prevalence. A manual search was performed by checking the reference lists of original reports and review articles, retrieved through the electronic searches, to identify studies not yet included in the computerized databases.
Inclusion and Exclusion Criteria
The inclusion criteria were:
Studies in the mentioned three databases with full text;
Population-based studies;
Studies provided sufficient information to estimate the pooled prevalence of AAA.
The exclusion criteria were:
Studies without specific sample origins;
Studies with overlapping time intervals of sample collection from the same origin;
Studies with a sample size less than 50;
Studies that failed to present data clearly enough or with obviously paradoxical data.
Data Extraction
All the potentially relevant papers were reviewed independently by two investigators through assessing the eligibility of each article and abstracting data with standardized data-abstraction forms. Disagreements were resolved through discussion. The following information, though some studies did not contain all of them, were extracted from the literatures: first author's name, publication date, country, design, age, gender, number invited, number screened, definition of AAA, risk factors and prevalence rate by different stratified factors, including areas difference, age, gender and diameter of aneurysms.
Data Analysis
The primary outcome of this meta-analysis was the prevalence rate of AAA, defined as the number of cases divided by the total number of study participants. To examine possible sources of bias, stratified analyses were conducted for the studies. We investigated the effect of potentially distorting factors, including areas difference, age, gender and diameter of aneurysms of included participants. Because of insufficient numbers of studies for individual years, studies were grouped into eight 3-year periods, 1988–1992, 1993–1995, 1996–1998, 1999–2001, 2002–2004, 2005–2007, 2008–2010 and 2011–2013. Publication bias was assessed for the included studies, by visually inspecting funnel plots and applying Egger's test. [10], [11] Risk factor associations were expressed as odds ratios (ORs) to obtain consistency across studies. All analyses were conducted using STATA 12.0 (STATA Corporation, College Station, TX, USA). A random-effects model was chosen for data analysis as this model better addresses heterogeneity between studies and study populations and was less influenced by extreme variations in sample size. Heterogeneity among study prevalence estimates was assessed by means of the Q statistic, with magnitude of heterogeneity evaluated with the I 2 index.
Results
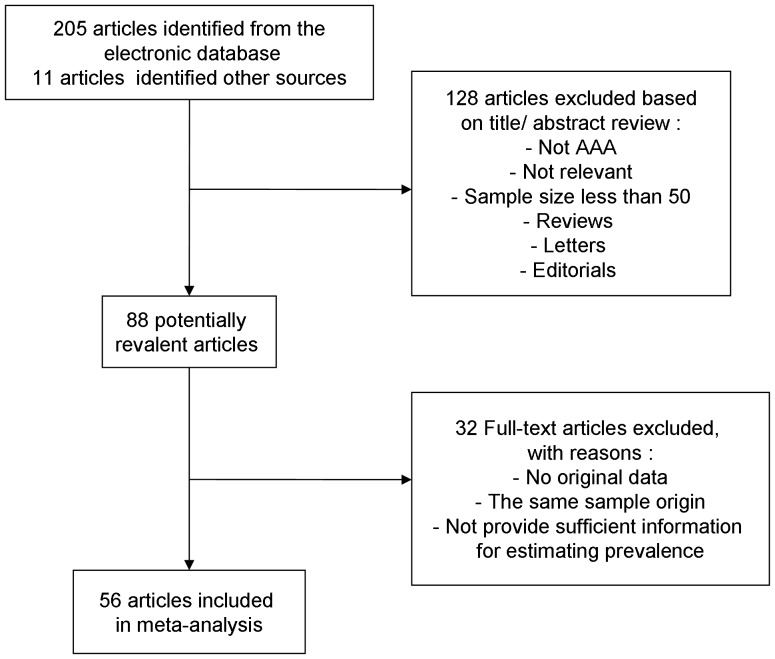
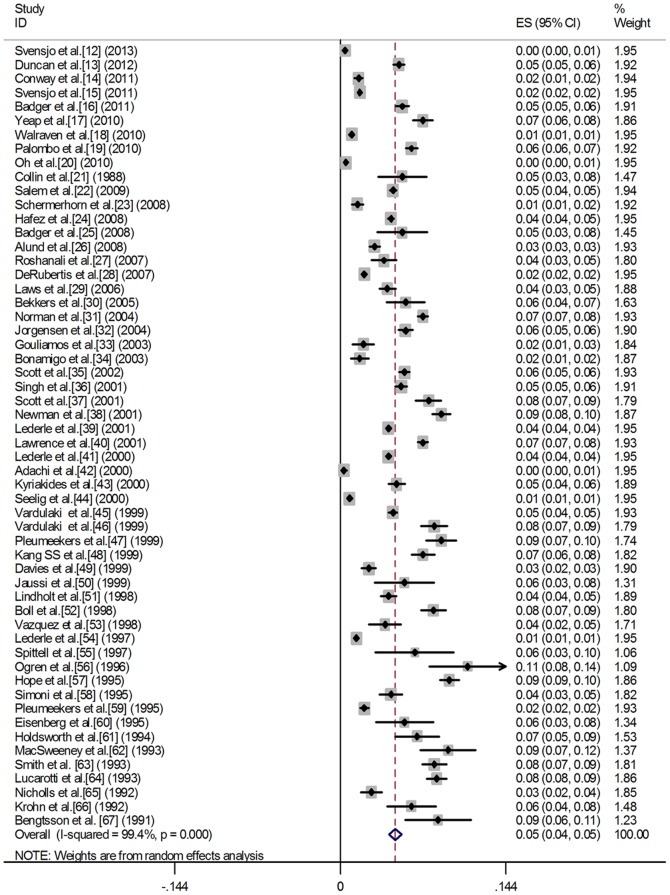
We screened 216 abstracts published from 1988 to 2013 and reviewed a total of 88 full-text articles. Of these, 32 were excluded for the following reasons: no original data, the same sample origin and not provide sufficient information for estimating prevalence. Thus, 56 studies were included in this meta-analysis. Figure 1 gives a schematic representation of the selection process and reasons for excluding studies. The characteristics of the 56 included studies are summarized in table 1. All studies were based on general population samples and used abdominal ultrasound as screening test. Figure 2 shows a forest plot of prevalence from individual studies and combined prevalence from random-effects models. The definitions used for AAA varied between studies, but most studies used a similar definition (a diameter greater than 30 mm).
Figure 1. Flow chart demonstrating those studies that were processed for inclusion in the meta-analysis.
