Abstract
Muscle injuries can be classified into strain injuries and contusions. Depending on the type of injury, different complications may occur, which in turn can be divided into early, intermediate and delayed complications. A prompt diagnosis of complications allows early treatment and permits to avoid harmful sequelae. Imaging studies, ultrasonography in particular, allow (recognizing) the assessment of complications whenever clinically suspected. In this article the most frequent complications of muscle injuries are presented.
Keywords: Muscle injuries, Complications, Ultrasound
Riassunto
Le lesioni muscolari possono essere classificate in lesioni contusive e lesioni distrattive. A seconda del tipo di lesione, possono insorgere complicanze differenti, che a loro volta possono essere suddivise in complicanze precoci, intermedie e tardive. Un rapido e preciso riconoscimento delle complicanze consente di intervenire precocemente e permette di evitare conseguenze dannose. L’imaging, in particolar modo l’ecografia, permette il riconoscimento di complicanze ogni qualvolta esse siano sospettate clinicamente. In questo articolo vengono presentate le complicanze più frequenti delle lesioni muscolari.
Introduction
Muscular injuries are characterized by a sequence of events which may (lead to the development of) progress to complications or recurrences.
Muscle has limited capacity for regeneration following injury, and part of the healing is achieved by scar tissue formation, which is in turn frequently related with the volume of muscle necrosis and the size of the haematoma. The presence of an intramuscular scar alters the normal muscle contraction vector reducing strength and increasing fatigue. Hence, the more severe is an injury, the more muscle biomechanics will be altered and muscle fatigue will be increased, resulting in an augmented risk of re-injury [1].
Relapses represent the most frequent complications of muscle injuries; they are generally favoured by diagnostic errors and improper treatment, particularly concerning the timing of return to activity. Recurrences represent a major topic in sport medicine: a prompt return to physical activity and a favourable recovery are the main challenge that the physician and the athlete have to deal with, particularly in the elite athlete [2, 3].
Muscular injuries complications can be classified according to their onset as early, intermediate and delayed complications (Table 1).
Table 1.
Classification of muscle injuries complications
| Early onset complications |
| Diagnostic errors |
| Deep vein thrombosis |
| Acute compartment syndrome |
| Intermediate onset complications |
| Early recurrences |
| Myositis ossificans |
| Infections |
| Rhabdomyolisis |
| Delayed onset complications |
| Post-traumatic fibrosis |
| Calcified myonecrosis |
| Muscle herniation |
| Delayed recurrences |
Early onset complications
Deep vein thrombosis (DVT) and compartment syndrome can be numbered among early onset complications diagnostic errors.
Diagnostic errors
There are two types of diagnostic errors: site-related errors and entity-related errors. Diagnostic errors could lead to improper treatment and untimely return-to-play after injury. To reduce diagnostic errors in soft-tissue injuries evaluation, it is recommendable to perform an imaging study in addition to an accurate clinical examination [4, 5].
There is evidence that an exclusive clinical examination may lead to misdiagnosis [4–6]. To reduce diagnostic errors, an imaging examination should be performed to confirm the clinical impression and to evaluate severity and size of the lesion. Among imaging modalities, ultrasonography (US) is a low-cost, non-invasive and reliable tool to assess soft-tissue trauma. In addition, it allows to exclude other diseases that can mimic muscular injuries, and to assess the size of the lesion, which can influence choice and duration of the treatment [4, 5].
Deep vein thrombosis
Concerning deep vein thrombosis, it has been reported in literature that it can be related to muscular injuries [7]. The presence of oedema or haematoma, caused by the muscular rupture, may lead to compression of popliteal or gastrocnemic veins, thus determining DVT. In a study on 24 patients with partial rupture of the medial head of the gastrocnemius muscle, five patients showed DVT at US examination [4, 5].
The classic presentation of DVT in the extremity is the triad of swelling, pain, and tenderness. However, DVT cannot be diagnosed reliably by clinical features alone, especially when a patient shows a recent history of soft-tissue injury [6]. After clinical examination, history should be taken to assess probability of DVT by means of a validated scoring system [8]. Finally, in case of highly suspected DVT, US should be performed. US evaluation for DVT should include compression, colour and spectral Doppler US with assessment of phasicity, and venous flow augmentation (Fig. 1). Evaluation of compression is the most reliable technique and compressibility is the primary diagnostic criterion for excluding DVT on US [8].
