Abstract
Vascular access used in the treatment of patients involves central and peripheral vein accesses and arterial accesses. Catheterization of central veins is widely used in clinical practice; it is a necessary part of the treatment of patients in various settings. The most commonly involved vessels are the internal jugular, subclavian, and femoral veins. The mechanical, infectious, and thrombotic complications of central venous catheterization are markedly reduced when the procedure is performed with real-time ultrasound guidance or (to a slightly lesser extent) ultrasound assistance. Ultrasound guidance is also used to create peripheral venous accesses, for catheterization of peripheral veins and for peripheral insertion of central venous catheters. In this setting, it increases the catheterization success rate, especially during difficult procedures (e.g., obese patients, children) and reduces complications such as catheter-related infections and venous thrombosis. Arterial cannulation is used for invasive monitoring of arterial pressure and for access during diagnostic or therapeutic procedures. Ultrasound guidance reduces the risk of catheterization failure and complications. It is especially useful for arterial catheterization procedures performed in the absence of a palpable pulse (e.g., patient in shock, ECMO). Imaging support is being used increasingly to facilitate the creation of vascular accesses under difficult conditions, in part because of the growing use of ultrasonography as a bedside procedure. In clinical settings where patients are becoming increasingly vulnerable as a result of advanced age and/or complex disease, the possibility to reduce the risks associated with these invasive procedures should motivate clinicians to acquire the technical skills needed for routine use of sonographic support during vascular access procedures.
Keywords: Vascular access, Ultrasound
Riassunto
Gli accessi vascolari utilizzati nella cura dei pazienti comprendono gli accessi venosi centrali e periferici e gli accessi arteriosi. L’incannulamento di accessi venosi centrali è manovra largamente diffusa nella pratica clinica e si rende necessaria per la cura dei pazienti in molteplici contesti. I vasi centrali che usualmente vengono incannulati sono la vena giugulare interna, la vena succlavia e la vena femorale. Nell’incannulamento venoso centrale sia l’eco-assistenza che l’eco-guida real time, pur con una leggera superiorità di quest’ultima, riducono drasticamente le complicanze meccaniche, infettive e trombotiche. La guida ecografica viene utilizzata anche per l’accesso venoso periferico, per l’incannulamento di vasi periferici e di vasi centrali a inserzione periferica (PICC). In questo contesto la guida ecografica aumenta il successo della manovra di incannulamento soprattutto in condizioni di difficoltà, come avviene nei pazienti obesi o nei bambini, e diminuisce le complicanze quali le infezioni catetere correlate e le trombosi venose. L’incannulamento arterioso viene utilizzato per il monitoraggio cruento della pressione arteriosa e per garantire un accesso in caso di manovre diagnostiche e terapeutiche. La guida ecografica riduce il rischio di insuccesso, le complicanze e può essere utile per l’incannulamento arterioso soprattutto nei casi in cui non e’ reperibile un polso (shock, pazienti in ECMO). Il supporto dell’imaging per gli accessi vascolari difficili si sta quindi diffondendo rapidamente anche perchè è sempre più frequente l’impiego degli ultrasuoni nella pratica clinica al letto del malato. Nei contesti clinici attuali dove i pazienti sono sempre più fragili perchè anziani e con patologie più complesse, avere la possibilità di ridurre i rischi connessi alle metodiche invasive deve spingere i clinici ad acquisire le abilità tecniche per l’utilizzo routinario del supporto ultrasonografico per gli accessi vascolari difficili.
Introduction
Vascular access used in the treatment of patients involves central and peripheral vein accesses and arterial accesses. Central venous catheterization is widely used in clinical practice for diverse purposes, including invasive hemodynamic monitoring, radiological studies, infusion of drugs that cannot be administered via peripheral vessels, administration of parenteral nutrition, vascular access in patients whose peripheral veins are difficult to catheterize, and miscellaneous procedures that require access to large-caliber vessels [1].
The traditional technique relies on the use of anatomical landmarks rather than ultrasound guidance. Even in expert hands, it is associated with a high failure rate and a host of complications, ranging from mechanical problems (which occur in 5–19 % of cases) to infectious and thrombotic events (2–26 %) [1–5]. Pneumothorax, hemothorax, arterial puncture, hematoma, nerve lesions, damage to the left thoracic duct, and air embolism are among the main mechanical complications. Their incidence increases sixfold after the third insertion attempt [1]. Other factors that can increase the incidence of complications are operator inexperience, the presence of anatomical variants and/or coexisting conditions such as clotting disorders, pulmonary emphysema, or hypovolemia, and difficulties related to conditions under which the procedure is performed (i.e., an emergency).
The central vessels that are most frequently catheterized are the internal jugular, subclavian, and femoral veins. Each has specific clinical indications and each offers benefits and drawbacks with respect to the others. Catheterization of the subclavian vein is associated with a higher risk of mechanical complications; infectious complications are a greater risk with femoral vein access, and the likelihood of arterial puncture is highest with cannulation of the internal jugular vein [6].
The use of ultrasound imaging support for central venous catheterization was first described in 1978 by Ullman and Stoelting [7], who used Doppler imaging to identify the internal jugular vein. In 1986, Yonei et al. [8] first described the use of real-time, two-dimensional ultrasound for cannulating the same vein. Since then, a number of studies [9–14] have demonstrated that real-time ultrasound guidance and, to a slightly lesser extent, ultrasound assistance markedly reduce the mechanical, infectious, and thrombotic complications of the central venous catheterization [15–17]. Compared with the conventional blind insertion, ultrasound assistance can also reduce the rates of catheterization failure and incorrect catheter placement, increase the likelihood of success at the first pass, shorten procedure times, and reduce costs. These findings have been confirmed in recently published practice guidelines [18, 19].
Most of the published data today refer to ultrasound guidance for catheterization of the internal jugular vein, but recent studies show that this technique can also be useful for accessing to the subclavian vein [14]. Sonographic support also enables the operator to check the position of the catheter tip and identify any complications that may have occurred (e.g., pneumothorax) [20].
Ultrasound guidance is also used to obtain peripheral venous access, for catheterization of peripheral veins, and for peripheral insertion of central catheters (PICC) [21, 22]. In this context, ultrasound guidance increases the success of catheterization, especially under difficult conditions (e.g., obese patients, children), and decreases the frequency of complications such as catheter-related infections and venous thrombosis.
Arterial cannulation is used for invasive arterial pressure monitoring and to ensure access for diagnostic and therapeutic interventions. Sonographic guidance reduces the risk of insertion failure and complications. It can be especially useful for arterial catheterization when the pulse is difficult to detect (e.g., during shock or extracorporeal membrane oxygenation) [23].