Table 1. Characteristics of population-based studies of abdominal aortic aneurysm.
| Serial number | Study | Publication year | Country | Design | Age(years) | Gender | Number invited | Number screened (%) |
| 1 | Svensjö et al.[12] | 2013 | Sweden | CSS | 70 | W | 6925 | 5140(74.2) |
| 2 | Duncan et al.[13] | 2012 | UK | PCS | 65–74 | M | 8355 | 8146 (97.5) |
| 3 | Conway et al.[14] | 2011 | UK | CSS | 65 | M | 6091 | 4216 (69.2) |
| 4 | Svensjö et al.[15] | 2011 | Sweden | CSS | 65 | M | 26256 | 22139 (84.3) |
| 5 | Badger et al.[16] | 2011 | UK | CSS | 65–75 | M | 13316 | 5931 (44.5) |
| 6 | Yeap et al.[17] | 2010 | Australia | CSS | 70–88 | M | n.a. | 3620 |
| 7 | Walraven et al.[18] | 2010 | Canada | CSS | 65–80 | M and W | 311066 | 79121 (25) |
| 8 | Palombo et al.[19] | 2010 | Italy | CSS | 65–92 | M and W | 15151 | 8234 (54.3) |
| 9 | Oh et al.[20] | 2010 | Korea | CSS | 12–98 | M and W | 6267 | 4939 (79) |
| 10 | Collin et al.[21] | 1988 | UK | CSS | 65–74 | M | 824 | 426 (51.7) |
| 11 | Salem et al.[22] | 2009 | UK | CSS | 65 | M | n.a. | 19014 |
| 12 | Schermerhorn et al.[23] | 2008 | USA | CSS | ≥65 | M and W | 30000 | 2005 (6.7) |
| 13 | Hafez et al.[24] | 2008 | UK | CSS | 64–81 | M | 350000 | 22961 (6.6) |
| 14 | Badger et al.[25] | 2008 | UK | CSS | 65–75 | M | 908 | 409 (45.0) |
| 15 | Alund et al.[26] | 2008 | Sweden | CSS | 20–98 | M and W | 9296 | 5924 (63.7) |
| 16 | Roshanali et al.[27] | 2007 | Iran | PCS | 13–80 | M and W | 1285 | 1175 (91.4) |
| 17 | DeRubertis et al.[28] | 2007 | USA | CSS | ≥50 | M and W | n.a. | 17540 |
| 18 | Laws et al.[29] | 2006 | UK | CSS | 65–80 | M | 4000 | 2870(71.7) |
| 19 | Bekkers et al.[30] | 2005 | Netherlands | CSS | Mean = 60.5 | M and W | 796 | 742 (93.2) |
| 20 | Norman et al.[31] | 2004 | Australia | RCT | 65–83 | M | 17516 | 12203 (70) |
| 21 | Jørgensen et al.[32] | 2004 | Norway | CSS | 55–74 | M and W | 5465 | 5392 (98.7) |
| 22 | Gouliamos et al.[33] | 2003 | Greece | CSS | 55–85 | M and W | n.a. | 850 |
| 23 | Bonamigo et al.[34] | 2003 | Brazil | CSS | ≥54 | M | n.a. | 1012 |
| 24 | Scott et al.[35] | 2002 | UK | RCT | 65–80 | M and W | n.a. | 9485 |
| 25 | Singh et al.[36] | 2001 | Norway | PCS | 55–74 | M and W | 6892 | 6386 (92.7) |
| 26 | Scott et al. [37] | 2001 | UK | RCT | 64–81 | M | 6058 | 2212 (36.5) |
| 27 | Newman et al.[38] | 2001 | USA | PCS | ≥65 | M and W | 5888 | 4734 (80.4) |
| 28 | Lederle et al.[39] | 2001 | USA | CSS | 50–79 | M and W | n.a. | 125722 |
| 29 | Lawrence et al.[40] | 2001 | Australia | RCT | 26–69 | M | 19583 | 12203 (62.3) |
| 30 | Lederle et al.[41] | 2000 | USA | CSS | 50–79 | M and W | n.a. | 52745 |
| 31 | Adachi et al.[42] | 2000 | Japan | CSS | 35–82 | M and W | 1881 | 1591 (84.6) |
| 32 | Kyriakides et al.[43] | 2000 | UK | CSS | 65 | M | 4823 | 3497 (72.5) |
| 33 | Seelig et al.[44] | 2000 | Germany | CSS | ≥50 | M and W | 14876 | 13166 (88.5) |
| 34 | Vardulaki et al.[45] | 1999 | UK | RCT | ≥50 | M | n.a. | 11291 |
| 35 | Vardulaki et al.[46] | 1999 | UK | RCT | ≥65 | M and W | 5000 | 2215 (44.3) |
| 36 | Pleumeekers et al.[47] | 1999 | Netherlands | CSS | ≥55 | M | 2217 | 1771 (79.9) |
| 37 | Kang et al.[48] | 1999 | USA | CSS | Mean = 67 | M and W | n.a. | 2477 |
| 38 | Davies et al.[49] | 1999 | UK | CSS | ≥50 | M and W | n.a. | 2281 |
| 39 | Jaussi et al.[50] | 1999 | Lausanne | CSS | Mean = 59 | M and W | n.a. | 301 |
| 40 | Lindholt et al.[51] | 1998 | Denmark | RCT | 65–73 | M | 4404 | 3342 (75.9) |
| 41 | Boll et al.[52] | 1998 | Netherlands | CSS | 60–80 | M | 2914 | 2419 (83.0) |
| 42 | Vazquez et al.[53] | 1998 | Belgium | CSS | 65 and 75 | M | 1773 | 727 (41) |
| 43 | Lederle et al.[54] | 1997 | USA | CSS | 50–79 | M and W | n.a. | 73451 |
| 44 | Spittell et al.[55] | 1997 | USA | CSS | ≥50 | M and W | n.a. | 200 |
| 45 | Ogren et al.[56] | 1996 | Sweden | CSS | Mean = 74 | M | 423 | 343 (81.1) |
| 46 | Hope et al.[57] | 1995 | USA | CSS | 65–90 | M and W | n.a. | 4741 |
| 47 | Simoni et al.[58] | 1995 | Italy | CSS | 65–75 | M and W | 2734 | 1601 (58.5) |
| 48 | Pleumeekers et al.[59] | 1995 | Netherlands | CSS | ≥55 | M and W | 10215 | 5419 (53) |
| 49 | Eisenberg et al.[60] | 1995 | USA | CSS | 13–94 | M and W | n.a. | 323 |
| 50 | Holdsworth et al.[61] | 1994 | UK | CSS | 65–79 | M | 800 | 628 (78.5) |
| 51 | MacSweeney et al.[62] | 1993 | UK | CSS | n.a. | M and W | n.a. | 561 |
| 52 | Smith et al.[63] | 1993 | UK | CSS | 65–75 | M | 3500 | 2669 (76) |
| 53 | Lucarotti et al.[64] | 1993 | UK | CSS | 65 | M | 5337 | 4232 (79) |
| 54 | Nicholls et al.[65] | 1992 | Australia | CSS | 60–80 | M and W | n.a. | 1225 |
| 55 | Krohn et al.[66] | 1992 | Norway | CSS | >60 | M | n.a. | 500 |
| 56 | Bengtsson et al. [67] | 1991 | Sweden | CSS | Mean = 74 | M | 499 | 364 (72.9) |
Abbreviation: AAA, asymptomatic abdominal aneurysm; CSS, Cross-sectional study; RCT, Randomized controlled trial; PCS, Prospective cohort study; W, Women; M, Men; 95% CI, 95% confidence interval; n.a., not applicable; n.r., no data or no data in appropriate format reported.
Figure 2. A forest plot of prevalence from individual studies and combined prevalence from random-effects models.
