Abstract
This presentation summarizes several of the rodent and non-human studies that we have conducted to help inform the efficacy and clinical utility of succimer (meso-2,3-dimercaptosuccincinic acid) chelation treatment. We address the following questions: (1) What is the extent of body lead, and in particular brain lead reduction with chelation, and do reductions in blood lead accurately reflect reductions in brain lead? (2) Can succimer treatment alleviate the neurobehavioral impacts of lead poisoning? And (3) does succimer treatment, in the absence of lead poisoning, produce neurobehavioral deficits? Results from our studies in juvenile primates show that succimer treatment is effective at accelerating the elimination of lead from the body, but chelation was only marginally better than the complete cessation of lead exposure alone. Studies in lead-exposed adult primates treated with a single 19-day course of succimer showed that chelation did not measurably reduce brain lead levels compared to vehicle-treated controls. A follow-up study in rodents that underwent one or two 21-day courses of succimer treatment showed that chelation significantly reduced brain lead levels, and that two courses of succimer were significantly more efficacious at reducing brain lead levels than one. In both the primate and rodent studies, reductions in blood lead levels were a relatively poor predictor of reductions in brain lead levels. Our studies in rodents demonstrated that it is possible for succimer chelation therapy to alleviate certain types of lead-induced behavioral/cognitive dysfunction, suggesting that if a succimer treatment protocol that produced a substantial reduction of brain lead levels could be identified for humans, a functional benefit might be derived. Finally, we also found that succimer treatment produced lasting adverse neurobehavioral effects when administered to non-lead-exposed rodents, highlighting the potential risks of administering succimer or other metal-chelating agents to children who do not have elevated tissue lead levels. It is of significant concern that this type of therapy has been advocated for treating autism.
Keywords: Succimer chelation treatment, Neurobehavioral deficits, Lead poisoning, Brain lead, Autism
Introduction
This paper summarizes some of the animal studies completed in our laboratory over the past decade or so that help inform the efficacy and clinical utility of chelation treatment as commonly practiced. As a brief overview of its mechanistic basis, chelation treatment can be simply described as the process of the chelate ligand forming a selective and stable coordination complex with the metal of interest, yielding a complex that is more readily excreted in urine or feces than the metal alone (or the metal complexed with other inherent biological ligands). The efficiency of forming the metal–chelate complex can be described in the simplest terms as the ratio of the chelate versus the unbound metal (depicted as E in Eq. 1), and can be expressed as a stability constant (β) as depicted in Eq. 2:
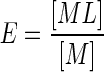 |
1 |
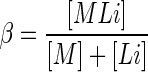 |
2 |
Such a relationship can even incorporate the concentrations or ‘activities’ of other endogenous elements in the body that may influence formation of the metal–chelate complex, such as shown here with calcium (Eq. 3):
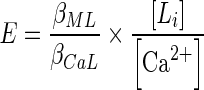 |
3 |
In reality, of course, these simple expressions do not tell us all that we need to know about how a chelating agent may be acting within the body. For example, it does not tell us about the presence and effect of competing ligands or other metals within the body, the kinetics of metal exchange with the ligand, the extent to which the chelating agent (i.e., the drug) may be metabolized and transported throughout the body, or how the chelating agent may be compartmentalized within the body.
We also know that in vitro studies may not provide sufficiently predictive information about how these important processes occur in vivo. As a result, animal or clinically based studies are needed. Given this, the objective of this presentation is to address important questions on chelation efficacy, using research animal model-based investigations that we have conducted with succimer over the past decade or so.