Equipment and technique
With two-dimensional (2D) ultrasound, vessels, needle, and surrounding structures can all be clearly identified. For optimal visualization, the ultrasound beam should be perpendicular to the long axis of the vessel, and the frequency should be increased to obtain maximum resolution and adequate depth of imaging. A linear-array transducer is normally used because it provides good visualization of the needle as it advances through the tissues with relatively little image deformation. The most widely used frequency is 7.5 MHz, although frequencies between 5 and 10 MHz can be used. During insertion of a central venous catheter, maximum sterility must be maintained. Infectious complications related to the use of CVCs are serious and potentially life threatening [15]. Sterile transducer covers are available for this purpose: first, gel is placed on the face of the transducer and then, with the aid of a second operator, the cover is applied, like a stocking. To maintain asepsis while the catheter is being positioned, sterile coupling gel must be used.
The catheter can be positioned with ultrasound assistance or ultrasound guidance. In the former case, ultrasound is used to identify the target vessel, but the needle is inserted without imaging support. This technique is helpful, but it does not guarantee successful catheterization. The target vein is often small in caliber, and it can easily be compressed during needle-insertion maneuvers. In addition, the skin puncture site should be slightly behind rather than right above the point where the vessel was identified; in this way, the needle enters the vein at an angle more in line with intraluminal flow and ensures entry of the catheter. Ultrasound guidance entails real-time imaging of the entire process, from venipuncture to final placement of the needle in the vessel.
Commercially available mechanical needle guides are available, which can be attached to the transducer. They provide greater stability during the advancement of the needle and are potentially useful for less-experienced operators. They also have several drawbacks, which have limited their large-scale use during vascular access procedures. Aside from high cost, the direction of the needle is established before puncture and cannot be modified during the phase of advancement. Furthermore, the guides tend to be specific for each machine, and they often have to be used with specially designed needles [24].
In our opinion, the free-hand technique with real-time guidance is a more flexible solution that can be used even if there is only one operator. If there are two operators, one holds the transducer and positions it over the vein, while the second inserts the needle into the vessel. If there is only one person, he/she operates the transducer with the non-dominant hand and the needle with the dominant hand, penetrating the skin under real-time guidance and visually following the progression of the needle through the tissues as it approaches the target vessel, which remains beneath the ultrasound beam. The latter method is the one we prefer because one often has limited space in which to work, and three hands take up more room than two. Moreover, during the procedure, the orientation of the transducer will have to be “fine-tuned,” and the operator handling the needle is the only one capable of making these adjustments with sufficient precision.
The vessel can be visualized with a transverse (short-axis) or longitudinal (long-axis) scan (Figs. 1, 2, respectively), and the orientation of the needle can be assessed “in-plane” (with the entire needle trajectory included in the ultrasound beam) (Fig. 2) or “out of plane” (with the needle oriented in such a way that it can be visualized only in cross section) [25] (Fig. 1).
Fig. 1.
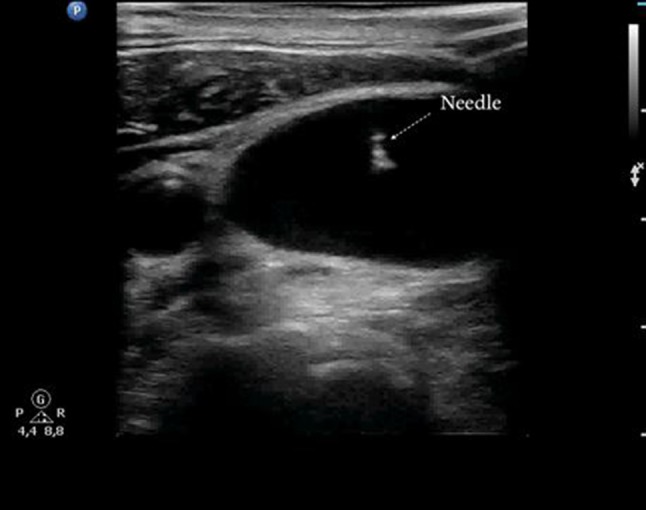
Short-axis view of the internal jugular vein. The tip of the needle is seen out of plane in the lumen of the vein
Fig. 2.
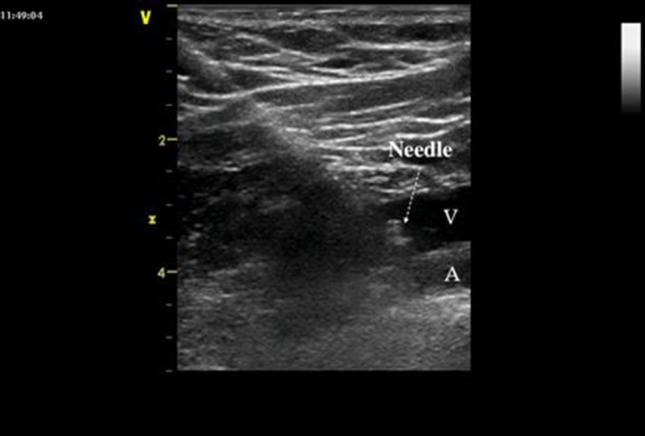
Long-axis view of the right subclavian vein. The full length of the needle can be seen with the tip lying within the vein. V subclavian vein, A subclavian artery
With the short-axis approach, the distance between the skin puncture site and the transducer should be equal to the distance between the skin surface and the target vessel. The needle should be held at an angle of 45° relative to the skin surface and out-of-plane relative to the transducer [26]. The operator should attempt to identify the needle, represented by a white or gray dot, although in some cases visualization is limited to the deformation of tissue and artifacts produced by the needle’s advancement. When the tip abuts the vein wall, additional pressure produces transient vessel deformation, which disappears once the wall has been penetrated. The presence of the needle in the vein should always be confirmed by aspiration of blood into the syringe.
With the long-axis approach, the skin is punctured at the base of the transducer. The needle is held at a 30° angle and oriented in-plane relative to the transducer [27]. The entire length of the needle is visible as it passes through the tissues, and vessel alignment should be maintained as the tip advances. Entry of the tip into the vessel should be confirmed by aspiration.
