Abstract
“Urine mobilization test,” “challenge test,” and “provoked urine test” are all terms used to describe the administration of a chelating agent to a person prior to collection of their urine to test for metals. There is no standard, validated challenge test. Despite recommendations by professional and government organizations against the use of provoked urine testing, the tests are still commonly used and recommended by some practitioners. Challenge testing utilizes a variety of chelating agents, including dimercaptosuccinic acid (DMSA), dimercaptopropanesulfonate (DMPS), and ethylenediaminetetraacetic acid (EDTA). The agents are given by a variety of routes of administration, doses used are inconsistent, and urine collection procedures vary. Additional problems with challenge tests include comparison of results to inappropriate reference ranges and creatinine correction of urine obtained within hours of chelator administration. Human volunteer studies demonstrate that mercury is detected in the urine of most people even in the absence of known exposure or chelator administration, and that urinary mercury excretion rises after administration of a chelator, regardless of exposure history and in an unpredictable fashion. Studies also demonstrate that challenge testing fails to reveal a “body burden” of mercury due to remote exposure. Chelating agents have been associated with adverse reactions. Current evidence does not support the use of DMPS, DMSA, or other chelation challenge tests for the diagnosis of metal toxicity. Since there are no established reference ranges for provoked urine samples in healthy subjects, no reliable evidence to support a diagnostic value for the tests, and potential harm, these tests should not be utilized.
Keywords: Provoked urine test, Challenge test, Urine mobilization test, Metal chelation
Introduction
“Urine mobilization test,” “challenge test,” and “provoked urine test” are all terms used to describe the administration of a chelating agent to a person prior to collection of their urine to test for metals. The result of this test is then interpreted in isolation or in some cases is compared to a pre-chelation urine test obtained from the same person. There is no standard, validated challenge test. Challenge testing utilizes a variety of chelating agents, including dimercaptosuccinic acid (DMSA), dimercaptopropanesulfonate (DMPS), and ethylenediaminetetraacetic acid (EDTA). These agents are given by a variety of routes of administration, including oral, rectal, intramuscular, and parenteral. Even a transdermal formulation of DMPS has been advertised as effective, although a recent study reveals that this formulation of DMPS does not produce detectable serum levels of the drug or result in increased urinary mercury excretion [1]. In addition to differences in the particular chelating agent, formulations, and doses used in these tests, the volume of urine collected for testing also varies widely and can range from a spot urine sample to a 24-h urine collection or may include a sample collected over any number of hours in between.
The utility of provoked urine tests for the diagnosis of metal poisoning has been addressed previously by the American College of Medical Toxicology (ACMT). In 2009, ACMT published a position statement recommending against the use of this test [2]. Similarly, authors from the ATSDR and CDC have detailed the problems with provoked urine tests and have concluded that they should not be used as diagnostic tools [3]. Yet despite these recommendations against the use of provoked urine testing by respected organizations, the test is still commonly used and recommended by some practitioners.
A Google search of the phrase “urine test for metal poisoning” reveals that the public is receiving conflicting messages regarding the use of these tests. When this search was performed in January of 2012, the first result was a website called QuackWatch.org, which explains not to use this test as well as the reasons why it should not be used [4]. The next search result, however, states the opposite: that the most accurate way to test for metal toxicity is a chelation challenge test [5]. The third highest result tells the reader that acute metal poisoning is rare but states that alternatively, chronic, low-level exposure to metals is common and results in retention of metal in the body and a vast array of adverse health effects. The site instructs the reader to do a DMSA challenge test and also suggests doses to use. A very interesting declaration made on this site is that any increase in heavy metal excretion after DMSA challenge is considered significant. A deeper investigation into the site reveals that treatment will cost about US$25 a day for 3 weeks [6]. The fourth result of a Google search on “urine testing for metal poisoning” is the website for a clinic in New York City. There is an explanation that the testing and treatment done at this clinic differs from that done in mainstream medical clinics. A test that they perform is a 24-h urine heavy metal test with a provoking agent [7].
With three of the top four sites returned in a Google search recommending the use of a challenge test, it becomes clear that this is a topic that deserves further inspection. Advocates of the test state that current and past exposures to metals, including mercury, result in increased body stores, and they assert that a challenge test will reveal one's body burden of the metal. They advise health providers to do a comprehensive search for exposure to metals when performing a patient evaluation. As part of this comprehensive search, some have gone so far as to advise the use of a website where the patient enters their zip code to determine if there have been heavy metals released by industry in their geographical area. It is suggested that air, high-fructose corn syrup, anytime lifetime history of dental amalgams, or even remote childhood exposure to secondhand smoke can be considered sources of exposure to metals necessitating further testing. If a source is not identified, it has been recommended that maternal exposures prior to conception be sought, even for adult patients [8]. In essence, it can be interpreted that one should continue searching for a history of exposure until any potential source is identified so that testing can be justified.