As background, it is important to first recognize that there has been quite a bit of research over the past several decades by Drs. Graziano, Aposhian, Dart, Maiorino, and colleagues, among others, that has provided the foundation for much of what we now understand about chelation treatment with succimer and other chelating agents [1–10]. Those studies characterized the metabolism of succimer (meso-2,3-dimercaptosuccinic acid, or DMSA) in human subjects after it first started gaining attention as a lead-chelating agent. They determined that following a therapeutic dose essentially all the DMSA in the human body is excreted as mixed disulfide compounds, with a majority of the compound within the circulation being mixed disulfides with plasma proteins, primarily albumin. The majority of the excretable DMSA in urine also appears as mixed disulfide compounds [7, 8]. Studies also showed that DMSA undergoes enterohepatic circulation [10]. Notably, it was shown that only a relatively small amount of the DMSA administered to humans is excreted within the first 2 to 4 h following dosing, while a substantial amount of it is still retained in the body after that period of time. Studies also provided some evidence that a patient’s lead poisoning status may alter how s/he metabolizes DMSA, with reduced renal clearance of DMSA and the DMSA-lead chelate in more severely lead-poisoned subjects [9, 10].
With this background, we would like to specifically focus the remainder of the presentation on the following questions:
What is the extent of body lead reduction with chelation, and specifically is brain lead reduced with chelation, and do reductions in blood lead accurately reflect reductions in brain lead?
Can succimer treatment alleviate the neurobehavioral impacts of lead poisoning?
Does succimer treatment, in the absence of lead poisoning, produce negative effects on neurobehavioral measures?
What is the extent of body lead reduction and elimination with succimer chelation versus the cessation of lead exposure alone?
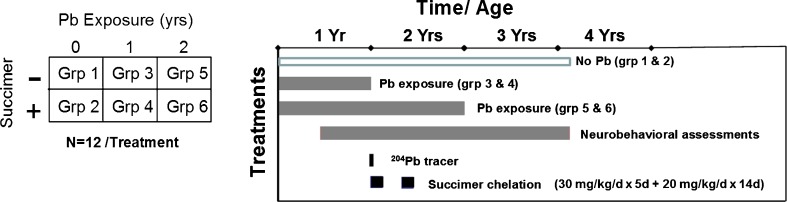
To address the first series of questions we will present results from a non-human primate study conducted several years ago [11]. This study utilized a 2 × 3 factorial design with two levels of succimer treatment (presence or absence) and three levels of lead exposure (no lead, daily lead exposure over the first year of life, lead exposure over the first 2 years of life). The animals were dosed orally with lead to achieve a target blood lead level of ∼40 to 45 mcg/dL. Animals were treated with a full chelation regimen (or vehicle) starting at 1 year of age (∼53 weeks) and again at age ∼65 weeks. The chelation regimen was comparable to that used clinically in lead-poisoned children; 30 mg succimer/kg/day divided into three daily doses for 5 days followed by a maintenance dose of 20 mg/kg/day divided into two daily doses for 2 weeks.
In addition, we utilized a stable lead isotope tracer methodology, in which animals were administered small amounts of stable 204Pb and/or 206Pb tracer prior to the start of chelation, in order to more precisely evaluate the movement and elimination of lead from the body with chelation. Animals were assessed with a battery of neurobehavioral tests spanning several years of testing. The general study design showing the timing and duration of lead exposure, chelation treatment, and neurobehavioral testing is shown in Fig. 1.
Fig. 1.
