Abstract
Lead exposure in children is one component leading to cognitive impairment. The Treatment of Lead-Exposed Children Trial (1994–2004) studied the effect of succimer in treating low levels of lead exposure (20–44 mcg/dL) in children 12 to 33 months old. While succimer was effective in reducing blood lead concentrations in the short term, treatment of blood lead levels did not result in any detectable improvement in a wide variety of measurements of cognitive or behavioral function. Furthermore, blood lead concentrations were not distinguishable between chelated and non-chelated individuals at 1 year. The most important treatment strategy is identification and termination of major sources of lead exposure.
Keywords: Succimer, Cognitive Impairment, Behaviorial Function, Elevated Blood Lead Levels, Treatment of Lead-Exposed Children Trial
Background
In the early 1990s, there was a broad scientific consensus that cognitive impairment followed lead exposure at low levels. Elevated blood lead levels (BLLs) at 2 years of age were associated with deficits identifiable beginning at 4 years old and beyond. Additionally, the Centers for Disease Control and Prevention (CDC) had redefined lead poisoning as BLLs >10 mcg/dL, generating thousands of new cases. The CDC recommended universal screening, helping to bring these new cases to physician’s offices. The Department of Health and Human Services had established as a Healthy People 2000 goal the elimination of childhood BLLs >25 mcg/dL [1]. Research had shown that succimer given orally, as well as parenteral EDTA, lowered BLLs, but no cognitive data had been collected. At that time, succimer was licensed only for children with BLLs greater than 45 mcg/dL. The Treatment of Lead-Exposed Children Trial (TLC) was a randomized controlled trial to determine the effect of succimer on BLLs, and more importantly, on the cognitive and behavioral impairments that were attributable to BLLs greater than 20 mcg/dL [2]. This paper summarizes the trial methods and findings previously published [2, 3], and offers a discussion on the role of chelation in the treatment of lead poisoning. Dr. Rogan was the principal investigator for the TLC and held the Investigational New Drug application for the use of succimer in children with BLLs <45 mcg/dL.
Trial Enrollment and Methods
From 1994–1997, the TLC took referral of individuals who were 12 to 33 months old at randomization. This age group was centered on the peak of BLLs in the USA, which is around 24 months of age. Individuals were enrolled in Baltimore, MD; Cincinnati, OH; Newark, NJ; and Philadelphia, PA. We considered those who had referral BLLs of 20 to 44 mcg/dL and we accepted referrals from private practitioners, city health departments, and anyone who wanted to refer individuals to our trial. The hospitals we worked with were established lead referral sites where children were sent to be treated for their lead poisoning or their lead exposure.
The pretreatment phase required participants to have a cleanable house. Succimer is not designed to treat children with continued severe lead exposure, but rather to be used in those children whose family has some control of their home environment. Our criteria for “clean houses” were within practice guidelines consistent with those used by the referring medical centers. Additional inclusion criteria were children in inner city populations. Two CDC-confirmed BLLs between 20 and 44 mcg/dL were required, as a standard practice for screening tests for BLLs that are higher than the reference range or a clinically important range is to repeat the measurement. A substantial number of tested individuals were enrolled in the trial, with duplicated test results in the high end of the target study range confirming lead poisoning. However, other individuals’ repeat testing did not confirm the higher BLL test results. There was a substantial amount of regression to the mean in these screening tests, with fewer than ½ of the initially screened positive samples being high on repeat testing.
After inclusion criteria were met, we made appointments to visit their homes. The cities we chose for our trial were historically full of houses with deteriorated lead-based paint (Fig. 1). The paint would frequently be used on windows, identifiable by its white, chalky appearance, and would have lead, lead oxide, some lead acetate, and linseed oil, often 50 % lead compounds by weight when it was formulated. Children ingested lead by playing with and eating the big flakes of paint, as well as the dust from it on their fingers, stuffed animals, and anything else they may put in their mouths. On this home visit, we determined whether we could reduce the deteriorated damaged paint enough to treat the child. If we concluded that we could treat him or her, we came back for another blood draw. We gave the family public health service brand multivitamins for the child. Ironically, early batches had low-level contamination with lead, and were recalled (Fig. 2).
