Abstract
Chelation for heavy metal intoxication began more than 70 years ago with the development of British anti-lewisite (BAL; dimercaprol) in wartime Britain as a potential antidote the arsenical warfare agent lewisite (dichloro[2-chlorovinyl]arsine). DMPS (unithiol) and DMSA (succimer), dithiol water-soluble analogs of BAL, were developed in the Soviet Union and China in the late 1950s. These three agents have remained the mainstay of chelation treatment of arsenic and mercury intoxication for more than half a century. Animal experiments and in some instances human data indicate that the dithiol chelators enhance arsenic and mercury excretion. Controlled animal experiments support a therapeutic role for these chelators in the prompt treatment of acute poisoning by arsenic and inorganic mercury salts. Treatment should be initiated as rapidly as possible (within minutes to a few hours), as efficacy declines or disappears as the time interval between metal exposure and onset of chelation increases. DMPS and DMSA, which have a higher therapeutic index than BAL and do not redistribute arsenic or mercury to the brain, offer advantages in clinical practice. Although chelation following chronic exposure to inorganic arsenic and inorganic mercury may accelerate metal excretion and diminish metal burden in some organs, potential therapeutic efficacy in terms of decreased morbidity and mortality is largely unestablished in cases of chronic metal intoxication.
Keywords: Chelating agents, Unithiol, Succimer, Dimercaprol, Arsenic, Mercury
The First Chelator in Medicine: an Historical Perspective
Because the use of chelation in medicine began with the treatment of arsenic and then mercury poisoning, this brief review of the clinical utility of chelation for intoxication by these metals appropriately begins with a historical note. The battlefields of World War I saw the weaponization of several toxic gases, notably chlorine, phosgene, and mustard gas. As America prepared for its eventual entry into the fray, chemistry laboratories at some universities, including the chemical weapons unit at Catholic University in Washington, DC, set about to develop a toxic warfare agent intended to be more lethal and rapid in onset than mustard gas [1]. The chemist who directed the unit, Capt. Winford Lewis, Ph.D., learned of the earlier thesis work of Catholic University doctoral candidate Father Julius Nieuwland, which focused on the chemical reactions of acetylene. As part of his thesis experiments in the early 1900s, Nieuwland reacted acetylene with arsenic trichloride. Nieuwland did not precisely identity the reaction product but he became acutely aware of its toxicity when contact with its vapors hospitalized him for a few days. Nieuwland abandoned further work on the reaction product because of this toxicity, but 15 years later his former thesis advisor recalled the experiments and suggested to Lewis that it might be investigated as a potential chemical weapon. Lewis complied and isolated the potent vesicant and respiratory irritant dichloro(2-chlorovinyl)arsine. Eventually known as “lewisite,” the agent was never used on World War I battlefields, but it was stockpiled as a chemical weapon by the USA and the axis powers prior to World War II.
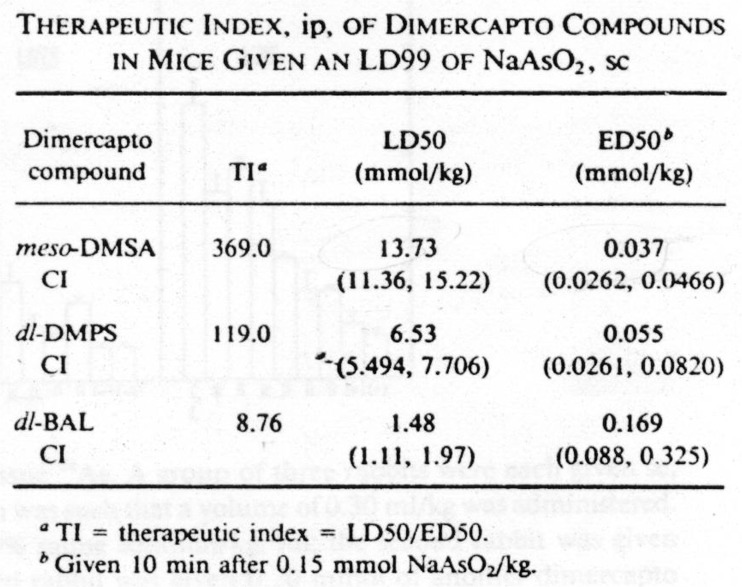
As conventional explosives fell on Britain during World War II, a team of biochemists at Oxford University—RA Peters, LA Stocken, and RHS Thompson—worked intensively to develop an antidote to lewisite. Building upon knowledge that inorganic arsenic and arsenical pharmaceuticals, as well as lewisite, appeared to inhibit the action of the pyruvate dehydrogenase enzyme complex by combining with vicinal thiol groups, the Oxford team synthesized and tested multiple thiol compounds as potential antidotes. They found that the oily dithiol compound 2,3-dimercapto-propanol was the best at reversing lewisite inhibition of aerobic respiration in epithelial cells and at enhancing survival in lewisite poisoned rats (Table 1) [2]. During the war, they shared their secret findings with colleagues in the USA, who referred to the new drug as “British anti-lewisite” or BAL. BAL (also known as dimercaprol) proved promising in unpublished experiments on the vesicant action of lewisite in human volunteers and in workers exposed during wartime factory accidents [3, 4].
Table 1.