Study design and timing of testing in the primate lead chelation study (Smith et al. [11]). [Reprinted from Toxicology and Applied Pharmacology with permission from Elsevier]
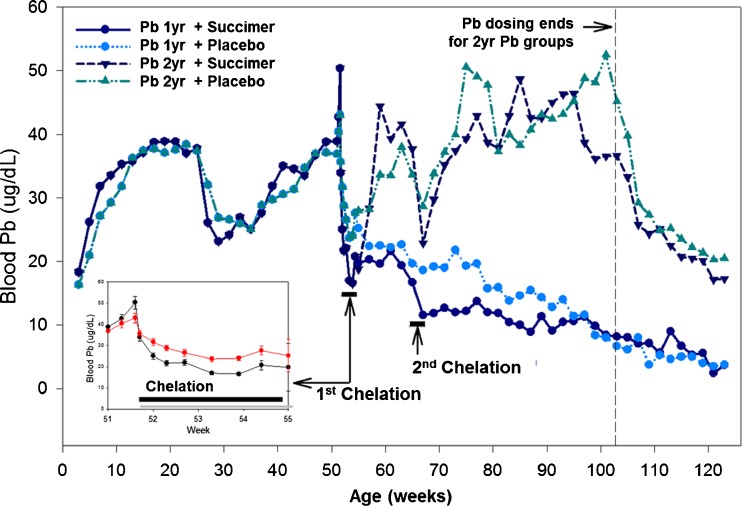
In Fig. 2 we show the blood lead profiles of animals administered oral lead for the first year of life. We want to point out that over the peri-weaning period blood lead levels dropped dramatically due to dietary changes (i.e., breast milk to solid food), similar to that which occurs in human infants. With this drop in blood lead levels, animals were administered higher doses of lead to bring their blood lead levels back to the target level of ∼40 to 45 mcg/dL. At 1 year of age, animals underwent their first chelation regimen—lead exposure ceased during chelation. After the first chelation regimen, animals remained off lead exposure (1 year lead-exposed groups) or were put back on lead exposure (2 years lead exposure groups) for their second year of life. A second chelation regimen was administered at age 65 weeks (Fig. 2).
Fig. 2.
Blood lead levels in the four groups of non-human primates receiving oral lead exposure for either the first 1 or 2 years of life. Each lead exposure group was divided into succimer or vehicle placebo groups, as indicated (n = 10–12/group). Inset panel shows expanded timeline over the first chelation regimen. (Smith et al. [11]). [Reprinted from Toxicology and Applied Pharmacology with permission from Elsevier]
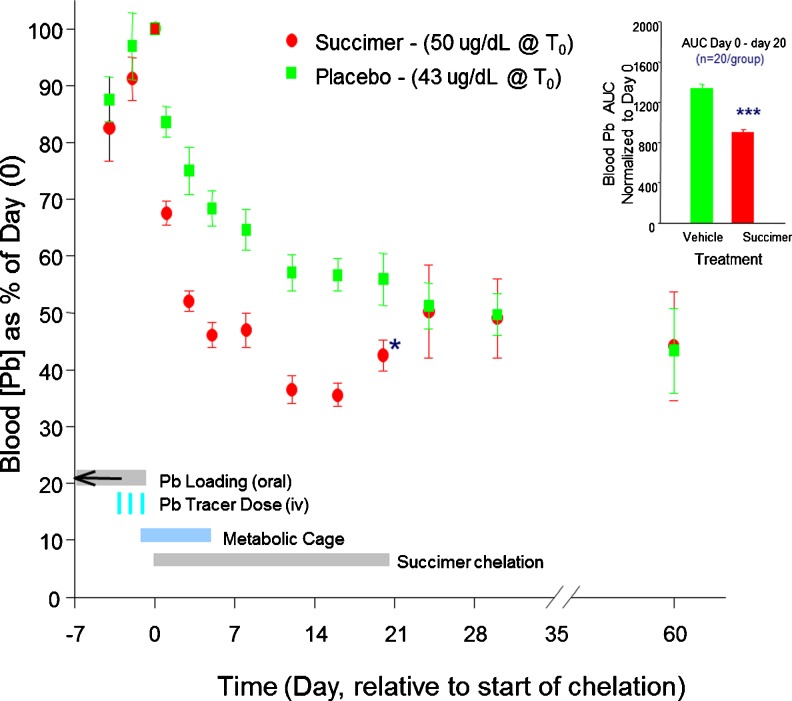
Figure 3 shows the relative reduction in blood lead levels over the first course of chelation, with blood lead levels normalized to the starting (chelation day 0) blood leads of ∼43 to 50 mcg/dL in the two treatment groups. The data clearly demonstrate a reduction in blood lead levels with chelation, though notably the reduction is most dramatic over the first several days of treatment.
Fig. 3.