Subgroup analysis
The prevalence of AAA ranged from 1.0% to 14.2% in men and from 0.2% to 6.4% in women. The pooled prevalence of AAA was 4.8% (4.3%, 5.3%). Pooled prevalence of all subgroups, according to geographical areas, gender, age and aneurysm diameter are presented in Table 2. The pooled prevalence of America, Europe, Australia and Asia were found to be 2.2% (2.2%, 2.2%), 2.5%(2.4%, 2.5%), 6.7% (6.5%, 7.0%) and 0.5%(0.3%, 0.7%), respectively. Male and female subgroups were 6.0% (5.3%, 6.7%) and 1.6% (1.2%, 1.9%), respectively. Prevalence in 55–64 years, 65–74 years, 75–84 years and ≥85 years were1.3% (1.2%, 1.5%), 2.8% (2.7%, 2.9%), 1.2% (1.1%, 1.3%) and 0.6% (0.4%, 0.7%), respectively. Pooled prevalence of aneurysm diameters in 30–39mm, 40–49 mm and ≥50 mm were 3.3% (2.8%, 3.9%), 0.7% (0.4%, 1.0%) and 0.4% (0.3%, 0.5%), respectively. Results showed that the pooled prevalence in Australia was higher than America and Europe. The pooled prevalence in western countries was all higher than the Asia. The prevalence of AAA in the male population was higher than in females. In addition, the prevalence in 65–74 years was the highest of the four age categories. The prevalence of aneurysms with diameters between 30 and 39 mm was higher than those with aortic diameters of more than 40 mm.
Table 2. Prevalence of abdominal aortic aneurysm in older people by different stratified factors.
| Stratified factors | No. of Studies | Prevalence rate | Lower limit | Upper limit | Heterogeneity I 2 (%) | P from test of heterogeneity | Model |
| Total | 56 | 0.048 | 0.043 | 0.053 | 99.4 | 0.000 | REM |
| Area | |||||||
| America | 12 | 0.043 | 0.033 | 0.053 | 99.8 | 0.000 | REM |
| Europe | 37 | 0.051 | 0.044 | 0.059 | 98.9 | 0.000 | REM |
| 1988–1992 | 3 | 0.065 | 0.048 | 0.081 | 31.8 | 0.231 | REM |
| 1993–1995 | 6 | 0.065 | 0.036 | 0.094 | 98.3 | 0.000 | REM |
| 1996–1998 | 4 | 0.042 | 0.035 | 0.049 | 94.1 | 0.000 | REM |
| 1999–2001 | 9 | 0.053 | 0.034 | 0.073 | 99.1 | 0.000 | REM |
| 2002–2004 | 3 | 0.045 | 0.026 | 0.063 | 96.0 | 0.000 | REM |
| 2005–2007 | 2 | 0.047 | 0.032 | 0.062 | 66.4 | 0.085 | REM |
| 2008–2010 | 5 | 0.046 | 0.037 | 0.055 | 95.5 | 0.000 | REM |
| 2011–2013 | 5 | 0.028 | 0.014 | 0.043 | 99.3 | 0.000 | REM |
| Australia | 4 | 0.067 | 0.065 | 0.070 | 96.5 | 0.000 | REM |
| Asia | 3 | 0.005 | 0.003 | 0.007 | 94.6 | 0.000 | REM |
| Gender | |||||||
| Male | 49 | 0.060 | 0.053 | 0.067 | 99.3 | 0.000 | REM |
| Female | 23 | 0.016 | 0.012 | 0.019 | 95.8 | 0.000 | REM |
| Age (y) | |||||||
| 55–64 | 3 | 0.013 | 0.012 | 0.015 | 89.5 | 0.000 | REM |
| 65–74 | 9 | 0.028 | 0.027 | 0.029 | 97.7 | 0.000 | REM |
| 75–84 | 7 | 0.012 | 0.011 | 0.013 | 99.0 | 0.000 | REM |
| ≥85 | 2 | 0.006 | 0.004 | 0.007 | 83.2 | 0.000 | REM |
| Aneurysm diameters (mm) | |||||||
| 30–39 | 11 | 0.033 | 0.028 | 0.039 | 98.3 | 0.000 | REM |
| 40–49 | 5 | 0.007 | 0.004 | 0.010 | 97.3 | 0.000 | REM |
| ≥50 | 9 | 0.004 | 0.003 | 0.005 | 90.3 | 0.000 | REM |
Abbreviation: AAA, asymptomatic abdominal aneurysm; No., number; REM, random effects model.
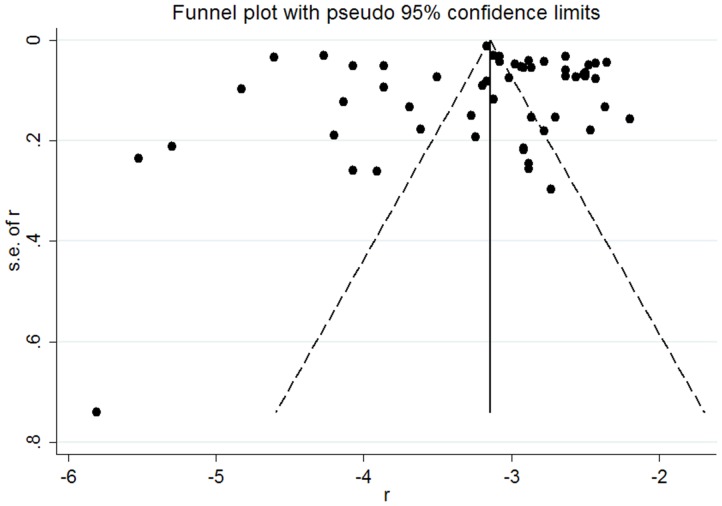
Analysis of heterogeneity and publication bias
We noted significant heterogeneity within studies and subgroups (P = 0.000, I2 = (83.2–99.8)). The visual examination of the funnel plots (Figure 3) and Egger's test did not reveal evidence of publication bias (P = 0.863).
Figure 3. Funnel plot assessing publication bias in the prevalence of AAA from 56 published studies.
Trends
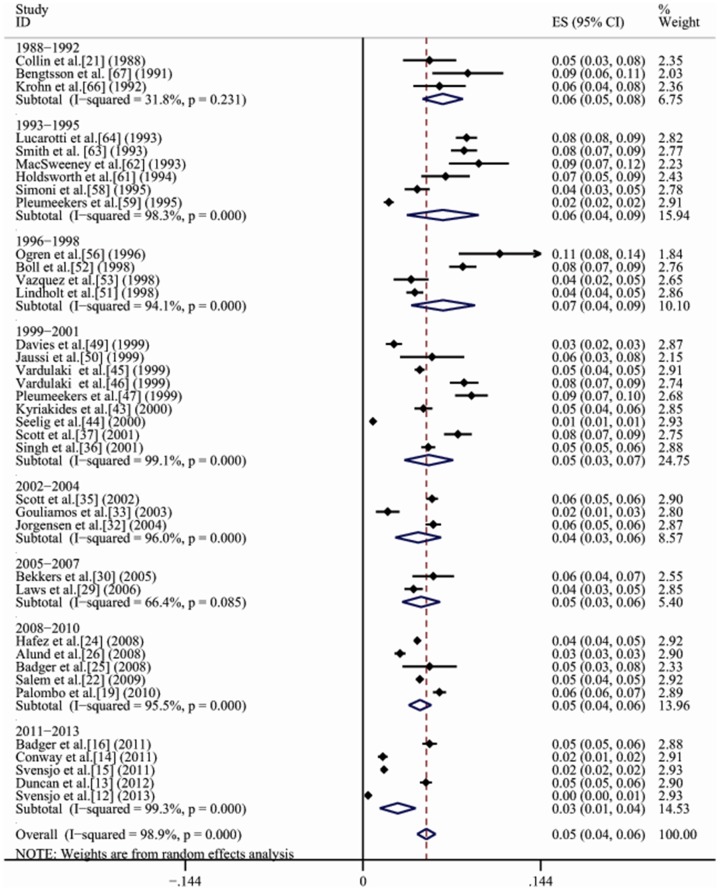
Studies evaluating secular trends in the prevalence of AAA were available only for Europe due to paper quantitative restrictions. Time trend analyses based on years of fieldwork showed that the prevalence of AAA for general population in Europe gradually decreased from 6.5% (95% CI, 4.8%–8.1%) in 1988–1992 to 2.8% (95% CI, 1.4%–4.3%) in 2011–2013 (Figure 4).