Efficacy of BAL in experimental Lewisite poisoning
| Percent decline in O2 uptake (skin cells + pyruvate tissue culture) after Lewisite (0.03 mM), 1 h | |
| Lewisite | 50 % |
| Lewisite + 2-mercaptoethanol (0.54 mmol) | 55 |
| Lewisite + BAL (0.27 mmol) | 6 |
| Survival (rats) after topical lewisite (≈35 mg/kg) | |
| Treatment begun at 30 min post-exposure | |
| Untreated | 0/27 |
| 2-Mercaptoethanol | 0/6 |
| BAL (50–70 mg/kg inunction) | 21/21 |
Data from Ref. [2]
Studies of the Clinical Efficacy of BAL in Arsenic Intoxication
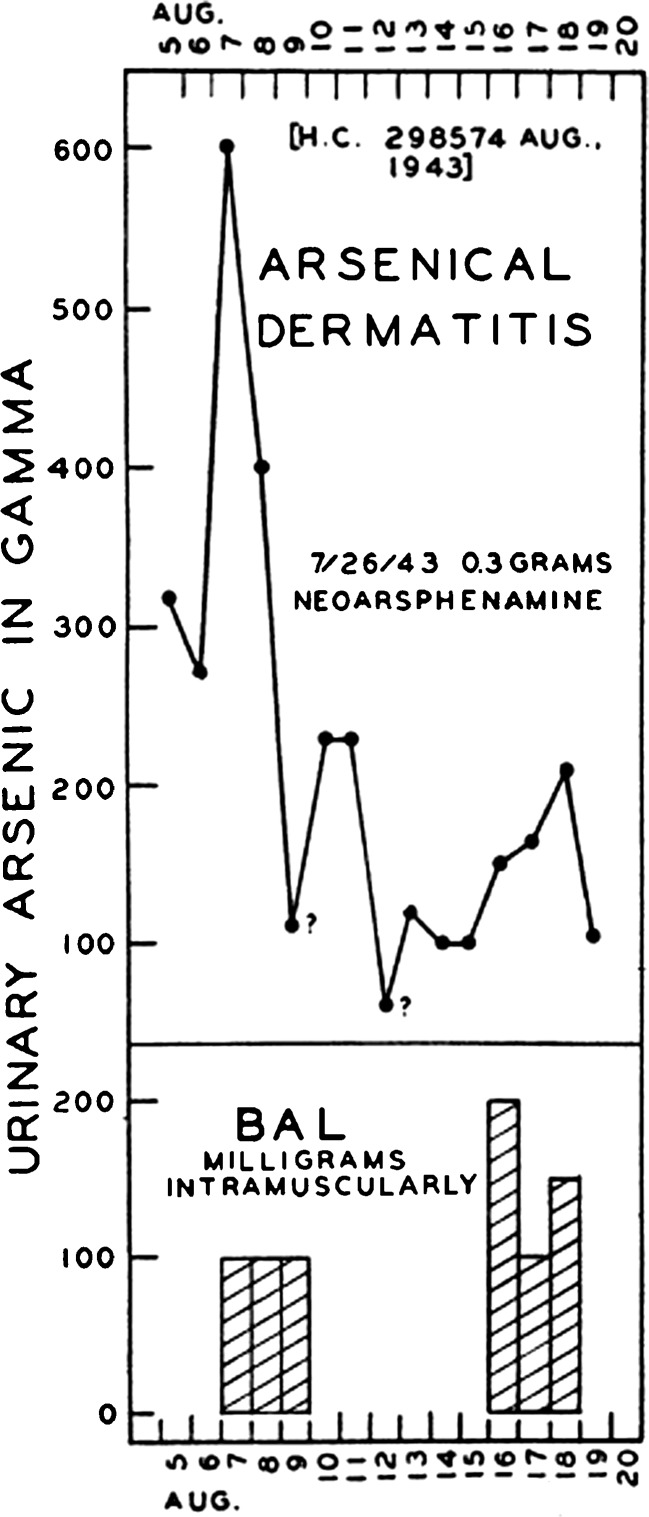
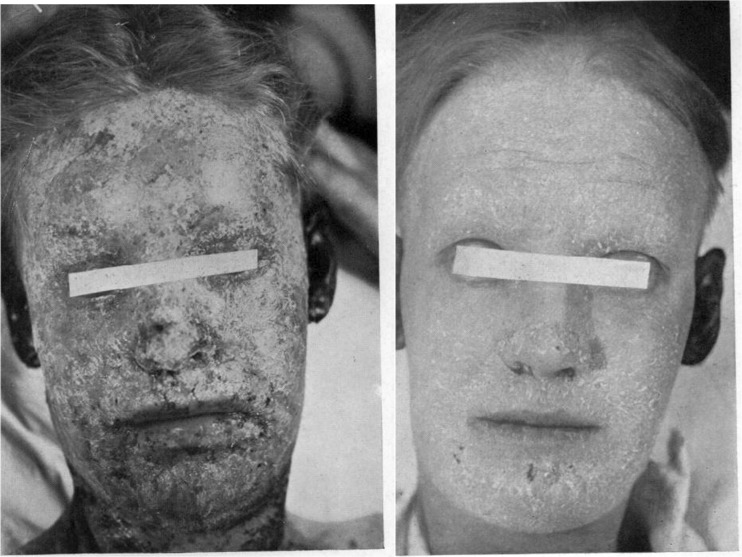
Although wartime use of lewisite that might have required battlefield trials of BAL by the Allies fortunately never transpired, soldiers and others of the day did experience the adverse effects of another class of organoarsenical, namely the many arsenic-based antibiotics in use since the early twentieth century to treat syphilis. Arsenical antibiotics such as neoarsphenamine administered in relatively high doses to treat syphilis were not infrequently accompanied by adverse effects such as dermatitis and hepatotoxicity. Attempts by wartime physicians to ameliorate arsenical dermatitis with BAL met with some clinical success. BAL was associated with large increases in urinary arsenic excretion in patients with arsenical dermatitis (Fig. 1) [5]. A case series with historical controls found that a 6-day course of BAL therapy decreased the mean duration of arsenical dermatitis by two thirds, from 62.5 to 21.5 days (Fig. 2) [6].
Fig. 1.
Effect of BAL i.m. on urinary arsenic excretion in patient with arsenical dermatitis after neoarsphenamine. Gamma = micrograms. (Luetscher [5]) [Reproduced from the Journal of Clinical Investigation with the permission of the American Society For Clinical Investigation via Copyright Clearance Center.]
Fig. 2.