Laboratory Reporting: Invalid Testing Procedures and Inappropriate Reference Ranges
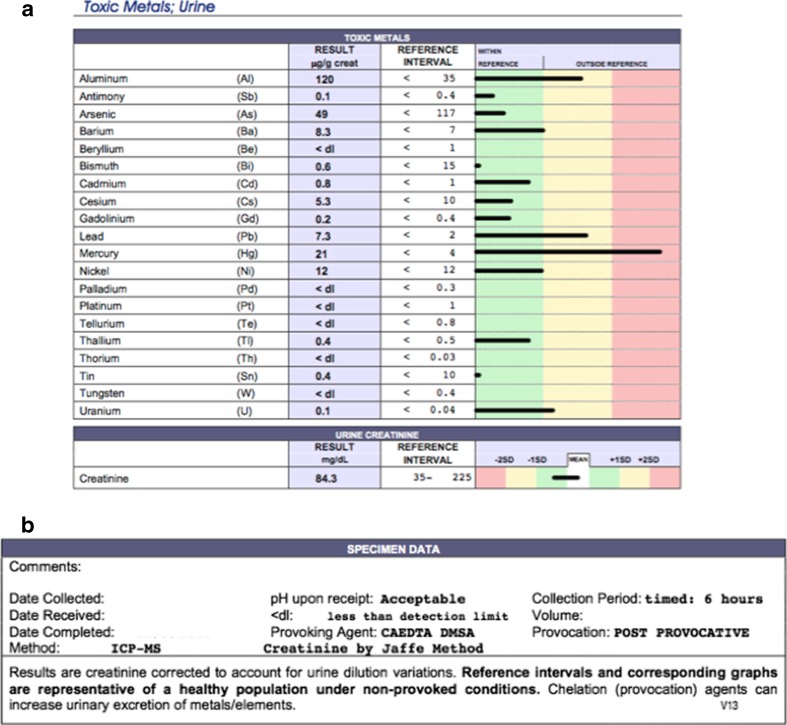
Once testing is performed using a provoked urine sample, a report is provided where “out-of-range” results are emphasized by placing them on a yellow or red background (Fig. 1a). It is easy for uninformed patients and providers to infer that a result that falls on the yellow or red background signifies poisoning (in Fig. 1a with mercury and, perhaps, lead and aluminum). The fine print at the end of the laboratory report indicating that it was a provoked test can easily be missed by patients and providers (Fig 1b). In addition, only a 6-h urine collection was done; this is important information because most of the metal will be pulled into the urine within the first 6 h after administration of a chelating agent [9]. Finally, the result was controlled for creatinine, which falsely elevates the concentration of metal reported.
Fig. 1.
Typical report provided to patients and providers on the results of a urine test for metals. a Sample laboratory report. b Fine print at the end of the laboratory report
The potentially misleading impact of controlling for creatinine in this setting can be seen in the following simplified example: A person excretes 1 g of creatinine (Cr) into the urine in 24 h and has a daily urine volume of 1 L. The same person excretes 0.4 μg/dL mercury into the urine over a day (or 4 μg Hg/L). So the urine mercury excreted over the course of 1 day is equal to 4 μg/g Cr. If urine is collected for 6 h and controlled for creatinine, the mercury level would be expected to continue to be 4 μg/g Cr (since 250 mg Cr, 1 μg Hg, and 250 mL of urine are expected to have been collected over 6 h). However, if a chelating agent were administered prior to collection of urine, the result would change. Assuming the excretion of mercury triples in the first 6 h after chelator administration and then returns to baseline, the 24-h excretion of mercury would increase to 6 μg, while the creatinine excreted over the same 24-h period would remain stable. However, if urine was collected only for the first 6 h and then controlled for creatinine, the 3 μg of Hg collected along with 250 mg of Cr would then be converted to 12 μg Hg/g creatinine. By simply cutting the urine collection period to 6 h and controlling for creatinine, the result reported to the patient and provider has doubled. Thus, in this example, creatinine correction is deceptive.
In addition to the use of a urine sample obtained over a short time interval and correction for creatinine after administration of a chelator, the result is then compared to a range taken from a unprovoked reference population. It is rarely reported how or from where the reference values are obtained, but it is clear that comparisons made with an incomparable reference population are misleading.
This leads to several questions:
Can a normal reference range for metals in urine after a dose of a chelator in healthy people be identified?