Fig. 1.
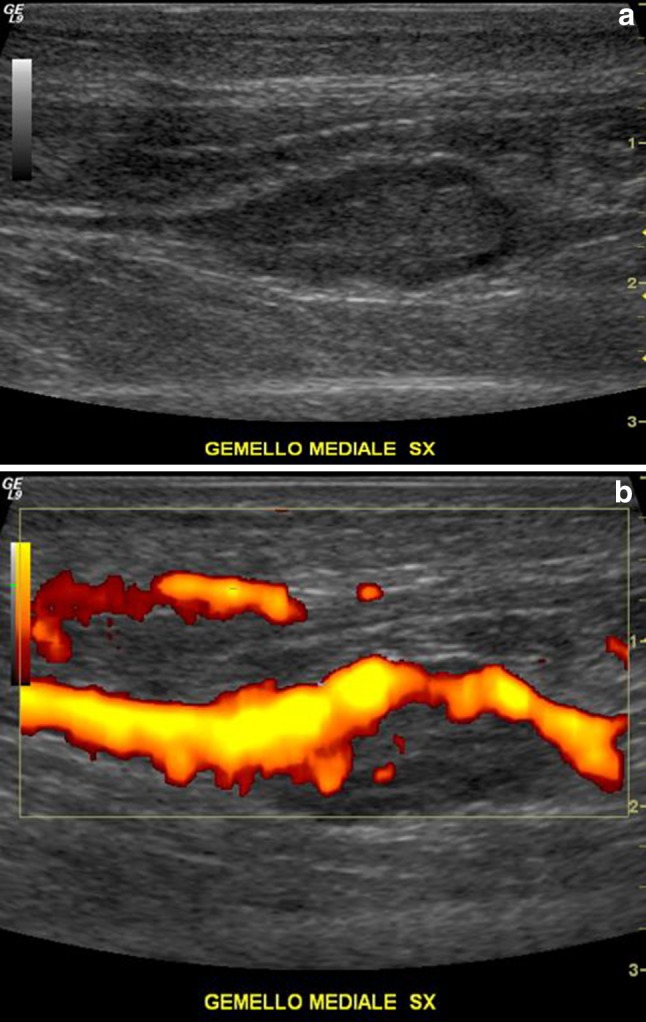
a Sagittal sonogram of the posterior lower leg demonstrating an acute gastrocnemic vein thrombosis. The thrombus is hypoechoic, filling the central portion of the vein. b Colour Doppler demonstrates venous flow only in the peripheral part of the vein, while the central part is occupied by the thrombus
Treatment by anticoagulants is recommended in selected cases to avoid embolic complications [4].
Acute compartment syndrome
Muscle strains or contusions may determine muscular rupture, which in turn can result in oedema or haematoma within the muscle, leading to an increase in tissue pressure in an inestensible compartment. Increased tissue pressure and metabolic insults from tissue necrosis determine microvascular disruption and progressive ischaemia acute compartment syndrome (ACS) [9].
Pain disproportionate to the injury and resistant to analgesic medication is the main symptom. It can be associated with neurological abnormalities, palpable tenseness and pulselessness [9, 10]. The anterior compartment of the leg is the most commonly affected [11].
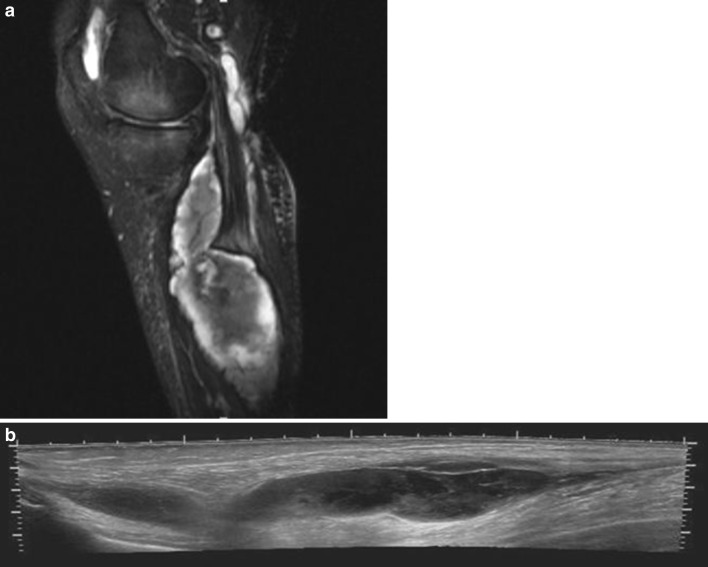
To confirm clinical diagnosis of ACS, direct intracompartmental pressure (ICP) measurement can be used, although also MRI has been advocated (Fig. 2). The best specificity and sensibility have been reached, however, by means of direct pressure measurement with near infrared spectroscopy (NIRS), a noninvasive method to detect haemoglobin saturation [11, 12]. Ultrasonography may be useful to detect and drain fluid collections percutaneously to decompress the compartment.