Figure 4. A forest plot of prevalence of AAA in Europe from 1988 to 2013.
Risk factors
Only 12 studies (nine among Europe, one among America, one among Australia and one among Asia) reported on risk factors for AAA. Table 3 gives combined odds ratios calculated by random or fixed-effects models and probabilities from tests of heterogeneity. Hypertension, smoking, coronary artery disease, dyslipidemia, respiratory disease, cerebrovascular disease, claudication and renal insufficiency were risk factors for AAA in Europe; Smoking and coronary artery disease were risk factors for AAA in America; Smoking, diabetes mellitus, coronary artery disease, dyslipidemia and respiratory disease were risk factors for AAA in Australia; Hypertension and smoking were risk factors for AAA in Asia.
Table 3. Combined odds ratios for the presence of abdominal aortic aneurysm from meta-analysis.
| Risk factor (yes vs. no) | Number of studies (bibliography number) | Combined odds ratio (95% CI) | p from test of heterogeneity |
| Hypertension | |||
| Total | 12 (12,15,17,19,20,32,38,53,58,59,63,67) | 1.26 (1.15, 1.39) | 0.000 |
| Europe | 9 (12,15,19,32,53,58,59,63,67) | 1.31 (1.17, 1.47) | 0.000 |
| America | 1 (38) | 1.08 (0.89, 1.33) | - |
| Australia | 1 (17) | 1.27 (0.93, 1.74) | - |
| Asia | 1 (20) | 2.35 (1.02, 5.43) | - |
| Smoking (previous or current) | |||
| Total | 11 (12,15,17,19,20,32,38,53,58,59,63) | 2.07 (1.87, 2.28) | 0.000 |
| Europe | 8 (12,15,19,32,53,58,59,63) | 1.93 (1.72, 2.16) | 0.000 |
| America | 1 (38) | 1.79 (1.27, 2.28) | - |
| Australia | 1 (17) | 4.03 (2.75, 5.90) | - |
| Asia | 1 (20) | 3.29 (1.43, 7.61) | - |
| Diabetes mellitus | |||
| Total | 10 (12,15,17,19,20,32,38,53,58,59,63) | 1.04 (0.90, 1.19) | 0.007 |
| Europe | 7 (12,15,19,32,53,58,59,63) | 0.97 (0.81, 1.17) | 0.073 |
| America | 1 (38) | 0.87 (0.63, 1.21) | - |
| Australia | 1 (17) | 1.65 (1.21, 2.23) | - |
| Asia | 1 (20) | 0.54 (0.18, 1.61) | - |
| Coronary artery disease | |||
| Total | 10 (12,15,17,19,32,38,58,59,63,67) | 1.82 (1.65, 2.00) | 0.000 |
| Europe | 8 (12,15,19,32,58,59,63,67) | 1.55 (1.37, 1.76) | 0.000 |
| America | 1 (38) | 1.91 (1.55, 2.35) | - |
| Australia | 1 (17) | 3.15 (2.44, 4.07) | - |
| Dyslipidemia | |||
| Total | 7 (12,15,17,19,20,53,58) | 1.36(1.19, 1.54) | 0.000 |
| Europe | 7 (12,15,19,53,58) | 1.31(1.13, 1.50) | 0.000 |
| Australia | 1(17) | 1.78(1.30, 2.43) | - |
| Asia | 1(20) | 0.25(0.06, 1.07) | - |
| Respiratory disease | |||
| Total | 6 (12,15,17,19,58,63) | 1.36 (1.19, 1.55) | 0.000 |
| Europe | 5(12,15,19,58,63) | 1.35 (1.16, 1.58) | 0.000 |
| Australia | 1(17) | 1.36 (1.04, 1.77) | - |
| Cerebrovascular disease | |||
| Total | 5 (12,15,32,58,59) | 1.28 (0.93, 1.77) | 0.070 |
| Europe | 5 (12,15,32,58,59) | 1.28 (0.93, 1.77) | 0.070 |
| Claudication | |||
| Total | 3 (12,15,59) | 3.00 (1.74, 5.19) | 0.330 |
| Europe | 3 (12,15,59) | 3.00 (1.74, 5.19) | 0.330 |
| Renal insufficiency | |||
| Total | 3 (12,15,19) | 1.20(0.95, 1.51) | 0.110 |
| Europe | 3 (12,15,19) | 1.20(0.95, 1.51) | 0.110 |
Discussion
The aim of this meta-analysis was to estimate prevalence rates of AAA in general population. To our knowledge, this is the first meta-analysis examining the prevalence of AAA in general population. 56 epidemiological studies were selected. Our analysis suggested that approximately 4.8% of the general population has AAA (6.0% for males and 1.6% for females). Results show that the pooled prevalence in Australia is higher than America and Europe. The pooled prevalence in western countries is higher than the Asia. The present meta-analysis indicated that the prevalence of AAA has decreased in Europe from 1988 to 2013. The prevalence of AAA in the male population is higher than in females. In addition, the prevalence in 65–74 years is the highest of the four age categories. The prevalence of aneurysms with diameters between 30 and 39 mm is higher than those with aortic diameters of more than 40 mm. The population prevalence of AAA varied widely which is not surprising considering the differences between studies in terms of their definition of AAA, area difference, age and gender distribution of study populations.
Necropsy reports provided the first information on AAA epidemiology. From Malmö, Sweden, a prevalence of 4.7% in men and 1.2% in women who were 65 to 74 years was reported [68] (10,413 necropsies with a 70% necropsy ate). Estimates of the prevalence of AAA can also be obtained from screening surveys. The reported prevalence of screening-detected AAA varies depending on the areas, gender, age, aortic diameters, and the criteria used to define an AAA.
