Abstract
Capillary forces arising during the evaporation of liquids from dense carbon nanotube arrays are used to reassemble the nanotubes into two-dimensional contiguous cellular foams. The stable nanotube foams can be elastically deformed, transferred to other substrates, or floated out to produce free-standing macroscopic fabrics. The lightweight cellular foams made of condensed nanotubes could have applications as shock-absorbent structural reinforcements and elastic membranes. The ability to control the length scale, orientation, and shape of the cellular structures and the simplicity of the assembly process make this a particularly attractive system for studying pattern formation in ordered media.
Cellular patterns arise frequently in nature on length scales ranging from microscopic to macroscopic as a result of spatially periodic and random perturbations (1–5); examples range from the morphogenesis of embryos to patterns in coffee stains. A film of aligned carbon nanotubes represents a unique, yet unstudied type of system in which pattern formation could arise from the collapse and reassembly of highly ordered, anisotropic, elastic, nanoscale rods with remarkable properties. We report the creation of intriguing two-dimensional cellular foams by the evaporation of liquids from such nanotube films (6, 7). Shrinkage and crack formation in the films caused by strong capillary forces during evaporation and strong van der Waals interactions between condensed nanotubes (8) result in the formation of visually striking, stable cellular patterns and contiguous foams. Patterns formed by nanotube aggregates differ significantly from other polygonal crack patterns (9–13) because of the inherent dimensions, strength, and flexibility of the nanotubes (14, 15). The length scale, orientation, and shape of the cellular structures can be controlled by varying the nanotube height and the rate of evaporation of liquid and by patterning the nanotube array. The nanotube foams also can be floated out to produce free-standing macroscopic films. The outstanding properties of the constituent nanotubes may lead to applications for these structures as shock-absorbent reinforcements and in nanofiltration devices.
Materials and Methods
Fabrication of Multiwalled Nanotube Arrays. Vertically aligned multiwalled nanotube (MWNT) arrays (Fig. 1a) were grown on rigid silica substrates by using a chemical vapor deposition process (7) based on the decomposition of ferrocene and xylene. Patterned MWNT arrays were fabricated by patterning silica (SiO2) on Si(100) (6) and exposing these patterned substrates to a mixture of ferrocene and xylene at 800°C. Nanotubes grow selectively on the patterned silica regions (6).
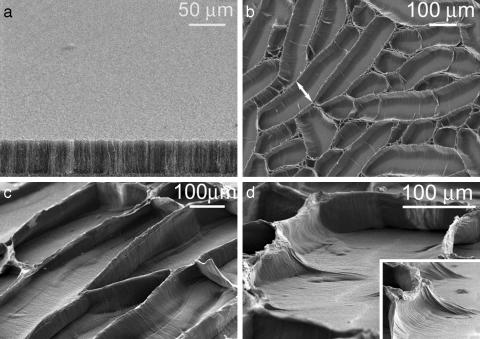
Fig. 1.
Formation of cellular patterns by the evaporation of liquids from vertically aligned carbon nanotube films. (a) Substrate-grown, vertically aligned MWNT arrays with an average nanotube diameter of 30 nm. (b) Scanning electron microscopy image showing a side view of cellular structures formed by the evaporation of water from plasma-oxidized, vertically aligned MWNTs. The arrow depicts the width of a cell. (c and d) Higher-magnification micrographs showing the cellular structures. The micrograph in d illustrates a “crack” along the floor of a nanotube “canyon” as well as the bending of nanotubes that gives rise to the open cellular structures. “Bent” nanotubes occupy the floor of the canyon in regions surrounding the crack. (d Inset) Wall of a cell. It can be seen that the alignment of nanotubes has been preserved.
Formation of Cellular Carbon Nanotube Foams. The aligned nanotube arrays were oxidized in an oxygen plasma created in a glow discharge chamber (Harrick Scientific, Ossining, NY) at room temperature and 0.6 torr (1 torr = 133 Pa) pressure for ≈10 min. Characterization of the oxidized MWNTs by Raman spectroscopy confirmed the preservation of their structural integrity. The oxidized MWNTs are wetted by a number of liquids including water. To generate the cellular structures, the arrays were immersed into a liquid, which then was allowed to evaporate at room temperature.