Fig. 2.
a Sagittal fat saturated T2-weighted magnetic resonance image of the lower leg demonstrating a ruptured baker cyst which eventually caused an acute compartmental syndrome of the lower leg. b Extended field-of-view longitudinal sonogram of the same patient, where debris can be observed within the ruptured cyst
Treatment is, by fasciotomy, with a single- or double-incision approach [8]. Compartment syndrome can also be chronic, as a result of neoplasm or associated with exercise, typically presenting with pain relieved by rest.
Intermediate onset complications
Early recurrences, myositis ossificans, rhabdomyolisis and infections are main intermediate onset complications.
Early recurrences
Early recurrences represent the most frequent complications of muscle injuries; incidence of repeat injury has been reported to be approximately 30 % [3].
Factors associated with recurrent muscle injuries include: extrinsic and intrinsic factors. Extrinsic factors are premature return-to-play, inadequate training, muscle strength imbalances, decreased flexibility, increasing age, and history of prior injury. Intrinsic factors are persistent weakness in the injured muscle, reduced extensibility of the musculo-tendon unit due to residual scar tissue and adaptive changes in the biomechanics and motor patterns of sporting movements following the original injury [11, 13].
The rate of recurrence is also related with the type and site of muscle injury, and highest rate of recurrence usually occurs after 1 or 2 weeks after muscular injury [3].
Because of the high risk of recurrent injury and variable convalescence period, imaging has a prognostic role in evaluating muscle injuries, particular for the elite athlete. It has been proved that a negative imaging study correlates with a low rate of recurrences compared with patient in which imaging studies showed any sign of injuries [11].
Myositis ossificans
Myositis ossificans (MO) is a benign condition defined as a heterotopic non-neoplastic bone or cartilage formation in or adjacent to muscle, typically growing in proximity to bone [14]. Myositis ossificans has been referred also as post-traumatic ectopic calcification in the muscle in literature. Myositis ossificans traumatica (MOT), which is related to muscle trauma should be distinguished from myositis ossificans progressiva, an inherited condition in which there is progressive extraskeletal ossification and from “neurogenic heterotopic ossification” in which paralysis has been implicated [15, 16].
The cause of MOT is usually blunt trauma. A history of preceding trauma, however, is lacking in 50 % of cases. Reinjury of an injured muscle seems to be a major risk factor in developing MOT [15].
Pain, swelling and a palpable mass are common symptoms although asymptomatic cases have been reported [15].
Myositis ossificans formation is a two-step process. The first step consists of degeneration and necrosis of the muscular tissue and occurs 1 or 2 weeks after the injury (early MO), while the second step is mesenchymal cell proliferation and bone formation and occurs 3 or 4 weeks after the injury (mature MO).