Our study shows that prevalence of AAA differs in areas. The prevalence in Australia is higher than America and Europe, and the prevalence in western countries is higher than the Asia. Presently, the world is embracing various large epidemiological studies which assess the current situation of AAA. A screening study done in the USA found 31 AAA in 2005 residents who aged over 65 years.[23] Another UK study screened 4216 residents and found only 69 patients with AAA (1.6%).[14] In the Australia, with the population of about 3620, 262 AAA was found in the community (7.2%). [17] However, studies from Japan and Korea reported a relatively low prevalence of AAA in Asians. [20], [42] Accurate data on AAA in the Asian population are very limited. A Hong Kong study screened total population and found only 0.14% patients with AAA. [69] However, there are few epidemiological studies in mainland China. In mainland China, research on causes of AAA has just started. Researches about causes focus on basic studies including animal and vitro tests. Population-based epidemiological studies are small in scale and sample size, however. Most researches are hospital-based single center and small studies. It needs continuousconclusion and perfectibility.
Prevalence of AAA showed a greater gender gap. Our meta-analysis confirmed this result. The pooled prevalence of AAA in males is higher than in females, 6.0% and 1.6%, respectively. AAA primarily affects men, who have a 5-fold greater prevalence of AAA compared with women in studies using ultrasound screening. [36], [57], [59] The prevalence of AAA has been reported as 1.3–8.9% in men and 1.0–2.2% in women in Western countries. [1], [70], [71] But it's important to note that the mortality rate associated with ruptured AAA among women is increasing, and that the rate of rupture is higher in women than in men. [72], [73] Female patients with AAA were only one 5th that of male patients, but one patient with AAA in three ruptured was female. [58]
Postmortem studies have suggested that 95% of deaths from ruptured AAA occur at or above the age of 65 years. It has, therefore, been recommended to focus screening at age 65 years to maximize the potential number of life years gained. In Bekkers et al. group [30]AAA started to occur at age 55 years with two patients already having significantly dilated abdominal aortas before the age of 60 years, with diameters of 55 and 68 mm, respectively. AAA was not found before the age of 50 years in both sexes, but the prevalence increased with age for both men and women. A one-time screening of men aged 60 to 65 years has also been shown to be cost-effective. [74], [75] After the age of 70 years AAA increased significantly only in men.
The majority of abdominal aorta diameters were between 30 and 40 mm. The rate of growth of abdominal aneurysms is relatively unpredictable with wide interindividual variability but seems to be increased in larger aneurysms. The mean expansion rate of AAA has been estimated to vary between 0.28 and 0.38 cm/year. [76], [77] All diameters were under 4 cm with low risk of rupture. [8] A second screening in patients with aortic diameters less than 30 mm has been shown to be of little practical value and is, therefore, not recommended. However, the following recommendations for subsequent surveillance have been made: patients with AAA between 3 and 4 cm should have an ultrasound after 1 year, between 4 and 4.5 cm after 6 months, and greater then 4.5 cm should be referred to a vascular surgeon.
Prevalence of AAA has been increasing for the past two decades, which possibly correlates to increased average life span and development of diagnostic tools and screening programs.[78] AAA screening in general population reported the prevalence of AAA from 1% to 7% of the general Western population [30], [31], [54] and 5% of men over 65 years of age. [79] A ruptured AAA can be fatal; therefore, a screening program is recommended for populations at increased risk. Currently, the U.S. Preventive Services Task Force recommends an ultrasound for AAA screening in men aged 65–75 years who have ever smoked. [80] This program has achieved reduced mortality in men aged 65–74 years. [79]
The population prevalence of AAA varied from region to region which is not surprising considering the differences between studies in terms of their definition of AAA, the age and sex distribution of study populations and the prevalence of risk factors. The results showed that hypertension, smoking, coronary artery disease, dyslipidemia, respiratory disease, cerebrovascular disease, claudication and renal insufficiency were risk factors for AAA in Europe; Smoking and coronary artery disease were risk factors for AAA in America; Smoking, diabetes mellitus, coronary artery disease, dyslipidemia and respiratory disease were risk factors for AAA in Australia; Hypertension and smoking were risk factors for AAA in Asia. Aortic aneurysms are a complex genetic disorder with environmental risk factors. The exact pathogenesis of abdominal aortic aneurysm has not been completely unraveled. It is clear, however, that it involves a series of known and unknown environmental factors acting over time. It is not known exactly which genetic risk factors make a person prone to aortic wall dilatation. Familial aggregation of AAA suggests that there are candidate genes that contribute to the development of AAA. The magnitude of the increased risk in first degree relatives suggests a genetic component, although the influence of a common lifestyle cannot be excluded. A recent molecular genetic study in an Irish population found no significant gene–disease associations.
A few limitations of this meta-analysis must be considered. First, the literature search was limited to articles published in English or Chinese. Nonetheless, no evidence of publication bias was found. Second, some characteristics of the subjects, such as ethnicity, which might exert an important influence on the prevalence of AAA, were not included in the meta-analysis. Finally, there is no general agreement on how to define an AAA. Moher et al. [81] demonstrated how various definitions strongly influence the reported prevalence of AAA, a finding confirmed by means of this study. Steinberg et al. [82] established normal standards for abdominal aortic diameters. They concluded that a diameter in excess of 30 mm was well above the average for both sexes and was considered to be the dividing line between ectasia and aneurysms. [83] This was the basis for the most accepted definition, described by McGregor et al. [84] in 1975, which defined an AAA as a maximum intracranial aortic diameter of 30 mm or more. Because it is widely used, there are several studies with which to compare when this definition is chosen, and there is no need to define the individuals according to age, sex, and body surface area (BSA) to calculate the normal aortic diameter.
Rupture of an AAA is fatal, and mortality is more than 50% before arrival at a hospital. Even if a patient survives the trip to the operating room, operation-related mortality has been described up to 70%. [85], [86] A routine screening for AAA during clinical transthoracic echocardiography (TTE) provides a low yield due to a low prevalence (0.5%) of AAA in general population. However, the detection of life-threatening but asymptomatic AAA may save lives. Therefore, a routine examination of the abdominal aorta during TTE, which involves little additional time, would appear to be an effective and efficient prevention strategy, especially in men over 60 years of age. When the cost is covered by governments, priorities have to be decided on the basis of the total budget and the need for screening of other diseases. On an individual basis, however, we must state that each person has the right to know what kind of disease may possibly affect him, and to decide whether to be screened or not, at his own expense.
Conclusion
AAA is common in general population. The prevalence of AAA is higher in Australia than America and Europe. The pooled prevalence in western countries is higher than the Asia. A higher prevalence of AAA is also found in 65–74 years and among males. The prevalence of aneurysms with diameters between 30 and 39 mm is higher than those with aortic diameters of more than 40 mm.
Supporting Information
PRISMA Checklist. Doi:10.1371/journal.pone.0081260.s001
(DOC)
Acknowledgments
The authors thank all the participants in this study.
Funding Statement
The authors have no support or funding to report.