The short-axis approach enables the operator to identify surrounding structures (arteries, nerves, pleura), and it is easier to perform, especially for beginners [27] (Fig. 3). However, the fact that the needle tip cannot be visualized increases the risk of overshoot, i.e., progression of the tip beyond the ultrasound beam with perforation of the posterior wall of the vessel and potential puncture of adjacent structures (arteries, pleura, nerves, etc.) [28, 29]. With the long-axis approach, needle advancement can be visualized during the entire procedure, and guidewire insertion is also facilitated. Nonetheless, it has its drawbacks. The presence of bony prominences may make it difficult to position the transducer for a longitudinal scan of the internal jugular or subclavian vein. In addition, more extensive training is required to successfully align the long axis of the needle with that of the transducer and of the vessel.
Fig. 3.
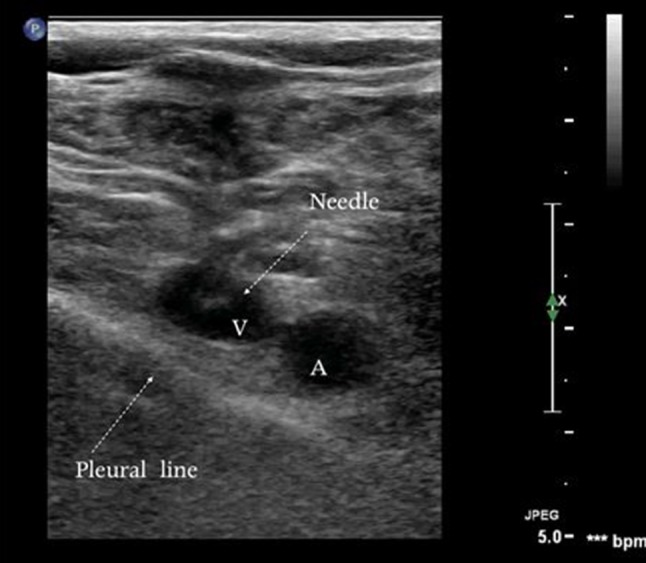
Short-axis view of the subclavian vein during catheterization. The pleural line is extremely close to the vein. V subclavian vein, A subclavian artery
When a catheter with a guidewire is being inserted with the two-hand technique, the operator inserts the needle tip into the vessel and then releases the transducer, using his/her non-dominant hand to stabilize the needle and advancing the guide wire with the dominant hand. With the short-axis approach, insertion of the guidewire is more complicated, especially when the angle between needle and vessel is too large and the tip hits the posterior wall. The angle should be reduced to 30° and mild tension exerted on the needle to facilitate the insertion of the guidewire. When the latter has been introduced, the transducer, which has remained sterile, can be used to verify its correct position within the vessel. The catheter can then be inserted as usual.
Approach to central venous catheterization
A pre-puncture ultrasound examination of the central vessels is useful for selecting the vein to catheterize. Anomalies may emerge in the course of the vein or its relation to the anatomic landmarks used for blind insertion. The size of a vessel may also differ considerably from that of the contralateral vessel [30]. In addition, in ICU patients or those who have in any case already had a central venous line, the scan frequently reveals venous thrombosis, which is often asymptomatic [31, 32]. Sonographic examination is the only way to avoid catheterizing a thrombosed central vein (Figs. 4, 5).
Fig. 4.
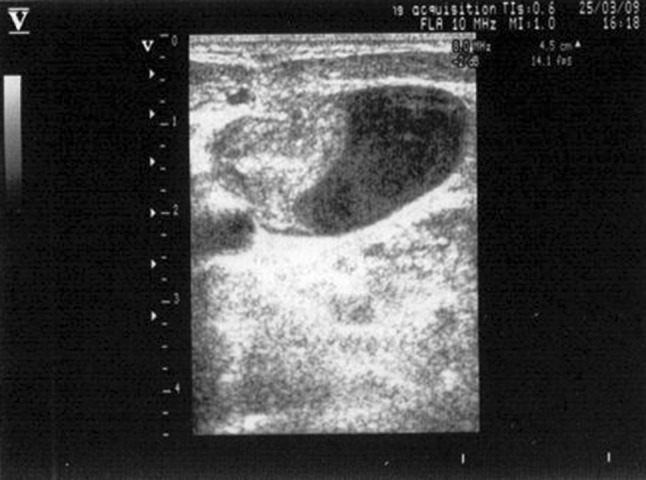
Endoluminal thrombus in an internal jugular vein
Fig. 5.
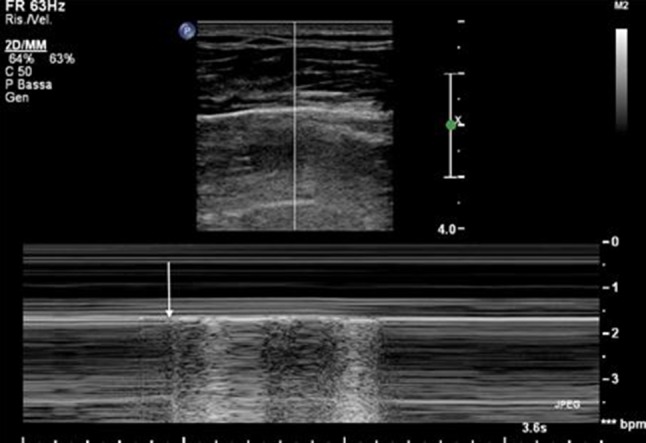
Lung point: the point of transition from PTX to normal lung observed during inspiration. In this M mode image, the arrow indicates the transition from the horizontal pattern of PTX to the granular pattern of a normally expanded lung. During expiration, the horizontal pattern is restored because the lung is once again separated from the pleura
Internal jugular vein
Traditional landmark-based cannulation of the internal jugular vein can be carried out with an anterior or a posterior approach [1]. In expert hands, the traditional technique is considered safe, but the reliability of anatomic landmarks is questionable because anatomic variations involving this vein and its relation to the internal carotid artery are encountered in a substantial number of cases [33, 34]. When landmark-based insertion fails, success rates drop 25 % with each repeat attempt, and the number of attempts is closely correlated with the likelihood of complications [9], the most common of which is arterial puncture [6]. A number of studies [9–13] have shown that real-time ultrasound guidance increases the rates of successful internal jugular catheterization by 100 % (particularly on the first pass). It also reduces the rates of mechanical complications (e.g., arterial puncture, hematoma, pneumothorax) as well as infectious and thrombotic events [15–17].
In numerous practice guidelines [15, 18, 19], routine use of real-time ultrasound guidance is recommended, on the basis of high-quality evidence, for catheterizing the internal jugular vein, and if real-time guidance is not possible, ultrasound assistance [18]. Sonography can be used before catheterization to characterize vessel anatomy and verify patency, during catheterization to avoid trauma to structures close to the vessel (e.g., pleura, internal carotid artery), and at the end of the procedure to identify any complications (e.g., hematoma, pneumothorax).