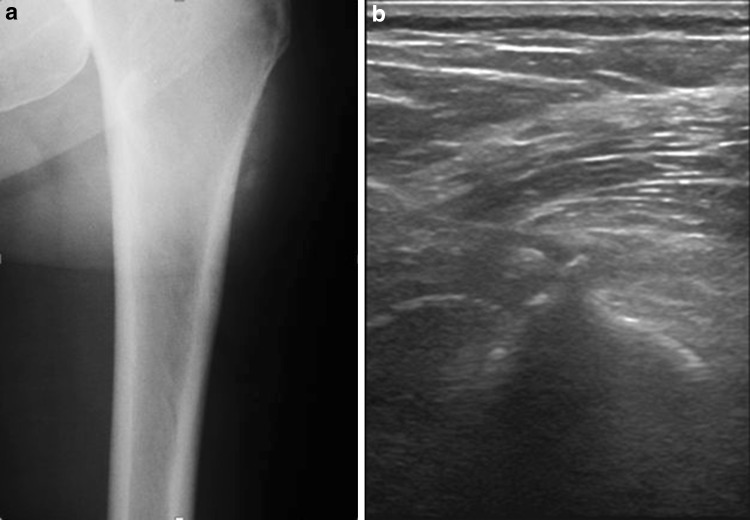
US has been proposed as a reliable method to diagnose MO. At US, MO appears as homogenous, hypoechoic, well-defined oval-shaped mass, with regular borders, with thickening of the surrounding muscle belly. A hyperechoic, ill-defined lamellar rim could be observed in early MO, while in mature stage a more defined rim with acoustic shadowing could be seen [1] (Fig. 3).
Fig. 3.
a Radiograph of a left femur showing oval-shaped extraskeletal calcifications. b Sagittal sonogram of the same patient showing homogenous, hyperechoic, well-defined oval-shaped masses, with acoustic shadowing
Ultrasonography findings of MO nevertheless are non-specific and can easily be confused with a soft-tissue sarcoma. In doubtful cases, a short-term follow-up is recommended to highlight the typical US changes that occur from early to mature MO [17].
The ability of US to differentiate early and mature MO has a pivotal role in treatment. In early stage the use of bisphosphonate and indomethacin in addition to rest, ice, compression, and elevation (RICE) of the affected limb had been advocated. Surgical excision should be performed only in cases of mature MO, since recurrence is more likely to occur in metabolically active lesions [14, 18].
Infections
Muscular infections may occur in muscles that have been compromised or injured by trauma, especially by blunt trauma, determining what is called “pyogenic myositis” or “pyo-myositis”. Pyomyositis is more prevalent in the tropics and in immunocompromised patients. In more than 90 % of cases of pyo-myositis the causative agent is S. aureus [19].
A history of recent blunt trauma is obtained in up to one-half of patients with pyomyositis, suggesting that local muscle injury created a “locus minoris resistentiae” that is subsequently infected [19].
Pyomyositis usually affects the large muscles of the lower extremities or trunk. The illness typically unfolds over several weeks. Most patients present with pain and tenderness localized to the body of a muscle, but occasionally patients will present with acute illness with marked systemic toxicity [19, 20].
Ultrasound appearance of pyomyositis may reveal diffuse muscle swelling with oedema and diffuse hyperaemia. If untreated pyo-myositis may lead to abscess formation, which at US may vary in appearance: its echogenicity can vary from hypoechoic to isoechoic. Posterior acoustic enhancement is characteristic of abscess. Internal debris is a common feature while gas bubbles may be seen with gas-forming organisms (Fig. 4). Internal septa are visible especially in chronic infections. Colour Doppler imaging usually reveals variable hyperaemia of the abscess wall and immediate surrounding tissues [19–21].
Fig. 4.
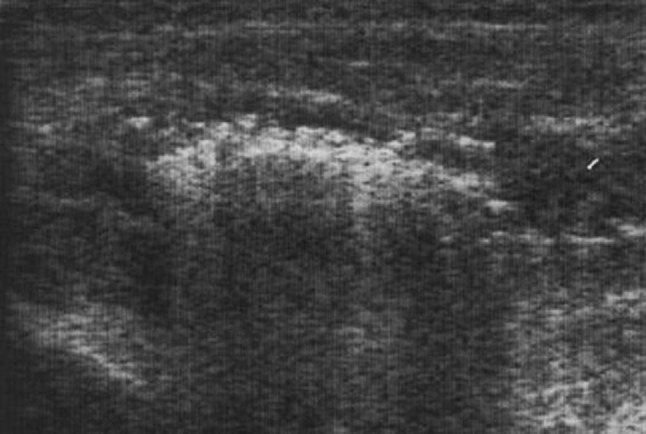
Transverse sonogram of a patient with pyomyositis of the rectus femoris muscle. Diffuse muscle swelling and partial posterior acoustic enhancement can be seen, suggesting a gas-forming organism infection
Iatrogenic muscle infections have been reported. Drainage of post-traumatic muscular haematomas may end up with abscesses. Some authors do not recommend drainage particularly in young athletes to avoid risk of secondary infections. Other clinicians advocate total drainage of the haematoma followed by a compression bandage to prevent recurrent haematoma, especially in the elite athlete [22].