Is there any diagnostic value to a challenge test?
Is there reliable scientific evidence to support the use of challenge testing?
Is challenge testing safe?
Before addressing these questions directly, a brief review of the historic use of urine metal challenge tests follows.
Historical Use of Challenge Testing
Use of challenge tests in medicine goes back at least 50 years, when deferoxamine was first used to detect iron poisoning. Early in the 1960s, deferoxamine was introduced to diagnose iron storage diseases [10]. During that time, deferoxamine was studied in a rabbit model by Keberle et al. [11]. Rabbits were loaded with iron and given deferoxamine, and there was a 16-fold increase in urine iron excretion, with development of a red-brown color of the urine. The normal rabbits, which had not been loaded with iron, also had a fivefold increase in urinary iron excretion after deferoxamine. So whether they were iron toxic or not in this animal model, there was an increase in urine iron after administration of this chelator.
Over time, the deferoxamine challenge test became a standard test used to diagnose acute iron toxicity. Patients were given intramuscular deferoxamine, and if a vin rose urine color developed within 4 to 6 h, it was interpreted as a presence of excess iron or iron toxicity and it would prompt treatment. But this deferoxamine challenge did not stand the test of time. It turned out that it was not a very good test to distinguish toxic from nontoxic patients, and ultimately it was recommended that the test be abandoned [12].
Also in the early 1960s, the EDTA challenge test for the diagnosis of lead poisoning was developed. A study was published in the journal Pediatrics which looked at the use of a lead mobilization test, which was described as a 24-h urine collection, followed by three doses of intramuscular calcium EDTA given at 8-h intervals over 24 h, and then followed by a post-chelation 24-h urine lead level. The test was done in a control group and in children identified as having either lead poisoning or suspected lead poisoning (history of pica and basophilic stippling). The lead-poisoned children were found to have higher urinary lead excretion than the controls, but all children had a rise in urinary lead concentrations after this challenge test. So even in the healthy children without suspected lead poisoning, there was an increase in urine lead concentration after administration of a challenge test [13]. As with the deferoxamine challenge test, the lead mobilization test became widely used by the 1980s, and even the CDC recommended its use to determine which patients would be expected to respond to chelation. But, as with deferoxamine, it did not stand the test of time and was ultimately abandoned. Reasons included concerns regarding safety, the fact that it was difficult to perform in children, and data showing that lead was initially mobilized primarily from the bone [14].
A commonly accepted challenge test, the penicillamine challenge test for copper is sometimes used as an adjunct to aid in the diagnosis of Wilson disease, but even its utility has been questioned. It has been recommended for use in symptomatic children when the diagnosis is suspected but basal urinary copper excretion is normal. In this test, d-penicillamine is administered orally at the onset, and 8 h into, a 24-h urine collection for measurement of copper. A study published in Hepatology in 2010 sought to reevaluate the conventional diagnostic criteria for Wilson disease, including the use of the penicillamine challenge test. The authors looked retrospectively at 40 subjects with a confirmed diagnosis of Wilson disease and at 58 controls that did not have Wilson disease but did have either another mild liver disease or had a sibling with Wilson disease. In the patients with Wilson disease, the basal 24-h urinary copper excretion was significantly higher than in the control subjects. But after a penicillamine challenge test, the copper excretion rose in both groups and was no longer significantly different between groups. The authors concluded this challenge test to be of little diagnostic value [15]. While other evidence suggests there may still be some applications for the penicillamine challenge test [16], it can be concluded that penicillamine can raise urinary copper levels in patients without Wilson disease.
Current Misapplication of Challenge Testing
With the possible exception of the penicillamine challenge test for the diagnosis of Wilson disease, there are currently no other challenge tests with accepted applications in conventional medicine today. Yet the use of various chelating agents in challenge tests is widespread. Mercury exposure, in particular to short-chain organic mercury compounds in pregnant woman and young children, is a common cause for concern. Common sources of exposure to mercury for the general population have been seafood ingestion, dental amalgams, and thimerosal in vaccines. However, since mercury is ubiquitous in the environment, nearly everyone has at least some low-level exposure to mercury throughout their lifetime, even in the absence of these specific exposures.
Determination of Normal Range for Post-Challenge Metal Excretion in Healthy Individuals
DMSA and DMPS are two drugs that can chelate mercury. DMSA was approved in 1990 by the US Food and Drug Administration for treatment of lead intoxication but also chelates mercury. DMPS is not approved for use in the USA but is available for compounding by pharmacies. DMPS has been used in Russia since the 1950s and has been approved for use in Europe since the 1970s. Over the last 20 years, numerous studies have been published which involve the use of both DMSA and DMPS in challenge tests in healthy populations. These studies may provide some insight into what happens to urine mercury levels when chelators are given to healthy people, perhaps allowing a rough determination of reference ranges in a provoked population.