In a case series with historical controls, BAL treatment of arsenical dermatitis from anti-syphilis therapy resulted in a mean decrease in the duration of rash from 62.5 to 21.5 days. Female patient at left is shown at time of peak dermatitis. Photo at right is of same patient, with rash markedly improved, 14 days after start of a 6-day course of intramuscular BAL. (Carleton [6]) [Reproduced from Quart J Med with permission from Oxford University Press.]
After BAL was introduced into the US pharmacopeia in the late 1940s, a few studies examined its efficacy in the treatment of intoxication by inorganic arsenic. One of the very few that examined efficacy in a controlled manner, albeit using historical controls, was conducted in pediatric patients with inorganic arsenic at Charity Hospital in New Orleans [7]. In 111 patients with inorganic arsenic exposure who received only supportive care, the average length of stay was 4.2 days, and there were three deaths; 46.2 % were symptomatic on admission and 29.3 % were symptomatic at 12 h. By comparison, in 42 patients treated with BAL, the average length of stay was 1.6 days, and there were no deaths; 47.6 % were symptomatic on admission, 0 % at 12 h. The small number of patients symptomatic on admission and at 12 h indicates that this study was dominated by patients with low dose arsenic exposure—a suboptimal population in which to examine clinical efficacy. Yet, the foregoing experience is reflective of the very limited manner in which the efficacy of BAL in arsenic poisoning was critically studied in humans.
Ampoules of BAL in peanut oil have remained on the US formulary for the intramuscular treatment of arsenic intoxication for more than 65 years. At high therapeutic doses, 4 to 5 mg/kg every 4 to 6 h, as many as two thirds of patients experience adverse effects. Nausea and vomiting are most common, but there may also be hypertension, lacrimation, and salivation, as well as pain associated with the repeated deep intramuscular injections. It should be used with extreme caution, if at all, in patients with peanut allergy.
Water-Soluble Analogs of BAL: DMPS (Unithiol) and DMSA (Succimer)
Alternatives to BAL were developed in the 1950s in the Soviet Union and China. Perhaps because of language barriers and cold war era limitations on information exchange, the recognition of these drugs in the West was limited for many years. Petrunkin in the Ukraine developed an analog of BAL, 2,3-dimercaptopropane sulfonic acid, also known as unithiol or DMPS (Fig. 3) [8]. It contains the two vicinal thiol groups found in BAL; however, unlike the hydroxyl group in BAL, the sulfonic acid group in DMPS renders the compound water soluble. Unlike BAL, it could therefore be administered intravenously as well as orally. Although unithiol (Унитиол in Russian) may seem like a misnomer for a dithiol compound, Russian colleagues have said the name reflected the drug’s universal utility in metal poisonings such as arsenic, lead, and mercury, as well as a variety of other conditions. Dimercaptosuccinic acid, known as succimer or DMSA (Fig. 3), was developed by Liang and Ding (formerly Ting) and colleagues in Shanghai, China in the late 1950s [9]. Professor Ding remains an active pharmacologist at age 92 years and recently contributed to a draft WHO monograph on succimer.
Fig. 3.
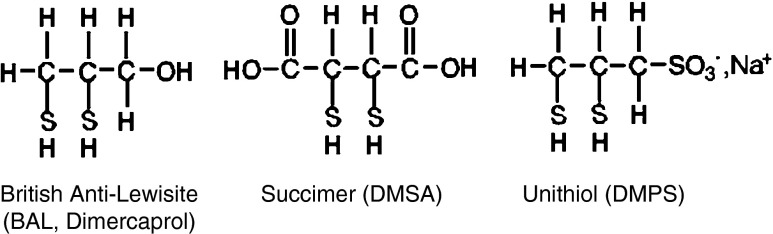
Structures of dithiol chelating drugs
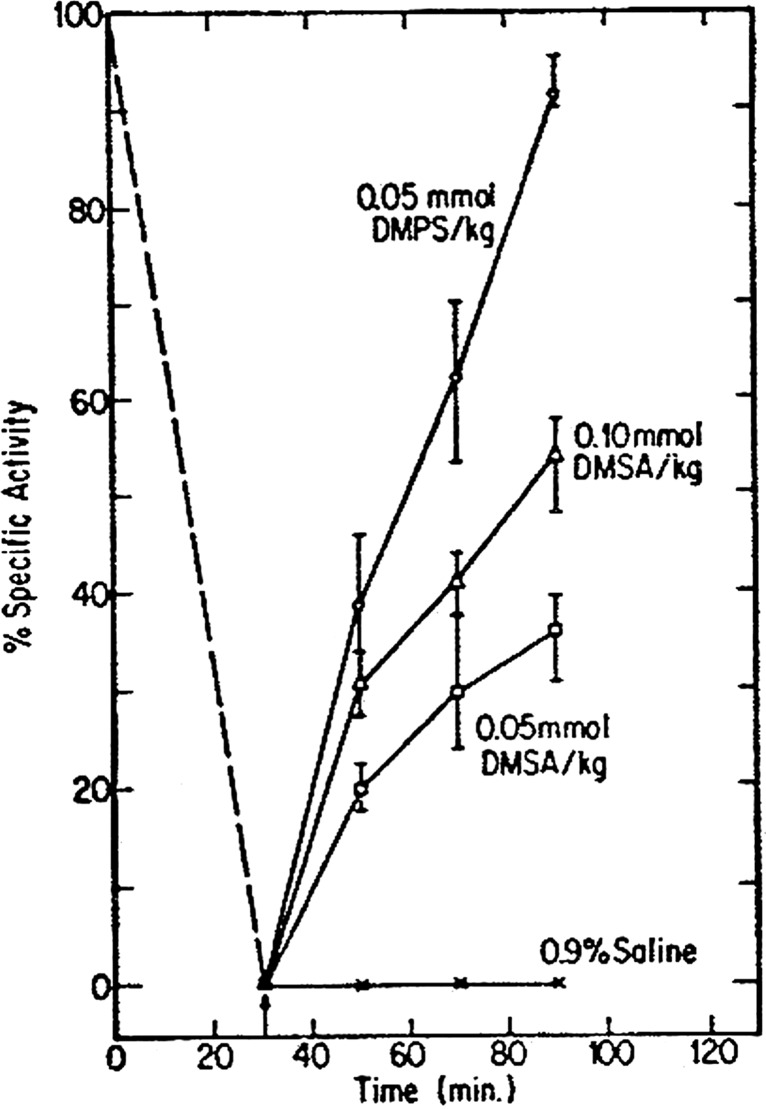
Animal experiments have established that prompt treatment with BAL, DMPS, and DMSA can avert the lethal effects of inorganic arsenic (e.g., sodium arsenite). As demonstrated by Aposhian and colleagues in a series of publications, DMPS and DMSA each has lower toxicity, and considerably higher therapeutic index, than BAL when used to treat acute arsenic poisoning (Fig. 4) [10]. Aposhian’s team also demonstrated that DMPS and DMSA reversed, in vivo, the inhibitory effect of arsenite on pyruvate dehydrogenase, with DMPS exhibiting greater antidotal potency in this regard (Fig. 5) [10].