Mean ± SE blood total lead levels (as % of day 0 pretreatment values) in succimer and placebo-vehicle-treated 1-year lead-exposed monkeys over the course of the first chelation treatment (days 0–20) and beyond. Placebo and succimer group n = 21–23/group for days 24 to 20, and n = 11/group for days 24 to 60. (Inset) The mean ± SE integrated area under the curve (AUC; days 0 to 20) for the placebo vehicle and succimer groups. ***Statistically different (p < 0.001) from placebo group (comparisons performed only on treatment day 20 and on AUC data). For reference, blood lead levels on day 0 were 43 and 50 mcg/dL for the placebo and succimer groups, respectively (p = 0.08). Data from Smith et al. [11]. [Reprinted from Toxicology and Applied Pharmacology with permission from Elsevier]
It should be noted that while there is a dramatic drop in blood lead levels in the succimer-treated group with treatment, there is also a dramatic drop in the placebo-vehicle group, reflecting the efficacy of the cessation of lead exposure alone. However, when one looks at the integrated blood lead level over the treatment period, which is the area under the curve shown in Fig. 3, there is an overall substantial and significant reduction in blood lead in the succimer versus vehicle group, as expected. It is noteworthy, however, that there is a substantial rebound in blood lead levels in the succimer-treated group at the cessation of treatment, such that if one assessed chelation efficacy a few days after treatment ended, the data would indicate no measurable difference between the succimer and vehicle groups (Fig. 3). This raises the issue that assessment of chelation efficacy based on blood lead reduction may depend in part on when exactly blood lead levels are evaluated relative to chelation.
The urinary and fecal elimination of lead was also evaluated with complete daily urine and feces collections from monkeys placed in metabolic cages over the first 5 days of succimer treatment. Results show a dramatic increase in the daily urinary lead elimination with succimer treatment as expected, though notably there is a dramatic reduction in lead diuresis over the first 5 days of treatment, even though animals continued to receive the same daily dose of succimer over that period (Fig. 4). Moreover, by the end of the 19-day treatment period, there was no measurable difference in the urinary lead elimination between succimer- versus vehicle-treated groups. This indicates that the majority of lead diuresis occurred within the first 5 days of treatment, with little additional urinary lead elimination after that. Overall, however, measures of the cumulative 5-day total urinary lead elimination show that there was a significant increase in lead diuresis with succimer treatment (Fig. 4, inset).
Fig. 4.
a, b Mean ± SE urinary excretion of total lead over the first chelation treatment regimen in 1-year lead-exposed monkeys. Left panel shows daily 24-h total urinary lead elimination, while inset panel shows mean 5-day cumulative total lead in placebo-vehicle- and succimer-treated groups. Placebo and succimer group n = 24/group over days 0 to 5, and n = 8/group for days 10 and 20. ***Statistically different (p < 0.001) from placebo group (comparisons performed only on data in b). Data from Smith et al. [11]. [Reprinted from Toxicology and Applied Pharmacology with permission from Elsevier]
Interestingly, however, when one assesses the inter-animal differences in the urinary elimination of lead with succimer treatment, it is evident that there were substantial differences between animals in how they responded to chelation (Fig. 5). Based on differences in lead diuresis, some animals may be characterized as ‘hyper’ responders to chelation, while others may be ‘hypo’ responders. The underlying reason(s) for these inter-animal differences are not known, but may reflect genetic/metabolic differences, since animals were administered the same body-weight-normalized regimen of succimer, and they started chelation treatment with approximately the same blood lead level and lead exposure history.
Fig. 5.