Several such studies come from the University of Arizona. In 1992, Dr. Aposhian and colleagues gave a DMPS challenge test to healthy students and compared the response between those with and without mercury amalgam dental fillings. The investigators collected subjects' urine for 11 h prior to administration of 300 mg of DMPS and then performed a 9-h post-chelation urine collection. Prior to administration of DMPS, both groups had mercury present in the urine. The mean concentration was low in both groups at baseline, but significantly higher in the amalgam group. After the DMPS was administered, the levels rose in each group significantly and the difference between groups remained significant. An important point to be made is that even without any history of exposure to mercury, the control subjects had mercury detected at baseline and this mercury concentration increased after a single dose of DMPS [17]. Something that is emphasized by the authors of this study and others is the factor by which the mercury concentration increased. The authors point out that the group without amalgams had a mean factor increase in urine mercury excretion of 19 while the amalgam group had a 25-fold increase, but they did not provide the ranges for the groups. Individual subjects excreted anywhere from 12- to 70-fold more mercury after the dose of DMPS. So it seems from this study that healthy subjects without any evidence of mercury toxicity had up to a 70-fold increase in urine mercury excretion after a 300-mg dose of DMPS. There were also a few minor adverse effects of DMPS worth noting. One subject vomited and another developed a rash a week following treatment.
Another group of investigators (Archbold et al.) gave DMSA to healthy subjects and controlled for amalgams. This time the challenge test involved a fasting spot urine sample, followed by a 30-mg/kg oral dose of DMSA. A 2-h urine sample was discarded and then a 6-h urine sample was collected for measurement of urine mercury concentration. The authors found that urine mercury correlated with the number of amalgam surfaces. They reported a mean factor increase of 7 (7 times more mercury excreted after DMSA), but the range was 1 to 27 [18]. The focus on factor increase is interesting because advocates of chelation challenge tests sometimes assert that, based on an individual's exposure history, the factor increase can be predicted. If the result is not as predicted, that serves as an indicator of a problem with excretion of metals due to another treatable condition [8]. The results from the Archbold study provide a good example of how useless the factor increase actually is. One of their subjects with the lowest numbers of amalgam surfaces (six) had the highest incremental factor of 27. Conversely, a subject with one of the highest number of amalgam surfaces (36) had the lowest incremental factor of 1. While the mean factor increase of all patients was 7, the individual results were highly variable. So for any individual, one cannot predict the mercury excretion factor increase based on the number of amalgam surfaces. However, one can conclude that healthy subjects had up to a 27-fold increase in their urine mercury excretion after a single dose of DMSA.
In the Archbold study, the intention was to include 20 subjects; however, the study was terminated after the 14th subject due to an allergic reaction in subject 15. Six minutes after receiving DMSA, the subject developed vomiting, chest tightness, and an urticarial rash. The assertion by some chelation advocates that this represents a reaction to mercury being pulled into the blood, rather than an allergic reaction to DMSA, is implausible from a pharmacokinetic standpoint. DMSA cannot be orally absorbed, distributed into tissues, chelate mercury from tissues and pull it into blood, and produce clinical mercury toxicity within 6 min. Other adverse effects reported by subjects in this study included foul-smelling urine and nausea [18].
Fish ingestion is another common source of exposure to mercury for which some seek chelation. Ruha et al. evaluated the effect of a DMSA challenge test on mercury excretion in healthy people who ingest different amounts of fish and compared the response between groups. Following a 12-h urine collection, a 30-mg/kg oral dose of DMSA was administered, and then a 12-h post-chelation urine collection was obtained. All subjects, even those who did not eat any fish, had mercury detected in their urine at baseline. There was not a significant difference in urine mercury concentration between groups at baseline, although a trend toward increased concentrations was noted with increased fish intake. However, after a dose of DMSA, all groups had a significant increase in urinary mercury excretion, and the difference between groups reached statistical significance [19]. Although not reported in the manuscript, the group without fish ingestion had a mean factor increase of 4, the group with ingestion of one to two fish meals per week had a factor increase of 9.5, and the group with the highest exposure of three or more fish servings per week had a factor increase of 10.8. The conclusion was that urinary mercury concentration rises in everyone after a DMSA challenge, and the increase is greater with higher fish consumption.