Fig. 4.
Aposhian [10]. [Reproduced from Fundamental and Applied Toxicology with permission from Elsevier.]
Fig. 5.
Reversal, in vivo, of arsenite inhibition of renal pyruvate dehydrogenase activity by DMPS and DMSA. Mice were injected sc with sodium arsenite (0.10 mmol/kg) and 30 min later (as noted by arrow), DMPS, DMSA, or saline was injected i.p. Groups of three mice were sacrificed at specific times. Kidneys were removed and renal pyruvate dehydrogenase activity was assayed in tissue extracts. (Aposhian [10]) [Reproduced from Fundamental and Applied Toxicology with permission from Elsevier.]
The animal data on chelation for acute arsenic intoxication established the important principle that the efficacy of chelation is greatest when it is administered promptly (minutes to hours) after arsenic exposure. As the time interval between arsenic exposure and chelator increased, efficacy declined. Delayed chelation is diminished chelation. For example, in rabbits, a single injection of BAL 5 min after exposure to an organoarsenical resulted in 100 % survival, compared to no survival when treatment was initiated after an interval of 6 h [11]. In like manner, after exposure of mice to subcutaneous arsenite (0.14 mmol/kg sc), DMSA (0.25 mmol/kg, ip) resulted in 79 % survival if first given at 60 min and 55 % survival if first given at 120 min [12].
The extensive arsenic exposure arising from geogenic contamination of the aquifers used for drinking water in West Bengal, India, was the setting for one of the only controlled trials of chelation for chronic arsenic intoxication [13]. Guha Mazumder and colleagues conducted a randomized, single-blind, placebo-controlled trial of DMPS in 21 adults with chronic arsenic ingestion (average duration of exposure 20 years). All subjects had dermal manifestations of chronic arsenicism and had been removed from exposure for less than 3 months. Average urine arsenic concentration at the inception of the trial was 46 μg/L. Eleven inpatients received four 1-week courses of DMPS 100 mg qid over a 7-week period; ten inpatients received placebo. The primary outcome variable was the change in a single-blind clinical score that aggregated the findings of multiple signs and symptoms. The clinical score of both groups improved, with a statistically greater change in the DMPS group. Several aspects of the study design render findings of a therapeutic benefit of DMPS inconclusive, particularly the limitation that more than half of the clinical improvement was based on evaluation of relatively subjective endpoints such as weakness and dyspnea by a nonblinded clinical observer. In addition, the groups were unbalanced by gender (a factor in symptom reporting), and the impact of nonblinded, nonrandomized “symptomatic treatment” (e.g., bronchodilators) was not evaluated. A fully blinded skin biopsy evaluation by a pathologist failed to confirm the improvement in skin findings assessed by the clinical observer. A similar study conducted by Guha Mazumder et. al reported no benefit of DMSA in chronic arsenic intoxication [14].
Chelation for Mercury Intoxication
Hazardous exposure to mercury in its myriad forms has long been a major focus of clinical toxicology. Episodes of environmental exposure to elemental mercury vapor have remained a leading precipitant of emergency response by the US Agency for Toxic Substances and Disease Registry for many years [15]. Experience in the use of chelation to treat acute intoxication by mercury dates from the late 1940s, when BAL was first used in the treatment of patients acutely poisoned by ingestion of mercuric chloride. Also known as “corrosive sublimate” and sold in pills in the shape of coffins as a warning, this antiseptic chemical was widely used as a means of suicide in the 1920s through 1940s. In 1949, Longcope and Luetscher of Johns Hopkins reported their favorable experience using BAL in a large case series of patients with mercuric chloride intoxication [16]. Prior to the availability of BAL, of 86 patients who presented after consuming ≥1 g of mercuric chloride and treated by conventional supportive methods within 4 h, there were 27 deaths, or a case mortality rate of 31.4 %. By comparison, of 41 patients consuming ≥1 g of mercuric chloride and treated by BAL within 4 h, there were no deaths. This impressive positive finding was prominently reported in the Annals of Internal Medicine.
Animal experiments support the efficacy of dithiol chelators used promptly in the treatment of acute intoxication by inorganic mercury salts such as mercuric chloride. For example, Neilson and Anderson in 1990 treated rats poisoned with a large dose of mercuric chloride (109 mg/kg po) with either BAL (ip), DMSA (po), or DMPS (po) within 15 min. Nine of ten control rats receiving just the mercuric chloride died. A protective effect of a single (albeit quite large) dose of the chelators was evident, particularly in the case of DMPS, which saved all the animals (Table 2) [17]. In a lower dose rat model examining acute nephrotoxicity of mercuric chloride (1.4 mg/kg iv), immediate treatment with DMPS (54 mg/kg iv) averted oliguric renal failure. However, if the DMPS were administered after a delay of 24 h, the protective effect was lost (Table 3) [18]. The time dependence of efficacy was also shown in another rat model of mercuric chloride intoxication (1 mg/kg iv) [19]. Untreated, the mercuric chloride dose (1 mg/kg iv) yielded mortality in 8 of 18 animals (44 %) within 30 days, with mean survival time of 19 days. If DMPS (32 mg/kg [150 micromole] po qd × 5 days) were begun at 6 h following mercury exposure, the mortality declined to 1 of 18 (6 %), with survival time of 29 days in the one expiring animal. However, if the same DMPS regimen was instituted at 24 h post-exposure, mortality was 6 of 18 (33 %), with mean survival of 22 days.