The guidelines published by the Centers for Disease Control in Atlanta, Georgia [15] strongly advise ultrasound guidance for central venous catheterization because fewer unsuccessful attempts mean less trauma to the vessel and consequently a lower risk of infectious and thrombotic complications.
Subclavian vein
A subclavian approach is generally used to cannulate this vein with the aid of anatomic landmarks. For many years, the use of ultrasound to guide subclavian vein catheterization was controversial. In a review of CVC complications published in 2003, the use of ultrasound in this setting was considered more difficult and less reliable than blind placement based on the use of landmarks alone [1]. The difficulty is related to the vein’s course beneath the clavicle, which tends to shield it from the ultrasound beam. This limitation can be overcome, however, using a more lateral approach, beyond the first rib, closer to the shoulder, where the axillary vein merges with the subclavian vein [26, 35]. Insertion at this point offers an additional advantage: the subclavian vein is farther away from the subclavian artery and the pleura, so there is less risk of arterial puncture and pneumothorax—the most dangerous mechanical complications of subclavian vein catheterization [1]. However, the axillary vein runs deeper than the subclavian vein, sometimes lying more than 5 cm beneath the skin surface (particularly in obese patients). This makes it harder to visualize, and it may also be difficult to reach with needles commonly used for CVC. When the axillary vein is going to be catheterized (especially the one at the left), the catheter tip has to traverse a longer distance to reach the right atrium, so a longer (20–25 cm) catheter must be used [36].
A recent randomized prospective study showed that ultrasound-guided subclavian catheterization was superior to landmark-based insertion, with a higher rate of success (100 vs. 87.5 %), lower rates of postprocedural pneumothorax (0 vs. 4.9 %), and fewer venipunctures [14].
Femoral vein
The femoral vessels are often cannulated in emergency settings because they are easier to locate and their catheterization is less likely to produce mechanical complications [37]. These vessels are also used for a number of interventional radiology procedures. The landmark used for catheterization of the femoral vein is the femoral artery pulse, which can be palpated 1–2 cm below the inguinal ligament. The femoral vein generally runs medial to the pulse. However, placement of a CVC in the femoral vein is associated with a high failure rate in part because of the vessel’s variable position and its alignment with the femoral artery. In the inguinal region, the vein usually lies medial to the artery, but ultrasound imaging has shown that in a substantial number of cases the artery runs directly above the vein [38]. Femoral vein catheterization with ultrasound guidance is easier than landmark-based cannulation [39, 40]. In a study by Hilty et al. [39], which was conducted on patients who were in cardiac arrest, the superior performance of ultrasound-guided insertion was attributed to its ability to allow visualization of the vein even in the absence of a palpable femoral arterial pulse. Use of ultrasound for catheterization of the femoral artery or vein reduces vascular complications because it provides better definition of the local anatomy and decreases the chances of accidental puncture of other vessels [41, 42].
Approach to peripheral vascular catheterization
The radial, brachial, anterior tibial, and posterior tibial arteries are the ones generally catheterized for hemodynamic monitoring in intensive care settings, but the femoral and axillary arteries are also used for this purpose. The peripheral veins that are catheterized are those that run through the dermis and are palpable. In obese patients, intravenous drug users, and patients who have been hospitalized for long periods of time, palpable peripheral veins may not be available. Ultrasound can be used to access veins lying deeper in the arm (e.g., basilic, cephalic) that cannot be identified by palpation [43].
Peripheral arteries
The most widely used is the radial artery: it is easy to access, and it is not a terminal artery, so its possible occlusion will not compromise blood flow to the hand, which is also vascularized by the ulnar artery [44]. The presence of effective collateral circulation should always be verified with the Allen test [45]. The traditional technique involves palpation of the pulse, if possible over a bone plane (i.e., over the head of the radius in the case of the radial artery).
A recent meta-analysis found that the use of ultrasound facilitates and accelerates catheterization of the radial artery [23]. Ultrasound is not generally indicated for arterial cannulation, but it can be very useful when blind catheterization fails or when the peripheral pulse is difficult to locate [18]. Because the arterial walls are not completely compressible, the vessel can still be visualized sonographically even in the presence of hypotension or nonpulsatile flow. In the presence of pulsatile flow, the addition of color Doppler imaging further facilitates its identification by demonstrating the typical arterial flow pattern. Ultrasound-assisted arterial catheterization can be performed with either an over-the-needle cannula or with a guide wire. Strict asepsis must be maintained with the aid of a sterile transducer sheath (when a guide wire is being inserted) or sterile glove (when an over-the-needle cannula is being used).
Peripheral veins
Ultrasound is not indicated for routine catheterization of peripheral veins, but when access is expected to be complicated, imaging guidance can increase the chances of successful cannulation, thereby eliminating the need to resort to the placement of a central venous line [19]. Observational studies [22, 23] have shown that ultrasound guidance is superior to the traditional, free-hand technique, but this finding was not confirmed in a randomized study conducted in a small sample of patients [46], so the debate remains open. International practice guidelines [21] strongly recommend the use of ultrasound during central venous catheterization and PICC, whereas for accessing peripheral vessels, its use should be considered when difficulties are encountered.
Ultrasound-guided peripheral venous cannulation can be performed with a common over-the-needle catheter consisting of a metal needle covered by a Teflon sheath, which enhances the echogenicity and therefore the sonographic visibility of the catheter. To maintain an adequate level of asepsis, the gel should be applied to the face of the transducer and covered with a sterile sheath. Common alcohol-based skin disinfectants can be applied to the skin to facilitate the movement of the transducer and improve imaging. For PICCs, it is important to use maximal sterile-barrier precautions, identical to those used for CVC insertion. The use of ultrasound is always advisable in these cases because it increases success rates and reduces the number of venipunctures. Some authors maintain that ultrasound-guided placement of a PICC is associated with lower infection rates than CVC insertion [21].
Diagnosing complications of central venous catheterization
Insertion of a CVC still carries the risk of improper placement and mechanical complications [47]. For this reason, a chest X-ray is advisable to verify the position of the catheter tip and exclude the possibility of iatrogenic pneumothorax (PTX) [48]. The presence of the catheter tip in the right atrium is reported in 8–47 % of cases; even in expert hands, placement error rates range from 6 to 14 % [49].