Five-day cumulative urinary total lead excretion by individual succimer- and placebo-treated 1-year lead-exposed monkeys over the first chelation regimen. Each bar represents an individual animal. Symbols above the bar in the succimer group, when present, identify apparent hyper (@) or hypo (#) responders to succimer treatment. Data from Smith et al. [11]. [Reprinted from Toxicology and Applied Pharmacology with permission from Elsevier]
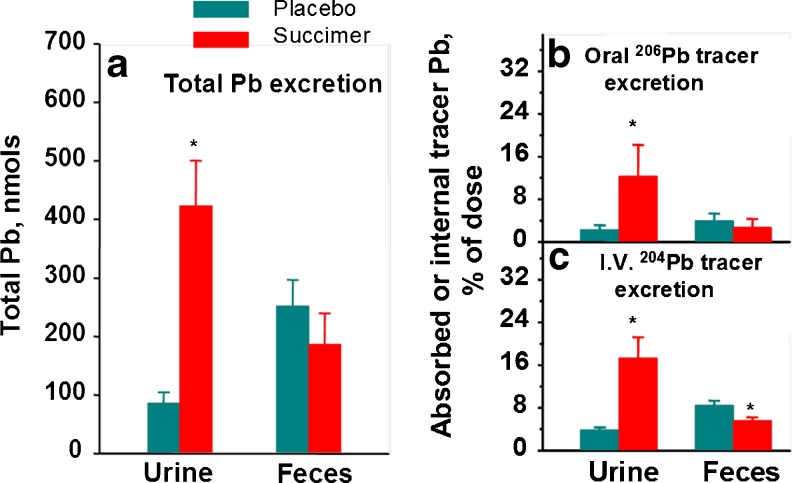
We also evaluated fecal lead elimination with chelation, using complete 24-h fecal samples collected over the first 5 days of treatment [12]. These data are summarized in Fig. 6 along with the urinary lead elimination data. Together these data show that the vast majority of body lead elimination with succimer chelation occurs via urine, with comparatively little eliminated via the fecal route. As noted earlier, we also administered several different stable lead isotope tracers to these monkeys prior to starting chelation, with stable 204Pb tracer administered via i.v. injection and stable 206Pb tracer administered orally. Use of these isotope tracers allowed us to more accurately evaluate elimination of endogenous lead via urinary versus fecal routes, as well as the GI absorption and subsequent elimination of lead present in the GI tract during chelation. Results from these studies substantiate the predominance of urinary lead elimination with succimer treatment, with urinary lead elimination increasing for both the i.v. administered lead tracer (204Pb) and the orally administered tracer (206Pb)—the latter reflecting elimination of oral 206Pb tracer absorbed into the body and subsequently eliminated. Notably, however, the i.v. 204Pb tracer data revealed that the increase in urinary lead elimination with succimer treatment came somewhat at the expense of a decrease in fecal lead elimination, evidenced by a significant reduction in fecal lead elimination with succimer treatment compared to placebo-vehicle (Fig. 6c).
Fig. 6.
Left panel a—effect of oral succimer on urinary and fecal excretion of total lead (inherent lead + stable lead isotope tracers). Right panel b—total urinary and fecal excretion of orally administered 206Pb tracer over the first 5 days of succimer treatment; right panel c—total urinary and fecal excretion of i.v. administered 204Pb tracer over the first 5 days of treatment. Bars are mean ± SE for the vehicle (n = 7) and succimer (n = 8–9) groups. *Significantly different from placebo-vehicle control (p < 0.05). Data from Cremin et al. [12]. [Reproduced with permission from Environmental Health Perspectives]
With the stable lead isotope tracer approach we were also able to evaluate the whole body elimination vs. retention of lead with chelation by assessing the total amount of orally administered 206Pb and i.v. administered 204Pb tracer that was recovered over the first 5 days of succimer treatment. Interestingly, both with orally and i.v. administered lead the amount of tracer recovered in feces and urine was proportionally small relative to the amount of the administered tracer dose (Fig. 7). The fact that animals retained a substantial amount of the administered lead tracers versus what was eliminated with succimer treatment over the 5-day period was surprising.
Fig. 7.