References
- 1. Sakalihasan N, Limet R, Defawe OD (2005) Abdominal aortic aneurysm. Lancet 365: 1577–1589. [DOI] [PubMed] [Google Scholar]
- 2. Gillum RF (1995) Epidemiology of aortic aneurysm in the United States. J Clin Epidemiol 48: 1289–1298. [DOI] [PubMed] [Google Scholar]
- 3. Castleden WM, Mercer JC (1985) Abdominal aortic aneurysms in Western Australia: descriptive epidemiology and patterns of rupture. Br J Surg 72: 109–112. [DOI] [PubMed] [Google Scholar]
- 4. Melton LJ 3rd, Bickerstaff LK, Hollier LH, Van Peenen HJ, Lie JT, et al. (1984) Changing incidence of abdominal aortic aneurysms: a population-based study. Am J Epidemiol 120: 379–386. [DOI] [PubMed] [Google Scholar]
- 5. Wilmink AB, Quick CR (1998) Epidemiology and potential for prevention of abdominal aortic aneurysm. Br J Surg 85: 155–162. [DOI] [PubMed] [Google Scholar]
- 6. van der Vliet JA, Boll AP (1997) Abdominal aortic aneurysm. Lancet 349: 863–866. [DOI] [PubMed] [Google Scholar]
- 7. Scott RA, Wilson NM, Ashton HA, Kay DN (1995) Influence of screening on the incidence of ruptured abdominal aortic aneurysm: 5-year results of a randomized controlled study. Br J Surg 82: 1066–1070. [DOI] [PubMed] [Google Scholar]
- 8. Scott RA, Vardulaki KA, Walker NM, Day NE, Duffy SW, et al. (2001) The long-term benefits of a single scan for abdominal aortic aneurysm (AAA) at age 65. Eur J Vasc Endovasc Surg 21: 535–540. [DOI] [PubMed] [Google Scholar]
- 9. Ashton HA, Buxton MJ, Day NE, Kim LG, Marteau TM, et al. (2002) The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet 360: 1531–1539. [DOI] [PubMed] [Google Scholar]
- 10. Sterne JA, Egger M (2001) Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol 54: 1046–1055. [DOI] [PubMed] [Google Scholar]
- 11. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Svensjo S, Bjorck M, Wanhainen A (2013) Current prevalence of abdominal aortic aneurysm in 70-year-old women. Br J Surg 100: 367–372. [DOI] [PubMed] [Google Scholar]
- 13. Duncan JL, Harrild KA, Iversen L, Lee AJ, Godden DJ (2012) Long term outcomes in men screened for abdominal aortic aneurysm: prospective cohort study. BMJ 344: e2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Conway AM, Malkawi AH, Hinchliffe RJ, Holt PJ, Murray S, et al. (2012) First-year results of a national abdominal aortic aneurysm screening programme in a single centre. Br J Surg 99: 73–77. [DOI] [PubMed] [Google Scholar]
- 15. Svensjo S, Bjorck M, Gurtelschmid M, Djavani Gidlund K, Hellberg A, et al. (2011) Low prevalence of abdominal aortic aneurysm among 65-year-old Swedish men indicates a change in the epidemiology of the disease. Circulation 124: 1118–1123. [DOI] [PubMed] [Google Scholar]
- 16. Badger SA, Jones C, Murray A, Lau LL, Young IS (2011) Implications of attendance patterns in Northern Ireland for abdominal aortic aneurysm screening. Eur J Vasc Endovasc Surg 42: 434–439. [DOI] [PubMed] [Google Scholar]
- 17. Yeap BB, Hyde Z, Norman PE, Chubb SA, Golledge J (2010) Associations of total testosterone, sex hormone-binding globulin, calculated free testosterone, and luteinizing hormone with prevalence of abdominal aortic aneurysm in older men. J Clin Endocrinol Metab 95: 1123–1130. [DOI] [PubMed] [Google Scholar]
- 18.van Walraven C, Wong J, Morant K, Jennings A, Jetty P, et al. (2010) Incidence, follow-up, and outcomes of incidental abdominal aortic aneurysms. J Vasc Surg 52: : 282–289 e281–282. [DOI] [PubMed] [Google Scholar]
- 19. Palombo D, Lucertini G, Pane B, Mazzei R, Spinella G, et al. (2010) District-based abdominal aortic aneurysm screening in population aged 65 years and older. J Cardiovasc Surg (Torino) 51: 777–782. [PubMed] [Google Scholar]
- 20. Oh SH, Chang SA, Jang SY, Park SJ, Choi JO, et al. (2010) Routine screening for abdominal aortic aneurysm during clinical transthoracic echocardiography in a Korean population. Echocardiography 27: 1182–1187. [DOI] [PubMed] [Google Scholar]
- 21. Collin J, Araujo L, Walton J, Lindsell D (1988) Oxford screening programme for abdominal aortic aneurysm in men aged 65 to 74 years. Lancet 2: 613–615. [DOI] [PubMed] [Google Scholar]
- 22. Salem MK, Rayt HS, Hussey G, Rafelt S, Nelson CP, et al. (2009) Should Asian men be included in abdominal aortic aneurysm screening programmes? Eur J Vasc Endovasc Surg 38: 748–749. [DOI] [PubMed] [Google Scholar]
- 23. Schermerhorn M, Zwolak R, Velazquez O, Makaroun M, Fairman R, et al. (2008) Ultrasound screening for abdominal aortic aneurysm in medicare beneficiaries. Ann Vasc Surg 22: 16–24. [DOI] [PubMed] [Google Scholar]
- 24. Hafez H, Druce PS, Ashton HA (2008) Abdominal aortic aneurysm development in men following a “normal” aortic ultrasound scan. Eur J Vasc Endovasc Surg 36: 553–558. [DOI] [PubMed] [Google Scholar]
- 25. Badger SA, O'Donnell ME, Sharif MA, Boyd CS, Soong CV (2008) Advantages and pitfalls of abdominal aortic aneurysm screening in high-risk patients. Vascular 16: 201–206. [DOI] [PubMed] [Google Scholar]
- 26. Alund M, Mani K, Wanhainen A (2008) Selective screening for abdominal aortic aneurysm among patients referred to the vascular laboratory. Eur J Vasc Endovasc Surg 35: 669–674. [DOI] [PubMed] [Google Scholar]
- 27. Roshanali F, Mandegar MH, Yousefnia MA, Mohammadi A, Baharvand B (2007) Abdominal aorta screening during transthoracic echocardiography. Echocardiography 24: 685–688. [DOI] [PubMed] [Google Scholar]
- 28. Derubertis BG, Trocciola SM, Ryer EJ, Pieracci FM, McKinsey JF, et al. (2007) Abdominal aortic aneurysm in women: prevalence, risk factors, and implications for screening. J Vasc Surg 46: 630–635. [DOI] [PubMed] [Google Scholar]
- 29. Laws C, Eastman J (2006) Screening for abdominal aortic aneurysm by general practitioners and practice-based ultrasonographers. J Med Screen 13: 160–161. [DOI] [PubMed] [Google Scholar]
- 30. Bekkers SC, Habets JH, Cheriex EC, Palmans A, Pinto Y, et al. (2005) Abdominal aortic aneurysm screening during transthoracic echocardiography in an unselected population. J Am Soc Echocardiogr 18: 389–393. [DOI] [PubMed] [Google Scholar]