Table 2.
Dithiol chelators in acute mercuric chloride intoxication in rats
| Chelator | Dose | Mortality | Percent |
|---|---|---|---|
| Control | 9/10 | 90 | |
| BAL (i.p.) | 400 μM (50 mg/kg) | 5/10 | 50 |
| DMSA (p.o.) | 1,600 μM (291 mg/kg) | 4/10 | 40 |
| DMPS (p.o.) | 1,600 μM (336 mg/kg) | 0/10 | 0 |
HgCl2 (109 mg/kg p.o.) was given to rats. A single dose of chelator was given 15 min later. Mortality was assessed within 14 days. (Data from Ref. [17])
Table 3.
Immediate DMPS prevents oliguric renal failure from i.v. HgCl2 in rats
| Urine volume (ml) | |||
|---|---|---|---|
| No DMPS | Immediate DMPS | DMPS after 24 h | |
| Day 1 | 14.5 | 10.0 | 14.5 |
| Day 2 | 0.7 | 6.0 | 1.0 |
| Day 3 | 4.0 | 15.5 | 7.0 |
| Histopathological tissue damage | + | − | + |
Rats (n = 4 per group) were given HgCl2 1.4 mg/kg (5 μmol) iv. DMPS 54 mg/kg (250 μmol) iv was given immediately or after an interval of 24 h. Urine output and renal histopathology were evaluated. (Data from Ref. [18])
DMPS was more potent than DMSA in reducing renal mercury content in rats after acute exposure to mercuric chloride (0.67 mg/kg iv) [20]. Equimolar regimens (100 μmol/kg ip 4×/week for 4 weeks) of DMSA or DMPS were begun 24 h post-exposure. In control rats receiving only the mercuric chloride, renal mercury content as a percent of administered radiolabeled mercury dose was 11.57 ± 0.04 % at the end of 4 weeks. By comparison, it declined to 5.73 ± 1.02 % of administered dose in the animals receiving DMSA and 0.71 ± 0.14 % of administered dose in the animals treated with DMPS (n = 6 animals per group).
Mercury and Arsenic Chelation: Limitations and Concerns
DMPS and DMSA are relatively well tolerated. Allergic reactions, predominantly skin rashes and exanthems, have been reported to affect between 1 to 10 % of subjects in some but not all studies. Mild gastrointestinal complaints may also occur in a minority of patients, and there are isolated reports of mild reversible increases in hepatic transaminases or decreases in white blood cell count. In the large randomized, placebo-controlled TLC study of pediatric lead chelation with DMSA (n = 780), there were no statistically significant differences in adverse signs or symptoms between the treatment and placebo groups [21]. DMPS and DMSA may result in increased excretion of zinc and copper, an effect of uncertain clinical significance. DMSA has been associated with adverse neurocognitive effects when administered to juvenile animals not overexposed to toxic metals [22].
Because the efficacy of chelation for acute symptomatic arsenic and inorganic mercury intoxication declines as the time interval between exposure and inception of treatment increases, a prompt initial dose of the chelator should be administered when diagnostic suspicion is high and should not be delayed for the days often required to obtain laboratory confirmation.
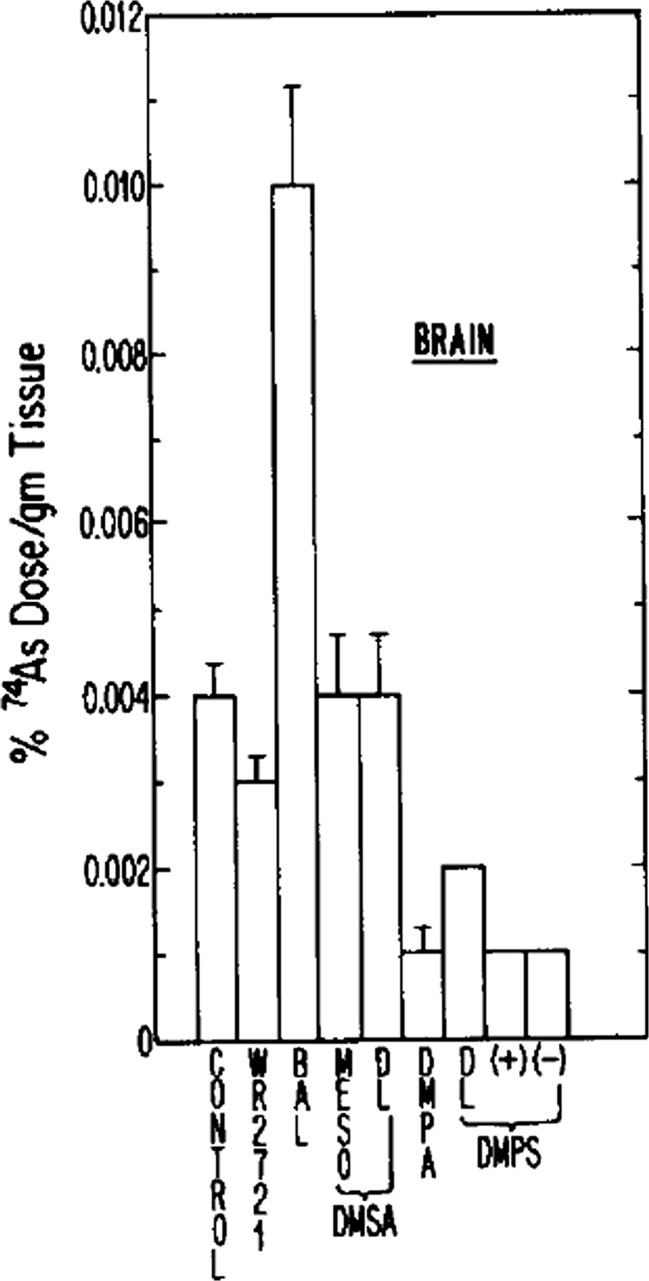
A caveat to consider in the use of chelating agents is that metal mobilization does not always equal metal excretion. Our understanding of how certain chelating agents may contribute to the redistribution of specific metals within the human body is incomplete. The net redistribution of tissue metal deposits, even when accompanied by increased excretion, might have undesirable consequences. This caveat is perhaps best demonstrated with BAL. Although BAL may exert protection against life-threatening effects of arsenic and inorganic mercury, it may at the same time redistribute a portion of these toxic metals to the brain, where subtler long-term adverse effects could conceivably occur. Aposhian and co-workers examined how various chelators administered 24 h after a subcutaneous dose of radiolabeled arsenite influenced the distribution of the radiolabeled arsenite in the brain of rabbits. Compared to saline, BAL more than doubled the arsenic content of the brain, whereas DMPS yielded a 75 % decrease (Fig. 6) [10]. A similar impact of BAL in the redistribution of mercuric ion to the brain of mice given 203Hg-radiolabeled phenylmercuric acetate has also been reported (Fig. 7) [23].