Rhabdomyolisis
The term rhabdomyolisis describes a condition in which there is necrosis of skeletal muscle. This condition is associated with release of myoglobin in circulation and subsequent renal impairment. Common causes are muscle trauma, especially crush injuries, extreme physical exertions and infections. Predisposing factors are drug use, particularly alcohol and diuretics, concomitant illness and training in hot environment [15, 21]. If untreated, rhabdomyolisis may lead to acute renal failure, hyperkalemia and disseminated intravascular coagulation.
Clinical signs are muscle weakness, tenderness and swelling. Typically, there is increased serum creatine kinase (CK), elevated creatinine and myoglobinuria [4, 15].
US of affected muscles shows muscle swelling with thickened myofibrils with loss of muscle striation (Fig. 5). Muscle may appear inhomogenously hypoechoic with hypoechoic pockets surrounding muscle, representing inflammatory fluid [4, 15].
Fig. 5.
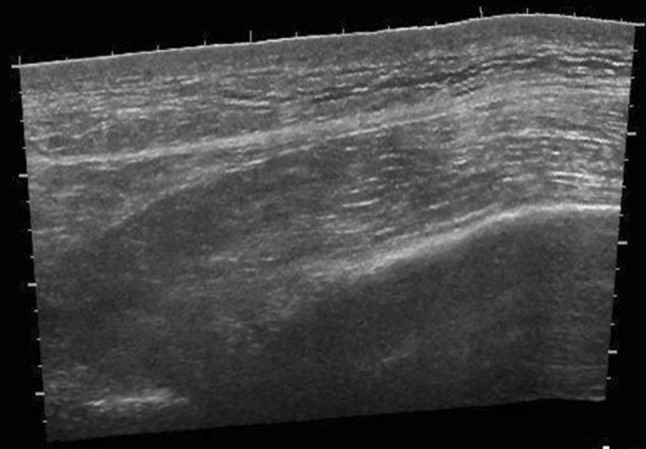
Sagittal extended field-of-view sonographic image of the right leg of a patient with rhabdomyolisis. Diffuse muscle swelling with loss of muscle striations can be seen
Treatment consists of hospitalization, hydratation, administration of mannitol and bicarbonate [15].
Delayed onset complications
Post-traumatic fibrosis
The healing of a muscle trauma can occur by means of two processes: through the regeneration of muscle fibres or through the formation of a fibrotic scar. In most cases, these processes are associated and are carried out simultaneously and competitively. In some cases, however, the balance between the two mechanisms can tilt towards the first or the second [1].
With regard to the regeneration of muscular fibres, it occurs through differentiation of satellite cells into myoblasts and then into myofibres, merging with the remaining damaged myocites.
Concerning fibrotic scar formation, the gap between the damaged muscle fibres is readily filled with a haematoma. In the first days after trauma an inflammatory response is created with phagocytes recalling and invading the lesion. The first clot is then organized and it will act as an initial web at which fibroblasts will be anchored. This newly formed tissue allows blocking lesion expansion. Fibroblasts start to produce type I collagen, thus creating a solid structure that will become the strongest point of the traumatized muscle tissue in about 10 days. Over time, the scar will tend to decrease the volume [1, 15].
The fibrotic scar alters the muscle mechanic, reducing the contractile capacity of the muscle, and therefore, the ability to develop strength comparable to healthy muscular tissue [15].
Although the majority of muscle lesions heal primarily through myofibres regeneration, in major trauma or recurrences healing occurs primarily through the formation of fibrotic scar [15].
Ultrasonography allows recognizing the various stages of healing of muscle tissue:
Haematomas: they can be intermuscular (between two muscle groups) or intramuscular (confined within a single muscle parenchyma). At US the presence of haematoma is suggested by the presence of hypoechoic or mixed echogenicity masses within the muscle. Longstanding haematomas may have peripheral hemosiderosis or calcifications. If the haematoma is causing pain and it is placing the limb at risk of ACS, US-guided evacuation may be necessary. This is usually performed 2 weeks after the initial injury [17, 23].