- 31. Norman PE, Jamrozik K, Lawrence-Brown MM, Le MT, Spencer CA, et al. (2004) Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. BMJ 329: 1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jorgensen L, Singh K, Berntsen GK, Jacobsen BK (2004) A population-based study of the prevalence of abdominal aortic aneurysms in relation to bone mineral density: the Tromso study. Am J Epidemiol 159: 945–949. [DOI] [PubMed] [Google Scholar]
- 33. Gouliamos AD, Tsiganis T, Dimakakos P, Vlahos LJ (2004) Screening for abdominal aortic aneurysms during routine lumbar CT scan: modification of the standard technique. Clin Imaging 28: 353–355. [DOI] [PubMed] [Google Scholar]
- 34. Bonamigo TP, Siqueira I (2003) Screening for abdominal aortic aneurysms. Rev Hosp Clin Fac Med Sao Paulo 58: 63–68. [DOI] [PubMed] [Google Scholar]
- 35. Scott RA, Bridgewater SG, Ashton HA (2002) Randomized clinical trial of screening for abdominal aortic aneurysm in women. Br J Surg 89: 283–285. [DOI] [PubMed] [Google Scholar]
- 36. Singh K, Bonaa KH, Jacobsen BK, Bjork L, Solberg S (2001) Prevalence of and risk factors for abdominal aortic aneurysms in a population-based study: The Tromso Study. Am J Epidemiol 154: 236–244. [DOI] [PubMed] [Google Scholar]
- 37. Scott RAP, Vardulaki KA, Walker NM, Day NE, Duffy SW, et al. (2001) The Long-term Benefits of a Single Scan for Abdominal Aortic Aneurysm (AAA) at Age 65. Eur J Vasc Endovasc Surg 21: 535–540. [DOI] [PubMed] [Google Scholar]
- 38. Newman AB, Arnold AM, Burke GL, O'Leary DH, Manolio TA (2001) Cardiovascular disease and mortality in older adults with small abdominal aortic aneurysms detected by ultrasonography: the cardiovascular health study. Ann Intern Med 134: 182–190. [DOI] [PubMed] [Google Scholar]
- 39. Lederle FA, Johnson GR, Wilson SE, Aneurysm D, Management Veterans Affairs Cooperative S (2001) Abdominal aortic aneurysm in women. J Vasc Surg 34: 122–126. [DOI] [PubMed] [Google Scholar]
- 40. Lawrence PF, Gazak C, Bhirangi L, Jones B, Bhirangi K, et al. (1999) The epidemiology of surgically repaired aneurysms in the United States. J Vasc Surg 30: 632–640. [DOI] [PubMed] [Google Scholar]
- 41. Lederle FA, Johnson GR, Wilson SE, Chute EP, Hye RJ, et al. (2000) The aneurysm detection and management study screening program: validation cohort and final results. Aneurysm Detection and Management Veterans Affairs Cooperative Study Investigators. Arch Intern Med 160: 1425–1430. [DOI] [PubMed] [Google Scholar]
- 42. Adachi K, Iwasawa T, Ono T (2000) Screening for abdominal aortic aneurysms during a basic medical checkup in residents of a Japanese rural community. Surg Today 30: 594–599. [DOI] [PubMed] [Google Scholar]
- 43. Kyriakides C, Byrne J, Green S, Hulton NR (2000) Screening of abdominal aortic aneurysm: a pragmatic approach. Ann R Coll Surg Engl 82: 59–63. [PMC free article] [PubMed] [Google Scholar]
- 44. Seelig MH, Malouf YL, Klingler PJ, Oldenburg WA, Atkinson EJ (2000) Clinical utility of routine screening for abdominal aortic aneurysm during echocardiography. Vasa 29: 265–268. [DOI] [PubMed] [Google Scholar]
- 45. Vardulaki KA, Prevost TC, Walker NM, Day NE, Wilmink AB, et al. (1999) Incidence among men of asymptomatic abdominal aortic aneurysms: estimates from 500 screen detected cases. J Med Screen 6: 50–54. [DOI] [PubMed] [Google Scholar]
- 46. Vardulaki KA, Prevost TC, Walker NM, Day NE, Wilmink AB, et al. (1998) Growth rates and risk of rupture of abdominal aortic aneurysms. Br J Surg 85: 1674–1680. [DOI] [PubMed] [Google Scholar]
- 47. Pleumeekers HJ, De Gruijl A, Hofman A, Van Beek AJ, Hoes AW (1999) Prevalence of aortic aneurysm in men with a history of inguinal hernia repair. Br J Surg 86: 1155–1158. [DOI] [PubMed] [Google Scholar]
- 48.Kang SS, Littooy FN, Gupta SR, Johnson GR, Fisher SG, et al. (1999) Higher prevalence of abdominal aortic aneurysms in patients with carotid stenosis but without diabetes. Surgery 126: : 687–691; discussion 691–682. [PubMed] [Google Scholar]
- 49. Davies AJ, Winter RK, Lewis MH (1999) Prevalence of abdominal aortic aneurysms in urology patients referred for ultrasound. Ann R Coll Surg Engl 81: 235–238. [PMC free article] [PubMed] [Google Scholar]
- 50. Jaussi A, Fontana P, Mueller XM (1999) Imaging of the abdominal aorta during examination of patients referred for transthoracic echocardiography. Schweiz Med Wochenschr 129: 71–76. [PubMed] [Google Scholar]
- 51. Lindholt JS, Juul S, Henneberg EW, Fasting H (1998) Is screening for abdominal aortic aneurysm acceptable to the population? Selection and recruitment to hospital-based mass screening for abdominal aortic aneurysm. J Public Health Med 20: 211–217. [DOI] [PubMed] [Google Scholar]
- 52. Boll AP, Verbeek AL, van de Lisdonk EH, van der Vliet JA (1998) High prevalence of abdominal aortic aneurysm in a primary care screening programme. Br J Surg 85: 1090–1094. [DOI] [PubMed] [Google Scholar]
- 53. Vazquez C, Sakalihasan N, D'Harcour JB, Limet R (1998) Routine ultrasound screening for abdominal aortic aneurysm among 65- and 75-year-old men in a city of 200,000 inhabitants. Ann Vasc Surg 12: 544–549. [DOI] [PubMed] [Google Scholar]
- 54. Lederle FA, Johnson GR, Wilson SE, Chute EP, Littooy FN, et al. (1997) Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Ann Intern Med 126: 441–449. [DOI] [PubMed] [Google Scholar]
- 55. Spittell PC, Ehrsam JE, Anderson L, Seward JB (1997) Screening for abdominal aortic aneurysm during transthoracic echocardiography in a hypertensive patient population. J Am Soc Echocardiogr 10: 722–727. [DOI] [PubMed] [Google Scholar]
- 56. Ogren M, Bengtsson H, Bergqvist D, Ekberg O, Hedblad B, et al. (1996) Prognosis in elderly men with screening-detected abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 11: 42–47. [DOI] [PubMed] [Google Scholar]
- 57. Alcorn HG, Wolfson SK Jr, Sutton-Tyrrell K, Kuller LH, O'Leary D (1996) Risk factors for abdominal aortic aneurysms in older adults enrolled in The Cardiovascular Health Study. Arterioscler Thromb Vasc Biol 16: 963–970. [DOI] [PubMed] [Google Scholar]
- 58. Simoni G, Pastorino C, Perrone R, Ardia A, Gianrossi R, et al. (1995) Screening for abdominal aortic aneurysms and associated risk factors in a general population. Eur J Vasc Endovasc Surg 10: 207–210. [DOI] [PubMed] [Google Scholar]