Looking at these studies collectively, it can be concluded that healthy subjects excrete mercury into the urine even without the use of a chelator. When a single dose of DMSA or DMPS is administered, healthy subjects without mercury poisoning exhibit a rise in urinary mercury concentration.
There are also studies comparing populations with occupational mercury exposure to control groups. Dentists, dental technicians, and non-dental personnel were compared by Aposhian et al. in 1995. At the time of this study, the investigators used a standard DMPS challenge protocol, which involved an 11-h pre-DMPS urine collection, a 300-mg oral dose of DMPS, and a 6-h post-DMPS urine collection. They chose the urine collection duration after Molin et al. demonstrated that most of the metal was chelated out in the first 6 h after DMPS [9, 20]. Prior to receiving DMPS, all groups demonstrated excretion of some amount of mercury at baseline, but exposed groups excreted more than the unexposed groups. After DMPS, all groups had a rise in urine mercury excretion. The levels reported in the exposed groups were higher than those in the control group, but all increased. As discussed earlier, the authors focused on factor increases, with the dental technicians having a mean 88-fold increase, while the dentists had a mean factor increase of 49, and the controls 35. However, the reported ranges demonstrate that the controls had anywhere from 14 to 132 times increase in their mercury excretion, whereas dental technicians had an 11 to 335 times increase. For any given individual, it is difficult to predict excretion based on their exposure history. From this study, it can be concluded that healthy controls, without history of exposure to mercury, had up to a 132-fold rise in urine mercury excretion after receiving a single 300 mg dose of DMPS [20].
Another similar study examined the effect of the DMPS challenge test in 11 subjects occupationally exposed to a calomel-containing skin lotion as well as in 8 users of the lotion and 9 control subjects [21]. The results are consistent with the previous study, with everyone excreting mercury at baseline prior to DMPS, even in the absence of exposure; although the exposed groups excrete more at baseline than the unexposed groups. All groups again exhibit a rise in urine mercury excretion after DMPS, with the exposed group having a greater mean increase. After DMPS, the healthy controls excrete up to 54 μg of mercury (or 73 μg Hg/L). This study also looked at urinary lead excretion after the DMPS. Although none of the groups studied had a history of lead exposure, they went from having about 5 μg of lead per 6-h urine collection to over 25 μg excreted in 6 h. So it seems that a dose of DMPS can also be expected to increase urinary lead concentrations in people without history of lead exposure [21].
Lack of Diagnostic Value to Challenge Testing for Determination of Body Burden of a Metal
A popular idea among chelation advocates is that the challenge test will reveal the body burden of a metal resulting from long-term or remote exposure. Molin and colleagues performed a study to determine if a DMPS challenge would provide an index of mercury body burden in occupationally exposed populations. They looked at four groups, including industrial workers from a chloralkali plant and a fluorescent tube factory with a mean of 11 years of exposure, dentists with a mean of 33 years of exposure, a group without occupational exposure but with amalgams, and a group that was without occupational exposure and amalgam free [9]. The results are generally similar to the previous studies discussed. All groups had measurable urinary mercury excretion at baseline, and exposed populations had greater baseline mercury excretion than unexposed populations. Mercury rose in everyone after a single dose of DMPS. The results of this study also nicely illustrate the effect of controlling for creatinine on the numerical result reported. The authors report the urine mercury concentration at 6 and 24 h after DMPS. In the control group, at 6 h, the mercury was reported to be 13 μmol/mol Cr. At 24 h, the result is 4.4 μmol Hg/mol Cr. The reason less mercury was reported after the 24-h urine collection than after the 6-h collection is that the result was corrected for creatinine. While correcting for creatinine can be useful in certain situations, in this circumstance (post-chelation challenge) it can be confusing. Correcting for creatinine while using only a 6-h urine collection obtained after administration of a chelator increased the numerical result by about a factor of 3. In the group of industrial workers in this study, the result went from 450 μg Hg/mol Cr with the 6-h collection down to 175 μg Hg/mol Cr at 24 h. The authors of this study had expected a large increase in urine mercury excretion after the challenge test because of a large body burden due to long-term occupational exposure. However, they found that the pre-DMPS urine mercury excretion was associated with the post-DMPS urine mercury excretion in all the groups. It was concluded that the challenge test did not reflect the long-term exposure or body burden [9].