Fibrous scars: at US they appear as hyperechoic or heterogeneous linear or stellate lesions attached to the epimysium or to the myotendineous junction (MTJ) (Fig. 6). The stellate form usually follows muscle contusion from a direct blow, whereas the linear form typically follows muscle strain and commonly surrounds the MTJ. The lesion does not show any change with contraction of the muscle belly [24, 25].
Fig. 6.
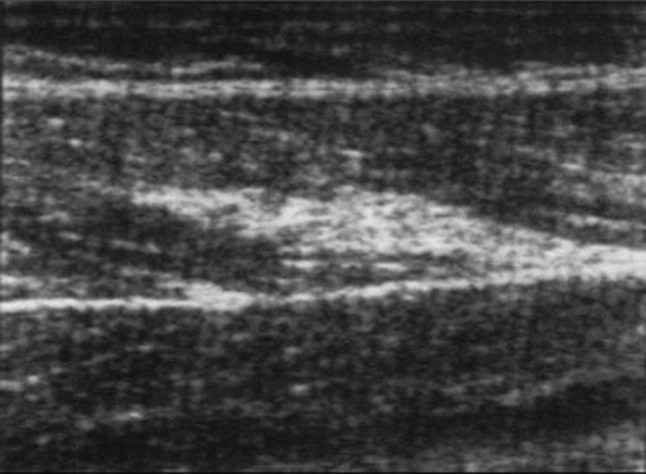
Sagittal sonogram of the left leg where a hyperechoic linear lesion attached to the epymisium of the rectus femoris can be seen consistent with posttraumatic fibrosis
Muscle herniation
Muscle hernia (MH) is a protrusion of muscle through a fascial defect [24]. Most commonly, the anterior compartment of the leg is affected. MH can be related to blunt or penetrating traumas or muscle hypertrophy [15].
Mostly asymptomatic, MH can be clinically suspected when a small superficial and painless soft-tissue lump is present and tends to reduce its volume with muscle contraction [4, 17].
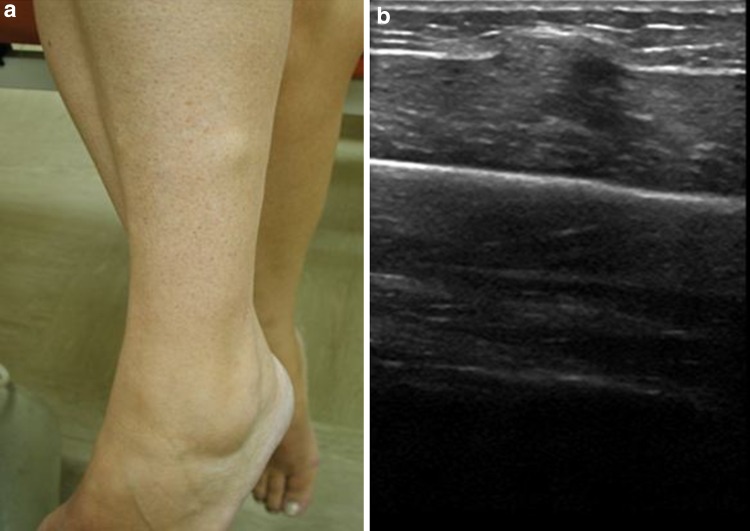
On US, normal muscle tissue may be seen protruding through a focal epimysial defect (Fig. 7). A dynamic study with contraction of the muscle under evaluation permits to identify a defect in perymisium through which muscle bulges with contraction.
Fig. 7.
a Patient with a lower leg muscle hernia. A lump can be observed in the lateral part of the lower leg. b Sagittal sonogram of the same patient. Muscle tissue can be seen protruding through an epymisial defect
Minimal pressure should be employed with the US probe as the hernia can be inadvertently reduced. Multiple herniations may indicate an underlying chronic compartment syndrome [17, 22].
Calcified myonecrosis
Calcific myonecrosis (CM) is a rare post-traumatic lesion, whose cause is unknown, although most of the cases reported in (the) literature are attributed either to compartment syndrome or nerve injury. The most common site of calcific myonecrosis is the anterior compartment of the lower leg [26, 27].
In this condition, an entire single muscle of the leg is replaced by a fusiform mass with central liquefaction and peripheral plaque-like calcification [26].