- 59. Pleumeekers HJ, Hoes AW, van der Does E, van Urk H, Hofman A, et al. (1995) Aneurysms of the abdominal aorta in older adults. The Rotterdam Study. Am J Epidemiol 142: 1291–1299. [DOI] [PubMed] [Google Scholar]
- 60. Eisenberg MJ, Geraci SJ, Schiller NB (1995) Screening for abdominal aortic aneurysms during transthoracic echocardiography. Am Heart J 130: 109–115. [DOI] [PubMed] [Google Scholar]
- 61. Holdsworth JD (1994) Screening for abdominal aortic aneurysm in Northumberland. Br J Surg 81: 710–712. [DOI] [PubMed] [Google Scholar]
- 62. MacSweeney ST, O'Meara M, Alexander C, O'Malley MK, Powell JT, et al. (1993) High prevalence of unsuspected abdominal aortic aneurysm in patients with confirmed symptomatic peripheral or cerebral arterial disease. Br J Surg 80: 582–584. [DOI] [PubMed] [Google Scholar]
- 63. Smith FC, Grimshaw GM, Paterson IS, Shearman CP, Hamer JD (1993) Ultrasonographic screening for abdominal aortic aneurysm in an urban community. Br J Surg 80: 1406–1409. [DOI] [PubMed] [Google Scholar]
- 64. Lucarotti M, Shaw E, Poskitt K, Heather B (1993) The Gloucestershire Aneurysm Screening Programme: the first 2 years' experience. Eur J Vasc Surg 7: 397–401. [DOI] [PubMed] [Google Scholar]
- 65. Nicholls EA, Norman PE, Lawrence-Brown MM, Goodman MA, Pedersen B (1992) Screening for abdominal aortic aneurysms in Western Australia. Aust N Z J Surg 62: 858–861. [DOI] [PubMed] [Google Scholar]
- 66. Krohn CD, Kullmann G, Kvernebo K, Rosen L, Kroese A (1992) Ultrasonographic screening for abdominal aortic aneurysm. Eur J Surg 158: 527–530. [PubMed] [Google Scholar]
- 67. Bengtsson H, Bergqvist D, Ekberg O, Janzon L (1991) A population based screening of abdominal aortic aneurysms (AAA). Eur J Vasc Surg 5: 53–57. [DOI] [PubMed] [Google Scholar]
- 68. Bengtsson H, Bergqvist D, Sternby NH (1992) Increasing prevalence of abdominal aortic aneurysms. A necropsy study. Eur J Surg 158: 19–23. [PubMed] [Google Scholar]
- 69. Cheng SW, Ting AC, Tsang SH (2003) Epidemiology and outcome of aortic aneurysms in Hong Kong. World J Surg 27: 241–245. [DOI] [PubMed] [Google Scholar]
- 70. Cabellon S Jr, Moncrief CL, Pierre DR, Cavanaugh DG (1983) Incidence of abdominal aortic aneurysms in patients with atheromatous arterial disease. Am J Surg 146: 575–576. [DOI] [PubMed] [Google Scholar]
- 71. Carty GA, Nachtigal T, Magyar R, Herzler G, Bays R (1993) Abdominal duplex ultrasound screening for occult aortic aneurysm during carotid arterial evaluation. J Vasc Surg 17: 696–702. [PubMed] [Google Scholar]
- 72.Brown LC, Powell JT (1999) Risk factors for aneurysm rupture in patients kept under ultrasound surveillance. UK Small Aneurysm Trial Participants. Ann Surg 230: : 289–296; discussion 296–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lindholt JS, Vammen S, Juul S, Fasting H, Henneberg EW (2000) Optimal interval screening and surveillance of abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 20: 369–373. [DOI] [PubMed] [Google Scholar]
- 74. Boll AP, Severens JL, Verbeek AL, van der Vliet JA (2003) Mass screening on abdominal aortic aneurysm in men aged 60 to 65 years in The Netherlands. Impact on life expectancy and cost-effectiveness using a Markov model. Eur J Vasc Endovasc Surg 26: 74–80. [DOI] [PubMed] [Google Scholar]
- 75. Multicentre Aneurysm Screening Study G (2002) Multicentre aneurysm screening study (MASS): cost effectiveness analysis of screening for abdominal aortic aneurysms based on four year results from randomised controlled trial. BMJ 325: 1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gadowski GR, Pilcher DB, Ricci MA (1994) Abdominal aortic aneurysm expansion rate: effect of size and beta-adrenergic blockade. J Vasc Surg 19: 727–731. [DOI] [PubMed] [Google Scholar]
- 77. Yeung JM, Heeley M, Gray S, Lingam MK, Manning G, et al. (2002) Does the angiotensin-converting enzyme (ACE) gene polymorphism affect rate of abdominal aortic aneurysm expansion? Eur J Vasc Endovasc Surg 24: 69–71. [DOI] [PubMed] [Google Scholar]
- 78. Acosta S, Ogren M, Bengtsson H, Bergqvist D, Lindblad B, et al. (2006) Increasing incidence of ruptured abdominal aortic aneurysm: a population-based study. J Vasc Surg 44: 237–243. [DOI] [PubMed] [Google Scholar]
- 79. Kim LG, RA PS, Ashton HA, Thompson SG (2007) Multicentre Aneurysm Screening Study G (2007) A sustained mortality benefit from screening for abdominal aortic aneurysm. Ann Intern Med 146: 699–706. [DOI] [PubMed] [Google Scholar]
- 80. Force USPST (2005) Screening for abdominal aortic aneurysm: recommendation statement. Ann Intern Med 142: 198–202. [DOI] [PubMed] [Google Scholar]
- 81. Moher D, Cole CW, Hill GB (1994) Definition and management of abdominal aortic aneurysms: results from a Canadian survey. Can J Surg 37: 29–32. [PubMed] [Google Scholar]
- 82. Steinberg CR, Archer M, Steinberg I (1965) Measurement of the abdominal aorta after intravenous aortography in health and arteriosclerotic peripheral vascular disease. Am J Roentgenol Radium Ther Nucl Med 95: 703–708. [DOI] [PubMed] [Google Scholar]
- 83. Steinberg I, Stein HL (1966) Arteriosclerotic abdominal aneurysms. Report of 200 consecutive cases diagnosed by intravenous aortography. JAMA 195: 1025–1029. [DOI] [PubMed] [Google Scholar]
- 84. McGregor JC, Pollock JG, Anton HC (1975) The value of ultrasonography in the diagnosis of abdominal aortic aneurysm. Scott Med J 20: 133–137. [DOI] [PubMed] [Google Scholar]
- 85. Kniemeyer HW, Kessler T, Reber PU, Ris HB, Hakki H, et al. (2000) Treatment of ruptured abdominal aortic aneurysm, a permanent challenge or a waste of resources? Prediction of outcome using a multi-organ-dysfunction score. Eur J Vasc Endovasc Surg 19: 190–196. [DOI] [PubMed] [Google Scholar]
- 86. Johnson G Jr, McDevitt NB, Proctor HJ, Mandel SR, Peacock JB (1980) Emergent or elective operation for symptomatic abdominal aortic aneurysm. Arch Surg 115: 51–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Checklist. Doi:10.1371/journal.pone.0081260.s001
(DOC)