Results and Discussion
Formation of Cellular Carbon Nanotube Foams. The evaporation of liquids from the interstices of the ordered nanotubes in the plasma-oxidized film produces remarkable cellular structures. Fig. 1 b–d shows the foam-like structure produced by the reassembly of nanotubes in the films during the drying process. Similar structures were formed with a variety of liquids including acetone, toluene, dimethylformamide, tetrahydrofuran, and methanol. The cellular structures were stable once formed despite the significant deformation of the constituent elastic nanotubes; the patterns were not affected by annealing the sample in vacuum at 800°C for 1 h or by submersion in water followed by another round of evaporation. The nanotube foams, once assembled, are quite elastic. The foams can also be floated out of the substrate, can be transferred to any other substrate, and are mechanically stable. To probe the role of the solvent in pattern formation further, we freeze-dried the wet nanotube films (16); no cellular structures were observed in the freezedried arrays, indicating that pattern formation requires evaporation of a liquid from the MWNT arrays.
Mechanism of Pattern Formation. We followed the real-time collapse and collective morphological evolution of the nanotubes into cellular structures by using an optical microscope, observing the macroscale features that develop and change in the films (schematic in Fig. 2 and optical micrographs in Fig. 3). These observations revealed that drying-induced microcrack formation (11, 17) and further in-plane shrinkage of the nanotube arrays accompanied by the bending of nanotubes results in the formation of the observed cellular structures (Figs. 2 and 3). Although mechanisms based on Marangoni convection and the nucleation and growth of holes have been proposed to explain the formation of cellular networks in dried nanoparticle suspensions (3, 18–20), the elongated polygonal structures seen in our samples (Figs. 1 and 3) are incompatible with these mechanisms, which would be expected to give rise to regular polygonal networks. In addition, the substrate-grown nanotubes cannot convect, as required for pattern formation by Marangoni convection.
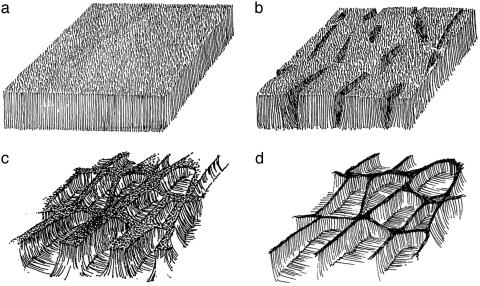
Fig. 2.
Schematic illustrating the formation of cellular structures in carbon nanotube films. (a) Film composed of vertically aligned carbon nanotubes. (b) The formation of cracks during the evaporation of liquid from the nanotube film. (c) A later stage in the process; the shrinkage of the array and the bending of nanotubes result in the formation of open cellular structures. (d) Schematic illustrating the final cellular structure.
Fig. 3.
The morphological evolution of cellular structures during the evaporation of water from a carbon nanotube film. (a) Optical micrograph during the early stages of structure formation, showing the appearance of cracks. (b and c) Optical micrographs of the same region at later stages of structure formation. The micrographs demonstrate that cell growth occurs via compaction and bending of the nanotubes. The cell dimensions are governed primarily by the initial crack length and spacing.
The open cellular structures formed by MWNT arrays are significantly different from other polygonal crack patterns, such as the shrinkage crack patterns in mud, clay, and cement, and columnar joints formed in cooling basaltic lava (1, 2, 9, 12, 13, 17). Unlike systems in which cracks tend to propagate until stopped by other preexisting cracks (9), crack propagation in the MWNT arrays is usually arrested before cracks meet (Fig. 3a). Consequently, these structures consist of a polygonal network of compacted MWNT rims instead of a polygonal network of cracks (20). These cellular structures do not evolve by means of cell division or coalescence (3). Although image analysis reveals that the average crack length is greater than the average cell width, the growth of an individual crack takes places on a time scale of seconds, whereas the subsequent shrinkage and bending of nanotubes (Fig. 3 b and c) is much slower and takes place on a time scale of minutes.
The formation of open cellular structures by the collapse (bending) and condensation of nanotubes (Figs. 1, 2, 3) is a result of the remarkable mechanical and structural properties of the MWNTs: their alignment, narrow spacing, high aspect ratios, flexibility, and mechanical strength. Although the evaporative flux during the drying of the nanotube film tends to reduce the height of liquid in the film (21), the bending of the nanotubes ensures that the liquid–vapor interface remains at the surface of the film. The narrow spacing between the MWNTs (on the order of tens of nanometers) (6) results in large, surface-tension-induced differences between the pressures of air and solvent. Assuming that MWNTs have an average spacing of 50 nm (22), we estimate the initial pressure difference to be 6 MPa (23). The pressure difference is inversely proportional to the spacing between the nanotubes and increases in magnitude during the consolidation of the nanotube film. The pressure difference exerts a bending moment on the nanotubes (23).