A study by Frumkin and colleagues addressed this same question of whether a challenge test estimates body burden, but studied a population with remote exposure. The authors administered a DMSA challenge to determine if it would reveal an increased body burden in people with remote occupational exposure to mercury. The study included chloralkali plant workers who had had long-term high-level exposure to mercury, but since the plant had closed 4 years before the study was done, the exposure was no longer ongoing. Exposure profiles were created for each subject which were determined using historical air sampling data, specific jobs, and other factors to come up with average, cumulative, and peak exposure profiles for each subject. They were then compared to controls [22]. The challenge test in this study consisted of a 24-h urine mercury level pre-DMSA, followed by two 10-mg/kg oral doses of DMSA separated by 8 h, and a 24-h post-DMSA urine mercury collection. The authors found that at baseline, the groups were not different at all. Excretion of mercury after the DMSA also did not differentiate the exposure groups. It is especially interesting to note that in the other studies with presumed ongoing exposure, the exposure groups have always had higher metal concentrations both at baseline and post-chelation than the unexposed groups, but in this study, the exposed population has been removed from the exposure. The authors concluded that the DMSA challenge is not useful in quantifying past mercury exposure.
Lack of Scientific Evidence to Support Challenge Testing
It is difficult to draw many firm conclusions about the DMPS or DMSA challenge tests based on the presented data for a variety of reasons. The urine mercury concentrations were reported differently in the different studies. The urine collection times varied. The dose of the chelating agent administered varied. The results may not be comparable between different chelators, and the overall numbers of patients included in each study were small. Unfortunately, it is not possible to determine normal reference ranges for a nationally representative provoked population from these few studies. However, some conclusions can be made. The first is that baseline urine mercury concentrations, obtained without prior administration of any chelating agent, are expected to be higher in currently exposed healthy populations than in unexposed populations. Second, DMPS and DMSA challenges produce a rise in urine mercury in all groups of patients, even those without any known exposure to mercury. This is similar to the effect of EDTA, penicillamine, and deferoxamine on other metals in unexposed populations, described earlier. Third, there is a great overlap in factor increases between and within different exposure groups. It is not possible to predict what factor increase will or should occur based on history of exposure.
Although reference ranges for provoked urine mercury concentrations in an unexposed population cannot be reliably determined, Table 1. provides a summary of those reported in the control and amalgam-only groups of the studies included in this discussion. All of the mean urinary mercury concentrations are higher than expected in an unprovoked, unexposed population and are higher than the typical normal reference ranges provided for interpretation of urine metal testing results. It is interesting to compare the results summarized in Table 1 to the sample report in Fig. 1a. Figure 1a shows a mercury concentration of 21 μg/g Cr. Looking at Table 1, this appears to be a normal and expected result in a healthy person after a dose of a chelator. Yet in Fig. 1a, there is a visual added in addition to the actual result, so that the patient and the provider see that the result is “in the red.” Considering that many providers and patients are not aware of the implications of the 6-h urine collection, the creatinine correction, and the use of a non-chelated reference population when two chelators have been administered (in this case—see Fig. 1b), it is understandable that metal toxicity might mistakenly be diagnosed.
Table 1.
Post-challenge urine mercury concentrations in healthy subjects
| Study | Chelator | Number of subjects | Hg excretion after chelator |
|---|---|---|---|
| Aposhian [17] | DMPS | 10 (no amalgam) | 5 ± 1 μg/9 h |
| 10 (amalgam) | 17 ± 3 μg/9 h | ||
| Archbold [18] | DMSA | 14 | 14 ± 14 μg/L |
| Ruha [19] | DMSA | 22 (no/1–2/≥3)a | 3/10/13; 33 μg/g Crb |
| Gonzalez-Ramirez [20] | DMPS | 13 | 27 ± 3 μg/6 h |
| or 37 ± 15 μg/L | |||
| Maiorino [21] | DMPS | 9 | 18 ± 7; 54 μg/6 hb |
| or 22 ± 10; 73 μg/Lb | |||
| Molin [9] | DMPS | 5 (no amalgam) | 3.2 μg/24 h |
| 18 (amalgam) | 14; 56 μg/24 hb | ||
| Frumkin [22] | DMSA | 101 | 8 ± 6; 28 μg/24 hb |
aIndicates the number of fish meals per week in the study participants
bData presented as mean; upper range
Is there a diagnostic value to the challenge test? There are no well-designed randomized controlled studies that compare the use of a DMSA or DMPS challenge test in subjects with metal poisoning to those without metal poisoning. In order to design such a study, the patients with metal toxicity would have to be diagnosed based on history of exposure and presence of clinical findings that are consistent with and specific for the particular metal toxicity. Some published studies describe patients that have vague non-specific symptoms and consider them to potentially have mercury toxicity. Then the patients are given a challenge test, and when there is an increase in the urine mercury excretion (an expected finding in anyone given a dose of a chelator), they are diagnosed with toxicity.