Fig. 6.
BAL redistributes arsenic to the brain of rabbits. Rabbits were injected with 25 μmol/kg sc radiolabeled sodium arsenite (74As), followed in 1 h by saline (control) or various antidotes (0.2 mmol/kg i.m.) (n = 3 per treatment group). At 24 h, the animals were sacrificed and the amount of 74As in the brain as a percent of administered dose was measured. Note that BAL more than doubled the arsenic content of the brain. DMPS yielded a substantial decrease in brain arsenic. (Aposhian [10]) [Reproduced from Fundamental and Applied Toxicology with permission from Elsevier.]
Fig. 7.
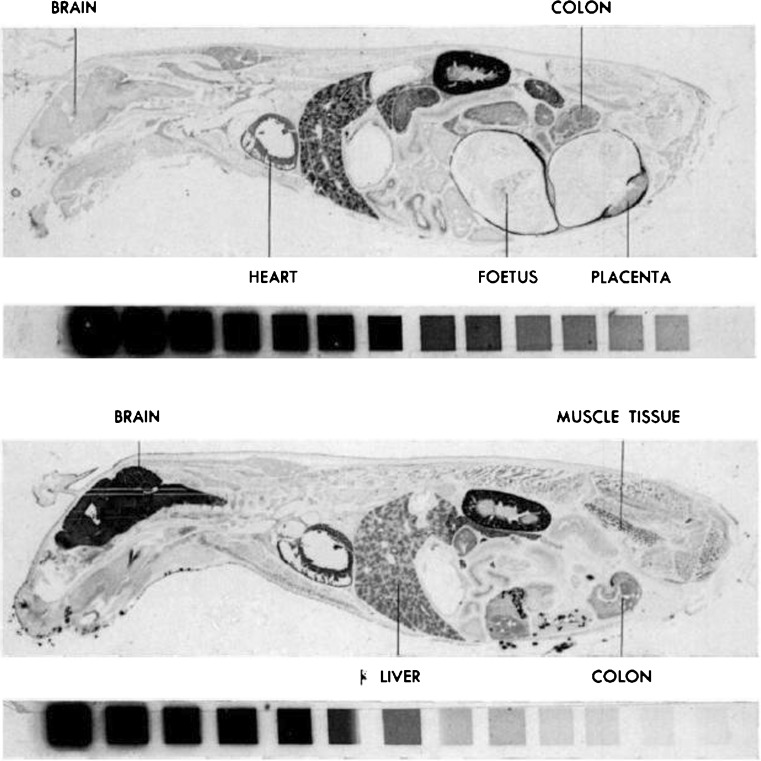
BAL redistributes mercuric ion to brain of mice. Autoradiograms of sagittal whole-body sections of mice 8 days after injection of phenylmercuric acetate (203Hg) (0.5 mg Hg/kg iv via tail vein) (upper panel) and phenylmercuric acetate (203Hg) (0.5 mg Hg/kg iv) plus simultaneous BAL 0.4 mg/kg (lower panel). The isotope reference scales accompanying the sections are shown; the activity ratio between adjacent steps is ½ (darker corresponds to more 203Hg). The upper panel is from a pregnant mouse. Note the prominent redistribution of 203Hg in the brain of the mouse administered BAL simultaneous with the phenylmercuric acetate. (Berlin [23]) [Reproduced from the Journal of Pharmacology and Experimental Therapeutics with permission from the American Society for Pharmacology and Experimental Therapeutics.]
The utility of chelation following acute overexposure to elemental mercury vapor is an important question in environmental and occupational toxicology. Cichini et. al had the opportunity to study the impact of DMPS and d-penicillamine on urine mercury excretion in 19 caisson workers who developed acute symptomatic intoxication from high dose elemental mercury vapor accidentally encountered when drilling a subway tunnel through rock containing natural elemental mercury deposits [24]. After having sustained a mean of 27 h of exposure (range ≈ 8 to 40 h), six patients were randomized to receive 600 mg DMPS per day p.o.; six received 300 mg DMPS per day p.o.; and seven received d-penicillamine 450 mg/day p.o. Baseline (pre-chelation) urine mercury concentration (all subjects) was 399 ± 98 μg/L. Measurement of 24 h urine mercury indicated that DMPS substantially increased mercury excretion, which over the course of a week of treatment was highest in the group receiving the 600 mg daily oral dose (mean ≈ 4,000 μg/24 h on day 2 of treatment). Interestingly, the study did not comment on the relative impact of the different chelation regimens on the workers’ neurological symptoms, an issue that has never been addressed in mercury vapor intoxication through a randomized clinical trial.