Fig. 1.

Typical deteriorated lead-containing paint from inner city housing identified in home visit for TLC study [used with permission of Walter Rogan MD]
Fig. 2.
Picture of vitamin supplement supplied for TLC study children [used with permission of Walter Rogan MD]
The next step was to clean the houses. Home cleanings were a neighborhood event. Neighbors would come outside to watch. Cleaning crews in hazmat suits would gather up all the dust. In particular, we found that the area behind radiators, which habitually were under windows, were a great source of dust (Fig. 3). After the cleaning, we randomized participants either to the placebo group or the succimer group. We tested BLLs a week later, and then 2 days after each 26-day course of treatment and again, 2 weeks later. Those who had BLL above 15 mcg/dL after active treatment received up to two more treatment courses. A similar proportion of those receiving placebo were given an additional two courses of placebo. Of note, the 26-day treatment courses were longer than the typical 19-day course of treatment recommended in the succimer package insert.
Fig. 3.

Home cleanup was done prior to randomized treatment with succimer or placebo [used with permission of Walter Rogan MD]
TLC Findings
In the 1990s, when we initiated this trial, lead poisoning affected mostly boys. Although 5 % of the overall group were primarily Spanish speaking, this was true for 25 % of the children at the Newark site. The majority of the other children in the trial were African-American. The children were usually living with their single moms who often hadn’t finished high school (HS), and were receiving public assistance (Table 1). This is essentially the same demographic of individuals affected by lead poisoning today.
Table 1.
Demographics of TLC participants
| Placebo N = 384; % |
Succimer N = 396; % |
|
|---|---|---|
| Female | 43 | 45 |
| Spanish speaking | 5 | 5 |
| African American | 76 | 78 |
| Parent without partner | 73 | 72 |
| Parent education < HS | 40 | 41 |
| On public assistance | 97 | 96 |
Used with permission from NEJM
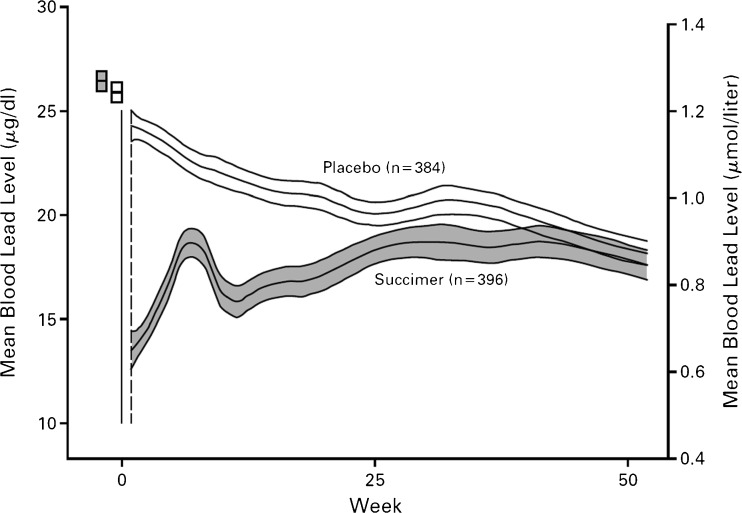
Succimer acutely lowered BLL, with BLL after 1 week of therapy averaging 13 mcg/dL (compared to 24 mcg/dL in the placebo group). However, lead then remobilized, presumably from bone, resulting in a mean rise to above 18 mcg/dL by week 7. The BLL dropped again with the second course of the drug (required in 83 % of the study group). However, the group that received the second course of the drug (those with BLLs greater than 15 mcg/dL) generally had increased BLL at the second and third BLL test (third round of treatment was required in 69 % of the entire treatment group). By 30 or 32 weeks, the placebo group had also demonstrated lower BLLs, similar to those of children in the general population in the USA at that time. The children in the succimer group had increased BLLs again, and after a year, everybody had similar BLLs, with the succimer group’s BLL an average of 2.7 mcg/dL lower than the placebo group’s mean of 17 mcg/dL. It was as though they had not taken the drug (Fig. 4).