The high aspect ratio of the MWNT enhances the magnitude of the pressure-induced deflection (23, 24). The maximum deflection (δ) of a beam caused by a uniformly distributed load can be expressed as δ = WL3/8EI, where W (equal to PDL) is the total force, L is the length, D is the diameter, E is the bending modulus, P is the pressure difference, and I (equal to πD4/64) is a cross-sectional second moment (24). Estimates of the value of the bending modulus for MWNTs, 30 nm in diameter, range from 0.1 to 1.28 TPa∥ (25, 26). Assuming a value of 1 TPa for the bending modulus of an MWNT, which is similar to the value of the modulus along the basal plane of highly oriented pyrolytic graphite, we estimate that a pressure difference of only 1.1 Pa is required to cause a maximum deflection of 10 μm for an MWNT that is 30 nm in diameter and 100 μm long. Assuming that the value of the bending modulus remains relatively constant, a pressure difference of only 5.5 kPa would be sufficient to cause a maximum deflection of ≈50 μm for each nanotube in a linear array consisting of 1,000 MWNTs. Capillary forces are therefore large enough to cause the bending of the MWNTs seen in Fig. 1. These large deformations are possible because of the extraordinary flexibility of the MWNTs and their resistance to fracture (14).
Although the deformation of nanotubes and the resulting formation of open cells can be attributed to the magnitude of the capillary forces, Fig. 1b also suggests that there is a length scale associated with these cellular structures. Real space image analysis reveals that the distribution of cell widths in the cellular structures is narrower than the distribution of crack lengths. The average cell width varies linearly with the length of the nanotubes in the array (i.e., with the height of the nanotube film) (Fig. 5, which is published as supporting information on the PNAS web site). This linearity can be explained by theoretical studies of crack pattern formation using spring-block models (17, 27). The adhesion between the nanotube film and the substrate hinders the contraction of the film at its base. Inhomogeneous shrinkage results in the accumulation of stress in the film. The length scale associated with the pattern (and hence the average cell width in the MWNT cells) would be expected to vary linearly with the film height if crack formation occurs because of a critical stress criterion (17, 27). Fig. 3 indicates that the cell width is governed, to a large extent, by the initial pattern of cracks; the length scale associated with the initial crack pattern (Fig. 3a) and the slow and uniform widening of the cracks (Fig. 3 b–c) result in the formation of cells having uniform widths. We also tested the effect of the rate of evaporation of the solvent (water) on pattern formation by allowing the structures to form under different ambient humidity conditions at room temperature. A lower value of the relative humidity (i.e., a faster rate of evaporation) favors crack formation and results in a decrease in the average cell width (11); the average cell width increases linearly with increasing relative humidity in the range of humidity values tested (relative humidity between 50% and 80%).
Controlling Structure Formation by Using Patterned MWNT Arrays. Although the results described above illustrate our ability to control the pattern length scale by varying experimental parameters, the use of patterned MWNT arrays provides striking control over the orientation and shape of the structures (Fig. 4). Fig. 4a shows structures formed by the evaporation of liquids from spatially periodic cylindrical MWNT arrays. The cylindrical MWNT arrays have compacted near their base to a third of their original diameter, forming “wine-glass” structures. This degree of compaction is consistent with MWNTs, initially separated by ≈50 nm, coming in close contact. This consolidation and the strong van der Waals interactions between condensed nanotubes (8) explain the stability of the cellular structures despite the extensive deformation of the constituent nanotubes. Fig. 4 b–e provides another example of our ability to control structure formation. Fig. 4 b–e shows cellular structures formed in patterned MWNT arrays located in different regions on the same substrate and therefore having the same film height. Although wider MWNT arrays showed cells oriented in multiple directions, unidirectional cells were seen below a certain width (Fig. 4b). More complex behavior was observed after further decreasing the width of the patterned arrays (Fig. 4 c–e). A transition region, consisting of cells oriented both perpendicular and parallel to the length of the pattern, were seen for arrays having an initial width of 200 μm (Fig. 4c). For arrays with smaller widths (Fig. 4d), cells were unidirectional but oriented parallel to the length of the pattern, whereas cell formation was not observed in arrays having widths <100 μm (Fig. 4e). Fig. 4e reveals a significant compaction of the array caused by capillary forces. Inward bending of the nanotubes at the edges of the pattern is also seen in the wider MWNT arrays (Fig. 4 b–d). We propose that this inward bending suppresses crack formation perpendicular to the length of the pattern (Fig. 4d) and parallel to the length of the pattern for the narrower patterns (Fig. 4e).