Animal studies have found that DMSA and DMPS are inefficient at reducing mercury content of the brain following inhalation exposure to elemental mercury vapor [25–27]. Buchet and Lauwerys conducted an elegant study of chelation with DMSA and DMPS in rats exposed to mercury vapor (244 μg/m3) by inhalation for 14 days [25]. As shown in the data in Table 4, the organ with the greatest mercury accumulation following mercury vapor exposure is the kidney, which is also the site of most mercury removal by chelation. The chelators did not reduce the mercury content of the brain, a key target organ in elemental vapor intoxication. This suggests that chelation might have limited utility in treating the neurological manifestations of acute or chronic overexposure to elemental mercury.
Table 4.
In subacute Hg vapor exposure, DMPS and DMSA reduce Hg concentration in kidneys not the brain
| Group | Hg concentration (μg/100 g body weight) | |
|---|---|---|
| Kidney | Brain | |
| Hg only (n = 8) | 2.78 ± 0.60 | 0.088 ± 0.017 |
| Hg + DMSA (n = 8) | 0.46 ± 0.20 | 0.076 ± 0.008 |
| Hg + DMPS (n = 8) | 0.10 ± 0.02 | 0.098 ± 0.030 |
| Control (n = 4) | 0.17 ± 0.15 | 0.0022 ± 0.0005 |
Three groups of rats (n = 8 per group) underwent 14 days of inhalation exposure to elemental mercury vapor (244 μg/m3). Seven days later, two groups were treated with 1 mmol/kg/day po DMSA (180 mg/kg/d) or DMPS (220 mg/kg/d) for 5 days and then sacrificed 24 h later. The 5-day chelation resulted in an extensive reduction in renal Hg, but brain Hg concentration was not reduced and remained elevated approximately 40-fold above the levels in the control rats. (Data from Ref. [25])
Experimental animal studies have established that DMPS and DMSA effectively reduce renal mercury content [28, 29]. In humans with chronic occupational exposure to mercury, administration of DMPS and DMSA greatly increase urinary mercury excretion [30, 31]. However, whether DMPS or DMSA might mitigate renal injury that has developed after chronic exposure to high doses of mercury has yet to be determined.
Conclusion
The chelating agents DMPS, DMSA, and BAL offer therapeutic benefit in acute intoxication by arsenic and inorganic mercury salts if administered promptly (within minutes to hours). DMPS and DMSA have a higher therapeutic index than BAL and, unlike BAL, do not redistribute arsenic or mercury to the brain. Although chelation for chronic intoxication by arsenic or mercury may accelerate metal excretion and diminish metal concentration in some tissues, potential therapeutic efficacy in terms of decreased morbidity and mortality for chronic metal intoxication is largely unestablished.
Acknowledgments
Acknowledgments
This publication was supported by the cooperative agreement award number 1U61TS000117-04 from the Agency for Toxic Substances and Disease Registry (ATSDR). Its contents are the responsibility of the authors and do not necessarily represent the official views of the Agency for Toxic Substances and Disease Registry (ATSDR).
Conflict of Interest
For the work under consideration for publication, Dr. Kosnett received an honorarium and reimbursement for travel through the ACMT/ATSDR Cooperative Agreement.
References
- 1.Vilensky JA. Dew of death: the story of Lewisite, America’s World War I weapon of mass destruction. Bloomington: Indiana University Press; 2002. [Google Scholar]
- 2.Stocken LA, Thompson RHS. British anti-lewisite. Dithiol compounds as antidotes for arsenic. Biochem J. 1946;40:535–548. doi: 10.1042/bj0400535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peters RA, Stocken LA, Thompson RHS. British anti-lewisite (BAL) Nature. 1945;156:616–619. doi: 10.1038/156616a0. [DOI] [PubMed] [Google Scholar]
- 4.Chiesman WE. Diagnosis and treatment of lesions due to vesicants. Br Med J. 1944;2(4359):109–112. doi: 10.1136/bmj.2.4359.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luetscher JA, Eagle H, Longcope WT, Watson EB. Clinical uses of 2,3-dimercaptopropanol (BAL). VIII. The effect of BAL on the excretion of arsenic in arsenical intoxication. J Clin Investig. 1946;25(4):534–540. doi: 10.1172/JCI101735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carleton AB, Peters RA, Thompson RHS. The treatment of arsenical dermatitis with dimercaptopropanol (BAL) Q J Med. 1948;17:49–79. [PubMed] [Google Scholar]
- 7.Woody NC, Kometani JT. BAL in the treatment of arsenic ingestion of children. Pediatrics. 1948;1(3):372–378. [PubMed] [Google Scholar]
- 8.Petrunkin VE. Synthesis and properties of dimercapto derivatives of alkylsulfonic acids. Ukr Khem Zh. 1956;22:603–607. [Google Scholar]
- 9.Liang Y, Chu C, Tsen Y, Ting K. Studies on antibilharzial drugs. VI. The antidotal effects of sodium dimercaptosuccinate and BAL-glucoside against tartar emetic. Acta Physiol Sin. 1957;21:24–32. [Google Scholar]
- 10.Aposhian HV, Carter DE, Hoover TD, Hsu CA, Maiorino RM, Stine E. DMSA, DMPS, and DMPA—as arsenic antidotes. Fundam Appl Toxicol. 1984;4:S58–S70. doi: 10.1016/0272-0590(84)90138-6. [DOI] [PubMed] [Google Scholar]