Intra-atrial placement of the CVC is not really necessary: central venous pressure can be read accurately at any point along the course of the superior vena cava (SVC) [50]. In addition, placement of the tip in the right atrium has been linked to perforation of the cardiac cavity. Precise quantification of this risk is difficult since there are only rare sporadic reports of this much-feared complication [51, 52]. In the American Society of Anesthesiologists database, which includes malpractice suits filed against anesthesiologists between 1970 and 2000, there are 110 events related to CVCs, including 16 cases of cardiac tamponade (13 of which were fatal) and 15 cases of hemothorax (14 fatal) [53]. Perforation of the vessel wall or the wall of the right atrium is associated with high mortality. Therefore, although the incidence of these events is low, measures must be taken to prevent their occurrence and to ensure prompt detection of perforations that do occur.
The use of anatomic landmarks is not a sufficiently reliable method for preventing misplacement of a CVC [54, 55]. In patients with sinus rhythm, the use of endocavitary EKG during catheter insertion has been shown to predict the correct placement of CVCs inserted via the right internal jugular or right subclavian vein. However, its performance was less promising with catheters introduced through the left internal jugular or left subclavian vein [56]. The main advantage of these checking systems is that they reduce the need to reposition the catheter after the post-insertion chest X-ray, which is still the reference method for verifying the correct placement [47]. However, there are no well-defined landmarks visible on chest X-rays that allow one to identify with certainty the boundary between the SVC and the right atrium.
Several radiological landmarks have been used to verify the extra-atrial position of the catheter tip [57], including the carina of the trachea [58]. Studies conducted on cadavers showed that the pericardial reflection rarely extends above the level of the carina. The segment of the SVC and the portion of the right atrium below the reflection are less resistant and more subject to perforation [59].
Even when the carina is used as the lower limit, however, problems can arise depending on where the catheter tip is located. For example, if the tip of a multilumen catheter is positioned in the area where the innominate (or brachiocephalic) vein drains into the SVC, the proximal most lumen may lie outside the SVC. In addition, this position is also associated with an increased risk of thrombosis if vasoactive drugs or hyperosmolar solutions are administered through the CVC. If the catheter is inserted at the left and the tip advanced into the upper portion of the SVC, the angle of incidence of the catheter may be too wide, and the tip can end up against the vessel wall. The risk of vessel wall perforation increases when the angle of incidence exceeds 40° [60].
According to the US Food and Drug Administration, the tip of the CVC should be placed in the SVC, parallel to the long axis of the vessel, at a point where it will not be able to slide into the right atrium [61]. Most catheter manufacturers include this recommendation on the instructions packaged with their products. In effect, placing the tip of the CVC in the area adjacent to the right atrium satisfies both of the above criteria.
Since the SVC is a relatively short vessel (circa 6 cm), precision placement is technically difficult. Indeed, it is probably impossible to achieve if one relies solely on the position relative to the carina, which is perceived differently on a portable chest X-ray taken at the patient’s bedside. Transesophageal echocardiography (TEE) is the most reliable method for verifying the position of the CVC tip: it allows almost full-length visualization of the SVC up to its junction with the right atrium [62]. TEE, however, is not available for routine use during CVC insertion, and it is difficult to use when the patient is unstable (e.g., secondary to severe respiratory distress) [63]. In contrast, transthoracic echocardiography (TTE) is widely available, and thanks to the introduction of increasingly portable scanners, it can be performed easily as a bedside procedure for rapid verification of catheter position. In some cases, however, the image quality may be low, and the presence of surgical wounds involving the abdomen can compromise visualization of the right atrium and vena cava.
The best window for visualizing the right atrium and the venae cavae is a subdiaphragmatic short-axis projection. Visualization will be limited to the segment of the vena cava that empties into the right atrium (atriocaval junction), i.e., exactly where the CVC tip needs to be. With a good-quality subdiaphragmatic projection, the catheter can often be visualized in the right atrium [64], but nonvisualization does not exclude its presence in the heart: it may simply be in a position that is invisible to the transducer. Identification of the tip can be facilitated by the administration of an acoustic contrast medium prepared by mixing the contents of two 10-ml syringes: one containing 9 ml isotonic saline, the other filled with 1 ml air [65]. TTE with the microbubble test identifies CVC misplacement with a sensitivity of 96 % and specificity of 93 %; findings are concordant with those of chest X-ray in 95 % of cases [66]. This makes it a valid alternative to chest X-ray for verifying the position of a CVC.
Ultrasound can also be used to detect the most feared mechanical complication of CVC insertion: postprocedural pneumothorax, which occurs with 0.1–0.2 % of CVCs inserted into the internal jugular and 1.5–3.1 % of subclavian lines [1]. Especially in ventilated patients, PTX can progress rapidly and produce a medical emergency.
Chest radiography is relatively insensitive in the identification of occult PTX because the air is initially distributed anterobasally, in the nondependent portions of the chest—the opposite of what occurs when the patient is in an upright position [67]. When a lordotic view is used to better explore the lung apices, the abdominal contents are especially likely to obscure the costomediastinal and costophrenic sinuses, where the air is often localized. It becomes visible only when the PTX increases in volume, extending upward toward the apex and laterally, where it causes separation of the pleural membranes [67].
With ultrasound, even small PTXs can be identified via systematic search for specific sonographic signs. The examination begins with a transverse scan along the parasternal line, from the third to the fifth intercostal spaces (deep sulcus area) and continues laterally toward the anterior axillary line. Ultrasound has been proved to be more sensitive and more specific than chest radiography in diagnosing occult PTX [68], which is manifested by the absence of “lung sliding” [characterized by high sensitivity (100 %) but low specificity (78 %)] and by identification of the “lung point,” which increases the specificity to 100 % while reducing sensitivity (79 %) [69]. Visualization of lung sliding in the deep sulcus area before insertion of the CVC facilitates the recognition of its absence after the procedure. For CVCs inserted at the left, disappearance of the parasternal cardiac window (identified prior to insertion) is an additional sign that should raise the suspicion of postprocedural PTX. Early identification is particularly important in ventilated patients because positive pressure can cause rapid expansion of the PTX. In addition to its value for detecting small PTXs, ultrasound can also be repeated when needed. It can thus be used to monitor the expansion rate and insert a chest tube before there are clinical signs of lung collapse. Postprocedural chest X-ray is still necessary in the subset of patients in whom ultrasound cannot be used due to the absence of a suitable acoustic window.
Conclusions
The use of imaging support for accessing difficult vessels is becoming increasingly widespread, in part because of the growing role of bedside ultrasonography in clinical practice. In the presence of patient vulnerability related to advanced age and/or complex disease, it is important to be able to minimize risks associated with invasive diagnostic/therapeutic procedures. To this end, clinicians should make every attempt to acquire the technical skills that will allow them to employ ultrasound support routinely during difficult vascular access procedures.