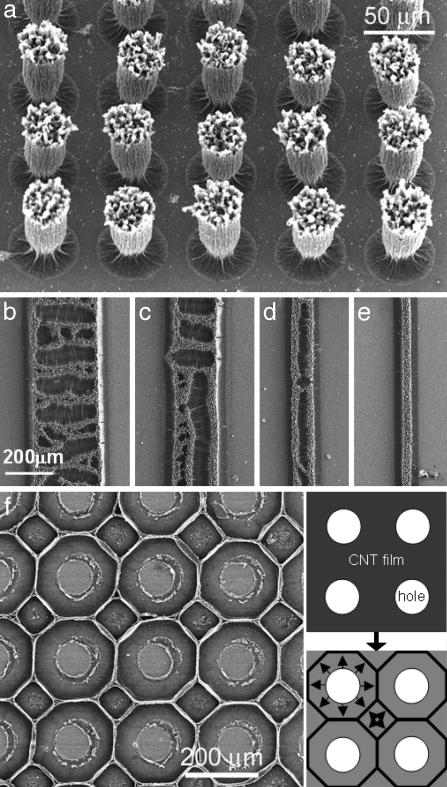
Fig. 4.
Control of structure shape and orientation using patterned carbon nanotube films. (a) Wine-glass structures obtained by the evaporation of liquids from cylindrical MWNT arrays. (b–e) The formation of directional cells in patterned nanotube films. The sample (consisting of aligned MWNTs having a length of 60 μm) was plasma-oxidized, immersed in acetone, and dried under ambient conditions. The micrographs show cellular structures formed in different regions on the same substrate. The array width influenced the direction of crack growth. The micrographs also reveal the inward bending of the nanotubes caused by capillary forces. (f) The formation of a two-dimensional carbon nanotube foam by the drying of a patterned MWNT array. The structure can be separated from the substrate forming a monolithic carbon nanotube foam. (f Inset) Schematic describing the formation of the foam from a patterned array consisting of spatially periodic circular holes 100μm in diameter.
Our ability to control structure formation in the MWNT arrays (Figs. 1 and 4) and the remarkable properties of the constituent nanotubes might engender several practical applications of these materials. The cellular structures are deformable and elastic; light-weight carbon nanotube foams may be attractive for impact energy absorption and acoustic damping applications (28). Fig. 4f illustrates another approach for controllably generating a carbon nanotube foam, using a patterned MWNT array containing circular holes. The consolidation of the rims during the drying of the patterned MWNT array results in the formation of a two-dimensional foam (Fig. 4f) consisting of mainly square and octagonal cells. A monolithic, free-standing carbon nanotube foam is readily obtained by separating the structure from the substrate by immersion into aqueous hydrofluoric acid. Straightforward extensions of this approach will allow the fabrication of macroscopic two-dimensional foams having cells of a variety of shapes and lateral extent of macroscale dimensions. The capillarity-induced nanotube consolidation and the resulting strong intertube interaction (8) will be critical for fabricating lightweight carbon nanotube foams having exceptional mechanical properties. The foams with predefined porosities and wall thickness could be used as elastic membranes and fabrics. These structures also provide an interesting example of the spontaneous generation of complex patterns from highly ordered media (4, 10, 12, 29). The ability to control the length scale, orientation, and shape of the structures and the simplicity of the structure-formation process makes this a particularly attractive system for studying pattern formation.
Supplementary Material
Acknowledgments
We thank Shripad Gokhale for assistance with the use of the microscope in the Wayner Laboratory and Yung Joon Jung for providing the patterned silica substrates. We acknowledge funding from the Rensselaer Polytechnic Institute National Science Foundation Nanoscale Science and Engineering Center for the directed assembly of nanostructures and Philip Morris USA.
Abbreviation: MWNT, multiwalled nanotube.
Footnotes
The wall thickness of the MWNTs may influence the value of the bending modulus; the wall thickness is ≈10 nm for the nanotubes used in this study.