The differential diagnosis of calcific myonecrosis is a calcified soft-tissue mass. History of trauma with compartment syndrome and imaging features consistent with a centrally liquefied and peripherally calcified fusiform mass will help to reach a correct diagnosis [23, 26].
Delayed recurrences
While in contusion injuries the risk of recurrence is greatest in the first few weeks in strain injuries there is a steady increase in the risk of relapse even after many weeks of return to activity. This is probably due to the fact that the regeneration of muscle tissue occurs even after a mature fibrotic scar is formed. Thus, the risk of recurrence in strain injuries remains high for many weeks after trauma has occurred and fibrotic scar has formed [2, 3].
In particular, concerning delayed recurrences, they are mainly determined by the fact that the injured muscle is more atrophic than the healthy, uninjured muscle. Moreover, it has been shown that delayed recurrences in muscle tears occur at a different location from original site of injury. Another factor that should be investigated is the role of NSAIDs in the treatment of soft-tissue injuries, as they may in fact significantly delay the healing of muscle injury and consequently increase the risk of reinjury [2, 3].
Conclusions
Muscle injuries are a major concern in terms of health and social costs particularly in sport medicine [28].
Complications of muscle injuries are characterized by different clinical and imaging features, their onset are influenced by the type, the entity of an injury and by patient status.
To promptly recognize complications and to avoid premature return to physical activity, an accurate diagnosis should be reached and precise imaging follow-up should be performed routinely in evaluating muscle injuries.
Conflict of interest
None.
References
- 1.Järvinen TA, Järvinen TL, Kääriäinen M, Kalimo H, Järvinen M. Muscle injuries: biology and treatment. Am J Sports Med. 2005;33:745–764. doi: 10.1177/0363546505274714. [DOI] [PubMed] [Google Scholar]
- 2.Orchard J, Best TM. The management of muscle strain injuries: an early return versus the risk of recurrence. Clin J Sport Med. 2002;12:3–5. doi: 10.1097/00042752-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Orchard J, Best TM, Verrall GM. Return to play following muscle strains. Clin J Sport Med. 2005;15:436–441. doi: 10.1097/01.jsm.0000188206.54984.65. [DOI] [PubMed] [Google Scholar]
- 4.Flecca D, Tomei A, Ravazzolo N, Martinelli M, Giovagnorio F. US evaluation and diagnosis of rupture of the medial head of the gastrocnemius (“Tennis Leg”) J Ultrasound. 2007;10(4):194–198. doi: 10.1016/j.jus.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bianchi S, Martinoli C, Abdelwahab IF, Derchi LE, Damiani S. Sonographic evaluation of tears of the gastrocnemius medial head (“tennis leg”) J Ultrasound Med. 1998;17(3):157–162. doi: 10.7863/jum.1998.17.3.157. [DOI] [PubMed] [Google Scholar]
- 6.Lutterbach-Penna RA, Kalume-Brigido M, Robertson BL, Jacobson JA, Girish G, Fessell DP. Deep vein thrombosis simulating hamstring injury on sonography. J Ultrasound Med. 2012;31:660–662. doi: 10.7863/jum.2012.31.4.660. [DOI] [PubMed] [Google Scholar]
- 7.Slawski DP. Deep venous thrombosis complicating rupture of the medial head of the gastrocnemius muscle. J Orthop Trauma. 1994;8(3):263–264. doi: 10.1097/00005131-199406000-00016. [DOI] [PubMed] [Google Scholar]
- 8.Heng Tan C, Bedi D, Vikram R. Sonography of thrombosis of the deep veins of the extremities: clinical perspectives and imaging review. J Clin Ultrasound. 2012;40:31–43. doi: 10.1002/jcu.20904. [DOI] [PubMed] [Google Scholar]