Conflict of interest
A. Vezzani, T. Manca, A. Vercelli, A. Braghieri, A. Magnacavallo declare that they have no conflict of interest.
Human and animal studies
The study described in this article did not include any procedures involving humans or animals.
References
- 1.McGee DC, Gould MK. Preventing complications of central venous catheterization. N Engl J Med. 2003;348:1123–1133. doi: 10.1056/NEJMra011883. [DOI] [PubMed] [Google Scholar]
- 2.Merrer J, De Jonghe B, Golliot F, et al. Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial. JAMA. 2001;286:700–707. doi: 10.1001/jama.286.6.700. [DOI] [PubMed] [Google Scholar]
- 3.Sznajder JI, Zveibil FR, Bitterman H, Weiner P, Bursztein S. Central vein catheterization: failure and complication rates by three percutaneous approaches. Arch Intern Med. 1986;146:259–261. doi: 10.1001/archinte.1986.00360140065007. [DOI] [PubMed] [Google Scholar]
- 4.Veenstra DL, Saint S, Saha S, Lumley T, Sullivan SD. Efficacy of antiseptic-impregnated central venous catheters in preventing catheter-related bloodstream infection: a meta-analysis. JAMA. 1999;281:261–267. doi: 10.1001/jama.281.3.261. [DOI] [PubMed] [Google Scholar]
- 5.Mansfield PF, Hohn DC, Fornage BD, Gregurich MA, Ota DM. Complications and failures of subclavian-vein catheterization. N Engl J Med. 1994;331:1735–1738. doi: 10.1056/NEJM199412293312602. [DOI] [PubMed] [Google Scholar]
- 6.Ruesch S, Walder B, Tramer M. Complications of central venous catheters: internal jugular versus subclavian access—a systematic review. Crit Care Med. 2002;30:454–460. doi: 10.1097/00003246-200202000-00031. [DOI] [PubMed] [Google Scholar]
- 7.Ullman JI, Stoelting RK. Internal jugular vein location with the US Doppler blood flow detector. Anesth Analg. 1978;57:118. doi: 10.1213/00000539-197801000-00024. [DOI] [PubMed] [Google Scholar]
- 8.Yonei A, Nonoue T, Sari A. Real-time ultrasonic guidance for percutaneous puncture of the internal jugular vein. Anesthesiology. 1986;64:830–831. doi: 10.1097/00000542-198606000-00033. [DOI] [PubMed] [Google Scholar]
- 9.Troianos CA, Jobes DR, Ellison N. Ultrasound-guided cannulation of the internal jugular vein. A prospective, randomized study. Anesth Analg. 1991;72:823–826. doi: 10.1213/00000539-199106000-00020. [DOI] [PubMed] [Google Scholar]
- 10.Karakitsos D, Labropoulos N, De Groot E, et al. Real-time ultrasound guided catheterization of the internal jugular vein; a prospective comparison to the landmark technique in critical care patients (ISRCTN61258470) Crit Care. 2006;10:R162. doi: 10.1186/cc5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mallory D, McGee W, Shawker T, et al. Ultrasound guidance improves the success rate of internal jugular vein cannulation. A prospective, randomized trial. Chest. 1990;98:157–160. doi: 10.1378/chest.98.1.157. [DOI] [PubMed] [Google Scholar]
- 12.Denys B, Uretsky B, Reddy P. Ultrasound-assisted cannulation of the internal jugular vein a prospective comparison to the external landmark guided technique. Circulation. 1993;87:1557–1562. doi: 10.1161/01.CIR.87.5.1557. [DOI] [PubMed] [Google Scholar]
- 13.Serafimidis K, Sakorafas G, Konstantoudakis G, et al. Ultrasound-guided catheterization of the internal jugular vein in oncologic patients; comparison with the classical anatomic landmark technique: prospective study. Int J Surg. 2009;7:526–528. doi: 10.1016/j.ijsu.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Fragou M, Gravvanis A, Dimitriou V, et al. Real-time ultrasound-guided subclavian vein cannulation versus the landmark method in critical care patients: a prospective randomized study. Crit Care Med. 2011;39:1607–1612. doi: 10.1097/CCM.0b013e318218a1ae. [DOI] [PubMed] [Google Scholar]
- 15.O’Grady NP, Alexander M, Burns LA, et al. Healthcare Infection Control Practices Advisory Committee. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2011;39:S1–S34. doi: 10.1016/j.ajic.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Debordeau P, Chahml D, LeGal G, et al. 2008 SOR guidelines for the prevention and treatment of thrombosis associated with central venous catheters in patients with cancer: report from the working group. Ann Oncol. 2009;20:1459–1471. doi: 10.1093/annonc/mdp052. [DOI] [PubMed] [Google Scholar]
- 17.Pronovost PJ, Needham D, Berhenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725–2732. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 18.Troianos C, Hartman G, Glas K, Skubas N, Eberhart R, Walker J, et al. Guidelines for performing ultrasound guided vascular cannulation: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr. 2011;24:1291–1318. doi: 10.1016/j.echo.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 19.Lamperti M, Bodenham AR, Pittiruti M, Blaivas M, et al. International evidence-based recommendations on ultrasound-guided vascular access. Intensive Care Med. 2012;38:1105–1117. doi: 10.1007/s00134-012-2597-x. [DOI] [PubMed] [Google Scholar]
- 20.Lichtenstein D, Menu Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill. Chest. 1995;5:1345–1348. doi: 10.1378/chest.108.5.1345. [DOI] [PubMed] [Google Scholar]
- 21.Nichols I, Humprey J. The efficacy of upper arm placement of peripherally inserted central catheters using bedside ultrasound and microintroducer technique. J Inf Nursing. 2008;31:165–176. doi: 10.1097/01.NAN.0000317703.66395.b8. [DOI] [PubMed] [Google Scholar]