Fig. 4.
Mean BLL and 95 % confidence intervals at baseline (small rectangles) and during TLC trial. Adapted from Reference [2] [used with permission from NEJM]
When we followed up these children at age five, we found that there was no evidence of an IQ effect. Approximately 90 % of each group was available for testing; no group improvement on performance IQ (mean 83), verbal IQ (mean 81.9), or full scale IQ (80.6) was identified. Neuropsychological testing showed there was no evidence of improvement on attention, language, sensory, motor, visuospatial, or memory testing; and behavioral testing showed there was no evidence of improvement on attention deficit-type behaviors or hyperactivity.
Another statistically significant but questionably clinically significant finding was that succimer-treated children grew 0.25 and 0.35 cm less than placebo-treated children at 12 and 34 months of follow-up, respectively.
Discussion
TLC was designed for 780 children to be able to detect with 80 % power a 3-point difference in IQ, which was the main outcome measured. The correlations between the IQ tests were higher and the retention rates were higher than expected; thus TLC’s results are very unlikely to suffer from a β error. Our steering group, having found a negative result at age five, which is preschool age, insisted that we follow up to age seven when most of the kids were starting second grade, and conduct a more comprehensive and more sensitive range of psychological tests. An unfortunate result of the screening protocol was that the children were tested at different ages centered on age five. At the age seven follow-up, children were tested within a couple of months of their seventh birthday. This substantially reduced the variation of the specific age at testing in the data and was appropriate for scientific rigor. It was, however, difficult to manage. Nonetheless, approximately 82 % (placebo) and 83 % (succimer) of each original group were evaluated at 7 years of age.
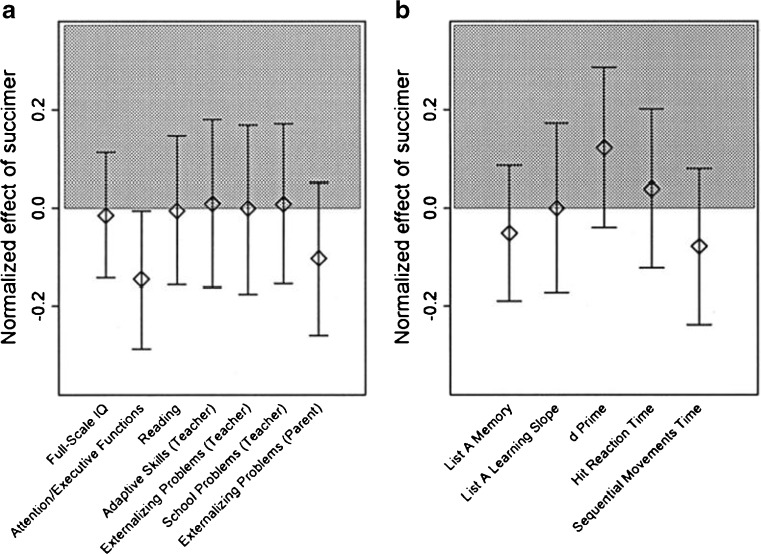
We did a preanalysis or predisclosure of the analysis results exercise in which we examined the correlation structure of these data. We did not want to simply examine all of the individual tests for a succimer effect, as that ruins type 1 error validity. That is, it’s very likely to have false positive results as a consequence of multiple testing. We reduced a large number of psychological tests to a smaller set that were orthogonal to each other so we were not testing the same hypothesis repeatedly, but calling it different names. There was still a little bit of multiple testing outcome. At one point, the placebo group did a little bit better for attention and executive function, but otherwise, everything was still flat. There was no improvement with succimer on any of the scales for cognition, behavior, or neuromotor speed or function (Fig. 5).