References
- 1.Weaire, D. & Rivier, N. (1984) Contemp. Phys. 25, 59–99. [Google Scholar]
- 2.Korneta, W. (2001) Philos. Mag. Lett. 81, 367–373. [Google Scholar]
- 3.Moriarty, P., Taylor, M. D. R. & Brust, M. (2002) Phys. Rev. Lett. 89, 248303. [DOI] [PubMed] [Google Scholar]
- 4.Kessler, M. A. & Werner, B. T. (2003) Science 299, 380–383. [DOI] [PubMed] [Google Scholar]
- 5.Deegan, R. D. & Bakajin., O. (1997) Nature 389, 827–829. [Google Scholar]
- 6.Wei, B. Q., Vajtai, R., Jung, Y., Ward, J., Zhang, Y., Ramanath, G. & Ajayan, P. M. (2002) Nature 416, 495–496. [DOI] [PubMed] [Google Scholar]
- 7.Wei, B. Q., Vajtai, R., Jung, Y., Ward, J., Zhang, Y., Ramanath, G. & Ajayan, P. M. (2003) Chem. Mater. 15, 1598–1606. [Google Scholar]
- 8.Girifalco, L. A., Hodak, M. & Lee, R. S. (2000) Phys. Rev. B Solid State 62, 13104–13110. [Google Scholar]
- 9.Colina, H. & Roux, S. (2000) Eur. Phys. J. E 1, 189–194. [Google Scholar]
- 10.Leung, K. T., Jozsa, L., Ravasz, M. & Neda, Z. (2001) Nature 410, 166. [DOI] [PubMed] [Google Scholar]
- 11.Lecocq, N. & Vandewalle, N. (2002) Eur. Phys. J. E 8, 445–452. [DOI] [PubMed] [Google Scholar]
- 12.Skjeltorp, A. T. & Meakin, P. (1988) Nature 335, 424–426. [Google Scholar]
- 13.Yuse, A. & Sano, M. (1993) Nature 362, 329–331. [DOI] [PubMed] [Google Scholar]
- 14.Falvo, M. R. & Clary, G. J. (1997) Nature 389, 582–584. [DOI] [PubMed] [Google Scholar]
- 15.Ajayan, P. M. (1999) Chem. Rev. (Washington, D.C.) 99, 1787–1799. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka, T., Morigami, M., Oizumi, H. & Ogawa, T. (1993) Jpn. J. Appl. Phys. 32, 5813–5814. [Google Scholar]
- 17.Shorlin, K. A., de Bruyn, J. R., Graham, M. & Morris, S. W. (2000) Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 61, 6950–6957. [DOI] [PubMed] [Google Scholar]
- 18.Stowell, C. & Korgel, B. A. (2001) Nano Lett. 1, 595–600. [Google Scholar]
- 19.Gelbart, W. M., Sear, R. P., Heath, J. R. & Chaney, S. (1999) Faraday Discuss. 112, 299–307. [Google Scholar]
- 20.Thiele, U., Mertig, M. & Pompe, W. (1998) Phys. Rev. Lett. 80, 2869–2872. [Google Scholar]
- 21.Scherer, G. W. (1990) J. Am. Ceram. Soc. 73, 3–14. [Google Scholar]
- 22.Drotar, J. T., Wei, B. Q., Zhao, Y.-P., Ramanath, G., Ajayan, P. M., Lu, T.-M. & Wang, G.-C. (2001) Phys. Rev. B Condens. Matter 64, 125417. [Google Scholar]
- 23.Namatsu, H., Kurihara, K., Nagase, M., Iwadate, K. & Murase, K. (1995) Appl. Phys. Lett. 66, 2655–2657. [Google Scholar]
- 24.Jincao, Y., Matthews, M. A. & Darvin, C. H. (2001) Ind. Eng. Chem. Res. 40, 5858–5860. [Google Scholar]
- 25.Poncharal, P., Wang, Z. L., Ugarte, D. & de Heer, W. A. (1999) Science 283, 1513–1516. [DOI] [PubMed] [Google Scholar]
- 26.Wong, E. W., Sheehan, P. E. & Lieber, C. M. (1997) Science 277, 1971–1975. [Google Scholar]
- 27.Kitsunezaki, S. (1999) Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 60, 6449–6464. [DOI] [PubMed] [Google Scholar]
- 28.Gibson, L. J. (2003) MRS Bull. 4, 270–271. [Google Scholar]
- 29.Bowden, N., Brittain, S., Evans, A. G., Hutchinson J. W. & Whitesides, G. M. (1998) Nature 393, 146–149. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.