- 22.Pittiruti M, Hamilton H, Biffi R, MacFie J, Pertkiewicz M. ESPEN guidelines on parenteral nutrition: central venous catheters (access, care, diagnosis and therapy of complications) Clinical Nutrition. 2009;28:365–377. doi: 10.1016/j.clnu.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 23.Shiloh A, Savel E, Paulin L. Ultrasound-guided catheterization of the radial artery: a systematic review and meta-analysis of randomized controller trials. Chest. 2011;139:524–529. doi: 10.1378/chest.10-0919. [DOI] [PubMed] [Google Scholar]
- 24.Matalon TA, Silver B. US guidance of interventional procedures. Radiology. 1990;174:43–47. doi: 10.1148/radiology.174.1.2403684. [DOI] [PubMed] [Google Scholar]
- 25.Chapman GA, Johnson D, Bodenham AR. Visualisation of needle position using ultrasonography. Anaesthesia. 2006;61:148–158. doi: 10.1111/j.1365-2044.2005.04475.x. [DOI] [PubMed] [Google Scholar]
- 26.Abboud PA, Kendall JL. Ultrasound guidance for vascular access. Emerg Med Clin North Am. 2004;22:749–773. doi: 10.1016/j.emc.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Blaivas M, Brannam L, Fernandez E. Short-axis versus long-axis approaches for teaching ultrasound-guided vascular access on a new inanimate model. Acad Emerg Med. 2003;10:1307–1311. doi: 10.1111/j.1553-2712.2003.tb00002.x. [DOI] [PubMed] [Google Scholar]
- 28.French JLH, Raine-Fenning NJ, Hardman JG, Bedforth NM. Pitfalls of ultrasound guided vascular access: the use of three four-dimensional ultrasound. Anaesthesia. 2008;63:806–813. doi: 10.1111/j.1365-2044.2008.05513.x. [DOI] [PubMed] [Google Scholar]
- 29.Blaivas M, Adhikari S. An unseen danger: frequency of posterior vessel wall penetration by needles during attempts to place internal jugular vein central catheters using ultrasound guidance. Care Med. 2009;37:2345–2349. doi: 10.1097/CCM.0b013e3181a067d4. [DOI] [PubMed] [Google Scholar]
- 30.Denys BG, Uretsky BF. Anatomical variations of internal jugular vein location: impact on central venous access. Crit Care Med. 1991;19:1516–1519. doi: 10.1097/00003246-199112000-00013. [DOI] [PubMed] [Google Scholar]
- 31.Hirsch DR, Ingenito EP, Goldhaber SZ. Prevalence of deep venous thrombosis among patients in medical intensive care. JAMA. 1995;274:335–337. doi: 10.1001/jama.1995.03530040063042. [DOI] [PubMed] [Google Scholar]
- 32.Timsit JF, Farkas JC, Boyer JM, et al. Central vein catheter-related thrombosis in intensive care patients: incidence, risk factors, and relationship with catheter-related sepsis. Chest. 1998;114:207–213. doi: 10.1378/chest.114.1.207. [DOI] [PubMed] [Google Scholar]
- 33.Troianos CA, Kuwik RJ, Pasqual JR, Lim AJ, Odasso DP. Internal jugular vein and carotid artery anatomic relation as determined by ultrasonography. Anesthesiology. 1996;85:43–48. doi: 10.1097/00000542-199607000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Gordon AC, Saliken JC, Johns D, Owen R, Gray R. US-guided puncture of the internal jugular vein: complications and anatomic considerations. J Vasc Interv Radiol. 1998;9:333–338. doi: 10.1016/S1051-0443(98)70277-5. [DOI] [PubMed] [Google Scholar]
- 35.Galloway S, Bodenham A. Ultrasound imaging of the axillary vein—anatomical basis for central venous access. Br J Anaesth. 2003;90:589–595. doi: 10.1093/bja/aeg094. [DOI] [PubMed] [Google Scholar]
- 36.Gibbs FJ, Murphy MC. Ultrasound guidance for central venous catheter placement. Hosp Physician. 2006;2006:23–31. [Google Scholar]
- 37.Maecken TM, Grau T. Ultrasound imaging in vascular access. Crit Care Med. 2007;35(2):S178–S185. doi: 10.1097/01.CCM.0000260629.86351.A5. [DOI] [PubMed] [Google Scholar]
- 38.Highes P, Scott C, Bodenham A. Ultrasonography of the femoral veins in the groin: implications for vascular access. Anesthesia. 2000;55:1198–1202. doi: 10.1046/j.1365-2044.2000.01615-2.x. [DOI] [PubMed] [Google Scholar]
- 39.Hilty WM, Hudson PA, Levitt MA, Hall JB. Real-time US-guided femoral vein catheterization during cardiopulmonary resuscitation. Ann Emerg Med. 1997;29:331–337. doi: 10.1016/S0196-0644(97)70344-5. [DOI] [PubMed] [Google Scholar]
- 40.Aouad MT, Kanazi GE, Abdallah FW, Moukaddem FH, Turbay MJ, Obeid MY, et al. Femoral vein cannulation performed by residents: a comparison between ultrasound-guided and landmark technique in infants and children undergoing cardiac surgery. Anesth Analg. 2010;111:724–728. doi: 10.1213/ANE.0b013e3181e9c475. [DOI] [PubMed] [Google Scholar]
- 41.Prabhu MV, Juneja D, Gopal PB, Sathyanarayanan M, Subhramanyam S, Gandhe S, et al. Ultrasound-guided femoral dialysis access placement: a single-center randomized trial. Clin J Am Soc Nephrol. 2010;5:235–239. doi: 10.2215/CJN.04920709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seto AH, Abu-Fadel MS, Sparling JM, Zacharias SJ, Daly TS, Harrison AT, et al. Real-time ultrasound guidance facilitates femoral arterial access and reduces vascular complications: FAUST (femoral arterial access with ultrasound trial) J Am Coll Cardiol Intv. 2010;3:751–758. doi: 10.1016/j.jcin.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 43.Constantino TG, Parikh AK, Satz WA, et al. Ultrasonography-guided peripheral intravenous access versus traditional approaches in patients with difficult intravenous access. Ann Emerg Med. 2005;46(5):456–461. doi: 10.1016/j.annemergmed.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 44.Brzezinski M, Luisetti T, London MJ. Radial artery cannulation: a comprehensive review of recent anatomic and physiologic investigations. Anesth Analg. 2009;109:1763–1781. doi: 10.1213/ANE.0b013e3181bbd416. [DOI] [PubMed] [Google Scholar]
- 45.Barber JD, Wright DJ, Ellis RH. Radial artery puncture. A simple screening test of the ulnar anastomotic circulation. Anaesthesia. 1973;28(3):291–292. doi: 10.1111/j.1365-2044.1973.tb00448.x. [DOI] [PubMed] [Google Scholar]