Fig. 5.
Follow-up at age 7 of neurocognitive impact of succimer treatment compared with placebo from the TLC study. Difference between succimer-treated and placebo groups are depicted by the shading, with improvement in the succimer group shaded gray. No statistically significant differences were found other than marginal improvement in attention/executive functions with placebo. Adapted from Reference [4] [used with permission from Pediatrics]
The only difference between the succimer and the placebo arms of the trial were that 41 % of families found it difficult to give succimer to their children as opposed to the 20 % of families who found it difficult to give placebo. Succimer has a terrible, sulfide smell. We tried to mimic the odor in the placebo, but it didn’t have as strong an odor as the full drug.
These findings are in the published papers [2, 4]. There were several additional interesting findings that we encountered in the TLC. The laboratory abnormalities identified in succimer’s package insert, such as elevated transaminases [5], occurred—but did not happen more frequently in succimer-treated children. This was a surprising finding. Another unexpected finding was that succimer did not prevent abrupt increases in BLL. Seven of the placebo children and 10 of the succimer children exceeded 44 mcg/dL and thus had to have their blinded treatment broken. A child who exceeded 44 mcg/dL on succimer went in the hospital and was given EDTA, and a child who was on placebo and exceeded 44 mcg/dL was given succimer. It mattered in terms of the treatment so we removed the blinding; but this rise while “on treatment” occurred at the same frequency in the two groups. An unexplained increase in trauma admissions occurred in the succimer-treated group. It was not statistically significant, but there were five versus zero in the placebo group. There were two head injuries, a burn, a near drowning, and a throat laceration. They had no common thread of any sort in the data that we saw. The history and physical examination data showed 15 % of the succimer children versus 10 % of the placebo children had evidence of trauma that included bruises, burn, scratches, and scrapes. Lead-exposed children have activity and behavioral disorders, but everyone in the TLC was lead-exposed. We were unable to draw any conclusions about the increase in trauma findings or admissions; these presumably represent statistical variations.
Role of Chelation in Lead Poisoning Treatment
Succimer is labeled for lead poisoning, but it is specifically labeled for use in children with BLLs greater than 45 mcg/dL. This designation likely enabled succimer to be granted orphan drug status when the drug was introduced in 1989. At that time, a BLL of 45 mcg/dL made lead poisoning a disease or disorder that affected fewer than 200,000 people in the USA, which is the definition of an orphan drug. It was easier to obtain approval for orphan drugs. Phase III trials supported by McNeil and the National Institute of Environmental Health Sciences, showed safety and efficacy for reduction of BLL only. They were not open trials. EDTA is labeled for children and adults for reduction of lead burden at no specific level, and dimercaprol is labeled as an adjunct to EDTA in adults and children. These are the three drugs that have lead as a treatment indication on the label in the USA.
The recommended succimer regimen is 350 mg/m2 per dose with a conversion for a 5 year old printed on the label in milligram per kilogram [5]. For young children, like 2 year olds who are commonly treated, the dose based on surface area is substantially larger than the dose based on weight. This is significant when calculating the dose for children who would not have the same dose until they were 5 years old.
There are no active recommendations on correct dosing for chelation in lead poisoning. Current treatment regimens are based on Julian Chisolm’s treatment regimens from several decades ago, which were published in the 1995 guidelines for the American Academy of Pediatrics Committee on Drugs in a statement that is now retired. The 1991 guidelines from the CDC in preventing lead poisoning in young children are now out of print. They offer clinical evidence rather than formal trials; there was no blinding. There is a Cochrane Collaborative Review on household dust reduction that has been published and updated [6], but it does not address chelation treatment of lead poisoning. There is also a World Health Organization monograph on succimer [7], but not on the general pharmacological approach.
Conclusion
Children with blood lead levels between 20 and 44 mcg/dL treated with succimer did not have better scores on cognitive, neuropsychological, or behavioral tests at 36 months of follow-up when they were 5 or 7 to 7 and a half years old. Additionally, while there is evidence that succimer is effective at acutely lowering blood lead levels in children, there are no current guidelines for appropriate treatment regimens. Clinically, the reasonable inference is that the way to prevent lead-associated defects is to prevent lead exposure.