Patient reports of poisoning or of symptom improvement after using a chelator are also considered supportive of a diagnosis of toxicity in some studies. One study specifically tested the diagnostic value of a DMPS challenge test in people who reported symptoms that they believed were due to amalgam fillings. A DMPS challenge test (2 mg/kg IV) was administered to 19 subjects who were healthy and without amalgams, 21 subjects who were healthy and had amalgams, 20 subjects with amalgams and with self-diagnosed mercury poisoning, and 20 subjects who had had their amalgams removed because of self-diagnosed mercury poisoning. The amalgam groups were found to excrete more mercury than the non-amalgam groups, but there was no difference in excretion between groups with and without symptoms. Thus, the symptomatic groups did not have a larger “body burden” detected with a challenge test [23].
A study which demonstrates the importance of proper research protocol (including blinding) looked at chelation therapy of patients with symptoms attributed to amalgam fillings. This double-blind, randomized controlled trial enrolled patients who absolutely attributed their symptoms to amalgams and in whom there was no alternative diagnosis. The subjects were randomized to be treated for 5 days with DMSA or to receive placebo. Both the DMSA and the placebo groups improved equally [24]. An important point from this and the previous study is that subjective improvement (attributable to placebo effect), correlated with deceptively manipulated and reported laboratory results that are the expected effect of exposure to a chelating agent (and demonstrated in asymptomatic individuals), does not provide evidence for either diagnostic or therapeutic benefit from chelation.
Safety Issues with Challenge Testing
Is there evidence at least that a challenge test is risk free? If chelation provides a placebo effect in some patients while removing small, inconsequential amounts of mercury into the urine, and causes no harm, then there may be some (albeit expensive) benefit. Unfortunately, adverse reactions are widely reported, and many were noted in the volunteer studies discussed. There is a potential for development of mineral deficiencies, allergic reaction, and obviously unpleasant effects such as foul smell, nausea, and vomiting. The cost is also an important factor. Treatments are very expensive. The transdermal formulation of DMPS purchased for a study cost US$420 at the “doctor discount” price, and this formulation was not even absorbed [1].
Conclusion
In summary, current evidence does not support the use of DMPS, DMSA, or other chelation challenge tests for the diagnosis of metal toxicity. Since there are no established reference ranges for provoked urine samples in healthy subjects, no reliable evidence to support a diagnostic value for the tests, and potential harm, these tests should not be utilized.
Acknowledgments
Conflict of interest
For the work under consideration for publication, Dr. Ruha received an honorarium (which she donated to MTF) and reimbursement for travel through the ACMT/ATSDR Cooperative Agreement. As relevant financial activities outside the submitted work, Dr. Ruha is a BTG and RDT paid speaker.
Footnotes
ATSDR disclaimer
This publication was supported by the cooperative agreement award number 1U61TS000117-04 from the Agency for Toxic Substances and Disease Registry (ATSDR). Its contents are the responsibility of the authors and do not necessarily represent the official views of the Agency for Toxic Substances and Disease Registry (ATSDR).
References
- 1.Cohen JP, Ruha AM, Curry SC, Biswas K, Westenberger B, Ye W, et al. Plasma and urine dimercaptopropanesulfonate concentrations after dermal application of transdermal DMPS (TD-DMPS) J Med Toxicol. 2013;9(1):9–15. doi: 10.1007/s13181-012-0272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charlton N, Wallace KL (2009) Post-chelator challenge urinary metal testing. AMCT position statement. http://www.acmt.net/cgi/page.cgi/zine_service.html?aid=2999&zine=show. Accessed 28 July 2013 [DOI] [PMC free article] [PubMed]
- 3.Risher JF, Amler SN. Mercury exposure: evaluation and intervention: the inappropriate use of chelating agents in the diagnosis and treatment of putative mercury poisoning. NeuroToxicology. 2005;26:691–699. doi: 10.1016/j.neuro.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Barrett S. How the urine toxic metals test is used to defraud patients. http://www.quackwatch.com/01QuackeryRelatedTopics/Tests/urine_toxic.html. Accessed 28 July 2013