- 11.Eagle H, Magnuson HJ, Fleischman R. Clinical uses of 2,3 dimercaptopropanol (BAL). I. The systemic treatment of experimental arsenic poisoning (marphasen, lewisite, phenyl arsenoxide) with BAL. J Clin Investig. 1946;25:451–466. doi: 10.1172/JCI101728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tadlock CH, Aposhian HV. Protection of mice against the lethal effects of sodium arsenite by 2,3-dimercapto-1-propane-sulfonic acid and dimercaptosuccinic acid. Biochem Biophys Res Commun. 1980;94:501–507. doi: 10.1016/0006-291X(80)91259-0. [DOI] [PubMed] [Google Scholar]
- 13.Guha Mazumder DN, De BK, Santra A, Ghosh N, Das S, Lahiri S, Das T. Randomized placebo-controlled trial of 2,3-dimercapto-1-propanesulfonate (DMPS) in therapy of chronic arsenicosis due to drinking arsenic-contaminated water. Clin Toxicol. 2001;39:665–674. doi: 10.1081/CLT-100108507. [DOI] [PubMed] [Google Scholar]
- 14.Guha Mazumder DN, Ghoshal UC, Saha J, Santra A, De BK, Chatterjee A, et al. Randomized placebo-controlled trial of 2,3-dimercaptosuccinic acid in therapy of chronic arsenicosis due to drinking arsenic-contaminated subsoil water. Clin Toxicol. 1998;36:683–690. doi: 10.3109/15563659809162616. [DOI] [PubMed] [Google Scholar]
- 15.Nickle RA. Mercury—top of the hit parade for eight years. Drug Chem Toxicol. 1999;22(1):129–142. doi: 10.3109/01480549909029727. [DOI] [PubMed] [Google Scholar]
- 16.Longcope WT, Luetscher JA. The use of BAL (British Anti-Lewisite) in the treatment of the injurious effects of arsenic, mercury, and other metallic poisons. Ann Intern Med. 1949;31:545–553. doi: 10.7326/0003-4819-31-4-545. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen JB, Andersen O. Effect of four thiol-containing chelators on disposition of orally administered mercuric chloride. Hum Exp Toxicol. 1991;10:423–430. doi: 10.1177/096032719101000610. [DOI] [PubMed] [Google Scholar]
- 18.Wannag A, Aaseth A. The effect of immediate and delayed treatment with 2,3-dimercapto-propane-1-sulphonate on the distribution and toxicity of inorganic mercury in mice and in foetal and adult rats. Acta Pharmacol Toxicol. 1980;46:81–88. doi: 10.1111/j.1600-0773.1980.tb02425.x. [DOI] [PubMed] [Google Scholar]
- 19.Planas-Bohne F. The excretion of two renal enzymes as influenced by mercuric chloride and DMPS. In: Brown SS, editor. Clinical chemistry and chemical toxicology of metals. New York: Elsevier; 1977. pp. 119–122. [Google Scholar]
- 20.Planas-Bohne F. The effect of 2,3-dimercaptopropane-1-sulfonate and dimercaptosuccinic acid on the distribution and excretion of mercuric chloride in rats. Toxicology. 1981;18:275–278. doi: 10.1016/0300-483X(81)90138-4. [DOI] [PubMed] [Google Scholar]
- 21.Treatment of Lead-Exposed Children (TLC) Trial Group Safety and efficacy of succimer in toddlers with blood lead levels of 20–44 Microg/dL. Pediatr Res. 2000;48(5):593–599. doi: 10.1203/00006450-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Smith DR. The scientific basis for chelation: animal studies and lead chelation. J Med Toxicol 2013;9(4). doi:10.1007/s13181-013-0339-2 [DOI] [PMC free article] [PubMed]
- 23.Berlin M, Rylander R. Increased brain uptake of mercury induced by 2,3-dimercaptopropanol (BAL) in mice exposed to phenylmercuric acetate. J Pharmacol Exp Ther. 1964;146:236–240. [PubMed] [Google Scholar]
- 24.Cichini GM, Petzl DH, Zeitlhofer J, Wolf C, Meisinger V, Strasser K, et al. Effekt von DMPS und d-Penicillamin bei inhalativer Intoxikation mit metallischem Quecksilber. Intensivmed Notf Med. 1989;26(6):303–306. [Google Scholar]
- 25.Buchet JP, Lauwerys RR. Influence of 2,3 dimercaptopropane-1-sulfonate and dimercaptosuccinic acid on the mobilization of mercury from tissues of rats pretreated with mercuric chloride, phenylmercury acetate or mercury vapors. Toxicology. 1989;54:323–333. doi: 10.1016/0300-483X(89)90067-X. [DOI] [PubMed] [Google Scholar]
- 26.Nerudová J, Cábelková Z, Frantík E, Lukás E, Urban P, Bláha K, et al. Mobilization of mercury by DMPS in occupationally exposed workers and in model experiments on rats: evaluation of body burden. Int J Occup Med Environ Health. 2000;13:131–146. [PubMed] [Google Scholar]
- 27.Aposhian HV, Morgan DL, Queen HLS, Maiorino RM, Aposhian MM. Vitamin C, glutathione, or lipoic acid did not decrease brain or kidney mercury in rats exposed to mercury vapor. Clin Toxicol. 2003;41:339–347. doi: 10.1081/CLT-120022000. [DOI] [PubMed] [Google Scholar]
- 28.Bridges CC, Joshee L, Zalups RK. Multi-drug resistance proteins and the renal elimination of inorganic mercury mediated by 2,3-dimercaptopropane-1-sulfonic acid and meso-2,3-dimercaptosuccinic acid. J Pharmacol Exp Ther. 2008;324:383–390. doi: 10.1124/jpet.107.130708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zalups RK, Bridges CC. Relationships between the renal handling of DMPS and DMSA and the renal handling of mercury. Chem Res Toxicol. 2012;25:1825–1838. doi: 10.1021/tx3001847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roels HA, Boeck M, Ceulemans E, Lauwerys RR. Urinary excretion of mercury after occupational exposure to mecury vapour and influence of the chelating agent meso-2,3-dimercaptosuccinic acid (DMSA) Brit J Ind Med. 1991;48:247–253. doi: 10.1136/oem.48.4.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonzalez-Ramirez D, Zuniga-Charles M, Narro-Juarez A, Molina-Recio Y, Hurlbut KM, Dart RC, Aposhian HV. DMPS (2,3-dimercaptopropane-1-sulfonate, Dimaval) decreases the body burden of mercury in humans exposed to mercurous chloride. J Pharmacol Exp Ther. 1998;287:8–12. [PubMed] [Google Scholar]