Acknowledgements and Funding
The Treatment of Lead-Exposed Children Trial was supported by the intramural research program of the NIH/NIEHS, the NIH Office on Minority Health, and the CDC. Unlabeled CHEMET® (succimer) and placebo for succimer capsules were an unrestricted gift from McNeil Consumer Products. A representative from McNeil was invited to attend TLC meetings as deemed appropriate by the TLC steering committee, but had no role in interpretation of results.
ATSDR Disclaimer
This publication was supported by the cooperative agreement award number 1U61TS000117-04 from the Agency for Toxic Substances and Disease Registry (ATSDR). Its contents are the responsibility of the authors and do not necessarily represent the official views of the Agency for Toxic Substances and Disease Registry (ATSDR).
Conflict delineations
For the work under consideration for publication, Dr. McKay received a consulting fee/honorarium and reimbursement for travel through the ACMT/ATSDR Cooperative Agreement. Relevant financial activities outside the submitted work included money paid by the state DPH and various private attorney’s firms to Dr. McKay and to Dr. McKay’s institution for consulting on cases involving chelation issues. Dr. McKay is on the Scientific Advisory Council for the Environmental Health Research Foundation, which addresses issues related to biomonitoring for environmental chemicals.
Footnotes
Editor’s Note
This paper is derived from a talk given by Walter Rogan, MD at the ACMT “Use and Misuse of Metal Chelation Therapy” held at the Atlanta GA CDC Conference Center February 29, 2012. Dr. Rogan has subsequently retired, and was unavailable to edit the transcript. He gave us his permission to do so, and requested that we take editorial responsibility.
References
- 1.Meyer PA, Pivetz T, Dignam TA, Homa DM, Schoonover J, Brody D. Surveillance for elevated blood lead levels among children – United States, 1997–2001. MMWR 2003;52(SS10):1–21. http://www.cdc.gov/mmwr/preview/mmwrhtml/ss5210a1.htm Accessed 30 Aug 2013 [PubMed]
- 2.Rogan WJ, Dietrich KN, Ware JH, Dockery DW, Salganik M, et al. The effect of chelation therapy with succimer on neuropsychological development in children exposed to lead. N Engl J Med 2001;344(19):1421–1426. http://www.nejm.org/doi/pdf/10.1056/NEJM200105103441902 Accessed 30 Aug 2013 [DOI] [PubMed]
- 3.The Treatment of Lead-exposed Children (TLC) trial: design and recruitment for a study of the effect of oral chelation on growth and development in toddlers. Paediatr Perinat Epidemiol 1998;12(3):313–333 [DOI] [PubMed]
- 4.Dietrich KN, Ware JH, Salganik M, Radcliffe J, Rogan WJ, et al. Effect of chelation therapy on the neuropsychological and behavioral development of lead-exposed children after school entry. Pediatrics. 2004;114(1):19–26. doi: 10.1542/peds.114.1.19. [DOI] [PubMed] [Google Scholar]
- 5.CHEMET® (succimer) Package Insert. Lundbeck LLC. Last accessed at: http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=0103deca-7f23-40bf-bf39-b89b16b599b2 Accessed 30 Aug 2013
- 6.Yeoh B, Woolfenden S, Lanphear B, Ridley GF, Livingstone N. Household interventions for preventing domestic lead exposure in children. Cochrane Database Syst Rev 2012;18: (4). CD006047. doi:10.1002/14651858.CD006047.pub3 Accessed 30 Aug 2013 [DOI] [PubMed]
- 7.Volans GN, Karalliedde L, Wiseman HM. Review of succimer for treatment of lead poisoning. World Health Organization, 2010. Last accessed at: http://www.who.int/selection_medicines/committees/expert/18/applications/succimer.pdf Accessed 30 Aug 2013