- 46.Stein J, George B, River G, Hebig A, McDermott D. Sonographically guided peripheral intravenous cannulation in emergency department patients with difficult intravenous access: a randomized trial. Ann Emerg Med. 2009;54:33–40. doi: 10.1016/j.annemergmed.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 47.Bishop L, Dougherty L, Bodenham A, et al. Guidelines on the insertion and management of central venous access devices in adults. Int J Lab Hematol. 2007;29:261–278. doi: 10.1111/j.1751-553X.2007.00931.x. [DOI] [PubMed] [Google Scholar]
- 48.Gladwin MT, Slonim A, Landucci DL, et al. Cannulation of the internal jugular vein: is a post procedural chest radiography always necessary? Crit Care Med. 1999;27:1819–1823. doi: 10.1097/00003246-199909000-00019. [DOI] [PubMed] [Google Scholar]
- 49.Taylor RW, Palagiri AV. Central venous catheterization. Crit Care Med. 2007;35(5):1390–1396. doi: 10.1097/01.CCM.0000260241.80346.1B. [DOI] [PubMed] [Google Scholar]
- 50.Fletcher SJ, Bodenham AR. Safe placement of central venous catheters: where should the tip of the catheter lie? Br J Anaesth. 2000;85:188–191. doi: 10.1093/bja/85.2.188. [DOI] [PubMed] [Google Scholar]
- 51.Ormel RM, McSwiney MM, Chamberlain-Webber RF. Fatal cardiac tamponade as a result of a peripherally inserted central venous catheter: a case report and review of the literature. Br J Anaesth. 2007;99:384–388. doi: 10.1093/bja/aem181. [DOI] [PubMed] [Google Scholar]
- 52.Booth SA, Norton B, Mulvey DA. Central venous catheterization and fatal cardiac tamponade. Br J Anaesth. 2001;87(2):298–302. doi: 10.1093/bja/87.2.298. [DOI] [PubMed] [Google Scholar]
- 53.Domino KB, Bowdle TA, Posner KL, Spitellie PH, Lee LA, Cheney FW. Injuries and liability related to central vascular catheters: a closed claims analysis. Anesthesiology. 2004;100(6):1411–1418. doi: 10.1097/00000542-200406000-00013. [DOI] [PubMed] [Google Scholar]
- 54.Lee JH, Bahk JH, Ryu HG, Jung C, Jeon Y. Comparison of the bedside central venous catheter placement techniques: landmark vs electrocardiogram guidance. Br J Anaesth. 2009;102:662–666. doi: 10.1093/bja/aep046. [DOI] [PubMed] [Google Scholar]
- 55.Na S, Kim JT, Kim HS, Bahk JH, Kim CS, Kim SD. Practical anatomic landmarks for determining the insertion depth of central venous catheter in paediatric patients. Br J Anaesth. 2009;102:820–823. doi: 10.1093/bja/aep078. [DOI] [PubMed] [Google Scholar]
- 56.Gebhard R, Szmuk P, Pivalizza E, Melnikov V, Vogt C, Warters R. The accuracy of electrocardiogram-controlled central line placement. Anesth Analg. 2007;104:65–70. doi: 10.1213/01.ane.0000250224.02440.fe. [DOI] [PubMed] [Google Scholar]
- 57.Wirsing M, Schummer C, Neumann R, Steenbeck J, Schmidt P, Schummer W. Is traditional reading of the bedside chest radiograph appropriate to detect intraatrial central venous catheter position? Chest. 2008;134:527–533. doi: 10.1378/chest.07-2687. [DOI] [PubMed] [Google Scholar]
- 58.Schuster M, Nave H, Piepenbrock S, Pabst R, Panning B. The carina as a landmark in the central venous placement. Br J Anaesth. 2000;85(2):192–194. doi: 10.1093/bja/85.2.192. [DOI] [PubMed] [Google Scholar]
- 59.Albrecht K, Nave H, Breitmeier D, Panning B, Troger HD. Applied anatomy of the superior vena cava: the carina as a landmark to guide central venous catheter placement. Br J Anaesth. 2004;92:75–77. doi: 10.1093/bja/aeh013. [DOI] [PubMed] [Google Scholar]
- 60.Stonelake PA, Bodenham AR. The carina as a radiological landmark for central venous catheter tip position. Br J Anaesth. 2006;96:335–340. doi: 10.1093/bja/aei310. [DOI] [PubMed] [Google Scholar]
- 61.Precautions necessary with central venous catheter. Washington DC: US Government Printing Office; 1989. pp. 15–16. [Google Scholar]
- 62.Reynolds N, McCulloch AS, Pennington CR, et al. Assessment of distal tip position of long-term central venous feeding catheters using transesophageal echocardiology. J Parenter Enteral Nutr. 2001;25:39–41. doi: 10.1177/014860710102500139. [DOI] [PubMed] [Google Scholar]
- 63.Beaulieu Y. Bedside echocardiography in the assessment of the critically ill. Crit Care Med. 2007;35:S235–S249. doi: 10.1097/01.CCM.0000260673.66681.AF. [DOI] [PubMed] [Google Scholar]
- 64.Maury E, Guglielminotti J, Alzieu M, Guidet B, Offenstadt G. An alternative to chest radiography after central venous catheter insertion? Am J Respir Crit Care Med. 2001;164(3):403–405. doi: 10.1164/ajrccm.164.3.2009042. [DOI] [PubMed] [Google Scholar]
- 65.Jauss M, Zanette E, For the Consensus Conference Detection of the right-to-left shunt with ultrasound contrast agent and transcranial Doppler sonography. Cerebrovascular Dis. 2000;10:490–496. doi: 10.1159/000016119. [DOI] [PubMed] [Google Scholar]
- 66.Vezzani A, Brusasco C, Palermo S, Launo C, Mergoni M, Corradi F. Ultrasound localization of central vein catheter and detection of postprocedural pneumothorax: an alternative to chest radiography. Crit Care Med. 2010;38:533–538. doi: 10.1097/CCM.0b013e3181c0328f. [DOI] [PubMed] [Google Scholar]
- 67.Alemen C, Alegre J, Armadans L, et al. The value of chest roentgenography in the diagnosis of pneumothorax after thoracocentesis. Am J Med. 1999;107:340–343. doi: 10.1016/S0002-9343(99)00238-7. [DOI] [PubMed] [Google Scholar]
- 68.Ball CG, Kirkpatrick AW, Laupland KB, et al. Factors related to the failure of radiographic recognition of occult posttraumatic pneumothoraces. Am J Surg. 2005;189:541–546. doi: 10.1016/j.amjsurg.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 69.Lichtenstein DA, Mezière G, Lascols N, et al. Ultrasound diagnosis of occult pneumothorax. Crit Care Med. 2005;33(6):1231–1238. doi: 10.1097/01.CCM.0000164542.86954.B4. [DOI] [PubMed] [Google Scholar]