- 9.Shadgan B, Menon M, Sanders D, Berry G, Martin C, Jr, Duffy P, et al. Current thinking about acute compartment syndrome of the lower extremity. Can J Surg. 2010;53(5):329–334. [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JC, Mitchell AW, Healy JC. Imaging of muscle injury in the elite athlete. Br J Radiol. 2012;85(1016):1173–1185. doi: 10.1259/bjr/84622172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armfield DR, Kim DH, Towers JD, Bradley JP, Robertson DD. Sports-related muscle injury in the lower extremity. Clin Sports Med. 2006;25:803–842. doi: 10.1016/j.csm.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 12.van den Brand JG, Nelson T, Verleisdonk EJ, van der Werken C. The diagnostic value of intracompartmental pressure measurement, magnetic resonance imaging, and near-infrared spectroscopy in chronic exertional compartment syndrome: a prospective study in 50 patients. Am J Sports Med. 2005;33:699–704. doi: 10.1177/0363546504270565. [DOI] [PubMed] [Google Scholar]
- 13.Heiderscheit BC, Sherry MA, Silder A, Chumanov ES, Thelen GD. Hamstring strain injuries: recommendations for diagnosis, rehabilitation and injury prevention. J Orthop Sports Phys Ther. 2010;40(2):67–81. doi: 10.2519/jospt.2010.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abate M, Salini V, Rimondi E, Errani C, Alberghini M, Mercuri M, et al. Post traumatic myositis ossificans: sonographic findings. J Clin Ultrasound. 2011;39(3):135–140. doi: 10.1002/jcu.20792. [DOI] [PubMed] [Google Scholar]
- 15.Best TM. Soft-tissue injuries and muscle tears. Clin Sports Med. 1997;16(3):419–434. doi: 10.1016/S0278-5919(05)70033-8. [DOI] [PubMed] [Google Scholar]
- 16.Falsetti P, Acciai C, Carpinteri F, Palilla R, Lenzi L. Bedside ultrasonography of musculoskeletal complications in brain injured patients. J Ultrasound. 2010;13(3):134–141. doi: 10.1016/j.jus.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Datir A, Connell DA (2010) Muscle injury and complications. In: Robinson P (ed.) Essential radiology for sports medicine. Springer Science + Business Media, LLC, New York, pp 199–215
- 18.King BJ. Post-traumatic ectopic calcification in the muscles of athletes: a review. Br J Sports Med. 1998;32:287–290. doi: 10.1136/bjsm.32.4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chau CL, Griffith JF. Musculoskeletal infections: ultrasound appearances. Clin Radiol. 2005;60:149–159. doi: 10.1016/j.crad.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Bureau NJ, Chhem RK, Cardinal E. Musculoskeletal infections: US manifestations. Radiographics. 1999;19:1585–1592. doi: 10.1148/radiographics.19.6.g99no061585. [DOI] [PubMed] [Google Scholar]
- 21.Sutera R, Iovane A, Candela F. Imaging ecografico delle complicanze delle lesioni muscolari da sport. Il Medico Sportivo. 2010;10(1):20–21. [Google Scholar]
- 22.Draghi F, Robotti G, Jacob D, Bianchi S. Interventional musculoskeletal ultrasonography: precautions and contraindications. J Ultrasound. 2010;13(3):126–133. doi: 10.1016/j.jus.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Counsel P, Breidahl W. Muscle injuries of the lower leg. Semin Musculoskelet Radiol. 2010;14(2):162–175. doi: 10.1055/s-0030-1253158. [DOI] [PubMed] [Google Scholar]
- 24.Lee JC, Healy J. Sonography of lower limb muscle injury. AJR Am J Roentgenol. 2004;182(2):341–351. doi: 10.2214/ajr.182.2.1820341. [DOI] [PubMed] [Google Scholar]
- 25.Woodhouse JB, McNally EG. Ultrasound of skeletal muscle injury: an update. Semin Ultrasound CT MRI. 2011;32:91–100. doi: 10.1053/j.sult.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Papanna MC, Monga P, Wilkes RA. Post-traumatic calcific myonecrosis of flexor hallucis longus. A case report and literature review. Acta Orthop Belg. 2010;76:137–141. [PubMed] [Google Scholar]
- 27.O’Dwyer HM, Al-Nakshabandi NA, Al-Muzahmi K, Ryan A, O’Connell JX, Munk PL. Calcific myonecrosis: keys to recognition and management. AJR Am J Roentgenol. 2006;187(1):W67–W76. doi: 10.2214/AJR.05.0245. [DOI] [PubMed] [Google Scholar]
- 28.Orchard JW, Best TM, Mueller-Wohlfahrt HW, Hunter G, Hamilton BH, Webborn N, et al. The early management of muscle strains in the elite athlete: best practice in a world with a limited evidence basis. Br J Sports Med. 2008;42(3):158–159. doi: 10.1136/bjsm.2008.046722. [DOI] [PubMed] [Google Scholar]