- 5.Nichols A. Heavy metal poisoning and safe detoxification. http://evenbetterhealth.com/heavy-metal-poisoning.asp. Accessed February 2012
- 6.The Great Plains Laboratory. Metals urine testing. http://greatplainslaboratory.com/home/eng/metals_urine.asp. Accessed 28 July 2013
- 7.Patients Medical (2013) Health AZ. http://patientsmedical.com. Accessed Feb 2012
- 8.Crinnion WJ. The benefit of pre- and post-challenge urine heavy metal testing: part 2. Alternat Med Rev. 2009;14(2):103–108. [PubMed] [Google Scholar]
- 9.Molin M, Schiitz A, et al. Mobilized mercury in subjects with varying exposure to elemental mercury vapour. Int Arch Occup Environ Health. 1991;63:187–192. doi: 10.1007/BF00381567. [DOI] [PubMed] [Google Scholar]
- 10.Fielding J. Differential ferrioxamine test for measuring chelatable body iron. J Clin Path. 1965;18:88–89. doi: 10.1136/jcp.18.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keberle H. The biochemistry of desferrioxamine and its relation to iron metabolism. Ann NY Acad Sci. 1964;119:758–768. doi: 10.1111/j.1749-6632.1965.tb54077.x. [DOI] [PubMed] [Google Scholar]
- 12.Proudfoot AT. Antidotes: benefits and risks. Tox Let. 1995;82/83:770–783. doi: 10.1016/0378-4274(95)03519-2. [DOI] [PubMed] [Google Scholar]
- 13.Whitaker JA, Austin W, Nelson JD. Edathamil calcium disodium (versenate) diagnostic test for lead poisoning. Pediatrics. 1962;29:384–388. [PubMed] [Google Scholar]
- 14.Chisolm JJ. Mobilization of lead by calcium disodium edetate. AJDC. 1987;141:1256–1257. doi: 10.1001/archpedi.1987.04460120018020. [DOI] [PubMed] [Google Scholar]
- 15.Nicastro E, Ranucci G, et al. Re-evaluation of the diagnostic criteria for Wilson disease in children with mild liver disease. Hepatology. 2010;52:1948–1956. doi: 10.1002/hep.23910. [DOI] [PubMed] [Google Scholar]
- 16.Vieira, et al. Urinary copper excretion before and after oral intake of D-penicillamine in parents of patients with Wilson's disease. Dig Liver Dis. 2012;44(4):323–327. doi: 10.1016/j.dld.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Aposhian HV, Bruce DC, Alter W, Dart RC, Hurlbut KM, Aposhian MM. Urinary mercury after administration of 2,3-dimercaptopropane-1-sulfonic acid: correlation with dental amalgam score. FASEB J. 1992;6:2472–2476. doi: 10.1096/fasebj.6.7.1563599. [DOI] [PubMed] [Google Scholar]
- 18.Archbold GP, McGuckin RM, Campbell NA. Dimercaptosuccinic acid loading test for assessing mercury burden in healthy individuals. Ann Clin Biochem. 2004;41:33–236. doi: 10.1258/000456304323019622. [DOI] [PubMed] [Google Scholar]
- 19.Ruha AM, Curry SC, Gerkin RD, Caldwell KL, Osterloh JD, Wax PM. Urine mercury excretion following DMSA challenge in fish eaters. Arch Pathol Lab Med. 2008;132:4–9. doi: 10.5858/133.1.87. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Ramirez D, Maiorino RM, Zuniga-Charles M, Xu Z, Hurlbut KM, Junco-Munoz P, et al. Sodium 2,3-dimercaptopropane-1-sulfonate challenge test for mercury in humans: II. Urinary mercury, porphyrins, and neurobehavioral changes of dental workers in Monterrey, Mexico. J Pharmacol Exp Ther. 1995;272(1):264–274. [PubMed] [Google Scholar]
- 21.Maiorino RM, Gonzalez-Ramirez D, Zuniga-Charles M, Xu Z-F, Hurlbut KM, Aposhian MM, et al. Sodium 2,3-dimercaptopropane-1-sulfonate challenge test for mercury in humans. III. Urinary mercury after exposure to mercurous chloride. J Pharmacol Exp Ther. 1996;277:938–944. [PubMed] [Google Scholar]
- 22.Frumkin H, Manning CC, Williams PL, Sanders A, Taylor BB, Pierce M, et al. Diagnostic chelation challenge with DMSA: a biomarker of long-term mercury exposure? Environ Health Perspect. 2001;109(2):167–171. doi: 10.1289/ehp.01109167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vamnes JS, Eide R, Isrenn R, Höl PJ, Gjerdet NR. Diagnostic value of a chelating agent in patients with symptoms allegedly caused by amalgam fillings. J Dent Res. 2000;79(3):868–874. doi: 10.1177/00220345000790031401. [DOI] [PubMed] [Google Scholar]
- 24.Grandjean P, Guldager B, Larsen B, Jørgensen PJ, Holmstrup P. Placebo response in environmental disease: chelation therapy of patients with symptoms attributed to amalgam fillings. J Occ Env Med. 1997;39(8):707–714. doi: 10.1097/00043764-199708000-00004. [DOI] [PubMed] [Google Scholar]