Effects of oral succimer on the retention of orally administered lead absorbed across the gastrointestinal tract (oral 206Pb tracer) and i.v. administered lead (i.v. 204Pb tracer) expressed as a percentage of the administered (204Pb tracer) or calculated (206Pb tracer) internal dose. The internal dose is the amount of the endogenous 204Pb tracer injected i.v. or the amount of the oral 206Pb tracer absorbed from the GI tract. The sum of the lead tracer retained over the first 5 days of succimer treatment is expressed as a percentage of the internal dose, which equals the amount of oral 206Pb tracer absorbed or the amount of 204Pb tracer that was injected. The bars represent mean ± SE for the vehicle (n = 7) and succimer (n = 9) groups. *Succimer group differs from the vehicle group according to an ANOVA (p < 0.05). Data from Cremin et al. [12]. [Reproduced with permission from Environmental Health Perspectives]
Overall, these data show that succimer treatment certainly facilitates the elimination of lead from the body, primarily via a urinary pathway. However, the substantial amounts of orally or i.v. administered lead tracer that were retained in the body in both vehicle- and succimer-treated groups after 5 days of treatment indicates that the vast majority of body lead from recent exposures is not affected by succimer chelation.
Is brain lead reduced with chelation, and do reductions in blood lead accurately reflect reductions in brain lead?
There is substantial interest in determining the extent to which succimer treatment reduces brain lead levels because the brain is a major target of lead poisoning. If one assumes that the brain tissue lead level is reflective of the potential toxicity to that tissue, then understanding how effectively chelation reduces brain lead levels might help us understand the extent to which treatment can alleviate toxicity in the central nervous system.
To address this question, we have conducted studies in rodents and adult primates. We will present results from the primate study first [13]. The study design allowed for five to six animals each per control and succimer treatment group. Animals were treated orally with lead for approximately 5 weeks, and then lead exposure ceased and 5 days later, treatment with succimer or placebo was initiated. As in the juvenile primate study presented above, we administered a stable 204Pb isotope tracer I.V. several days before succimer treatment began. The study utilized a prospective design to best investigate changes in brain lead levels with treatment; for this, a prefrontal cortex biopsy was collected from all animals after the cessation of lead exposure and the administration of the 204Pb tracer, but before succimer treatment began so we could evaluate, within an animal, the efficacy of succimer treatment to reduce brain lead levels. At the end of succimer treatment, the animals were sacrificed and full brain dissections were performed for analyses. The first set of results show for a representative animal the oral lead dose that was given and the animal’s resulting blood lead levels throughout the study, including before and after succimer treatment (Fig. 8).
Fig. 8.
Example of the oral lead doses (triangles, mg Pb/kg body wt.) and resultant blood lead levels over the lead exposure (filled circles) and chelation treatment (open diamonds) periods in a representative monkey from the succimer treatment group. The treatment schedule is indicated below the x-axis. Data from Cremin et al. [12]. [Reprinted from Toxicology and Applied Pharmacology with permission from Elsevier]
The blood lead profile over the course of succimer treatment was similar to the results presented above from the juvenile monkeys; i.e., as expected, blood lead levels declined more rapidly in succimer-treated animals than the placebo animals (Fig. 9), such that the total integrated area under that blood lead level curve was significantly lower for the succimer group. But when comparing blood lead levels at the end of succimer treatment on day 19 the blood lead levels in the succimer- and vehicle-treated groups were similar and not statistically different from one another, as with the juvenile primate study presented above. In this case, this is attributed to the reduction of blood lead levels in the vehicle group, which was also somewhat rapid, reflecting the efficacy of simply ending lead exposure.
Fig. 9.
Lead concentrations in blood of the vehicle- (filled circle, n = 4–5) and succimer- (open diamond, n = 5–6) treated groups over the succimer treatment period. The values presented are the means ± SE of blood lead concentrations. Statistical analyses were conducted on data adjusted to starting (day 0) blood lead levels for each animal, in order to control for variation in the initial blood lead concentrations among animals. Comparisons of mean blood lead values: means with different superscript letters (e.g., a, b, and c) are statistically significantly different from one another. Data from Cremin et al. [13]. [Reprinted from Toxicology and Applied Pharmacology with permission from Elsevier]
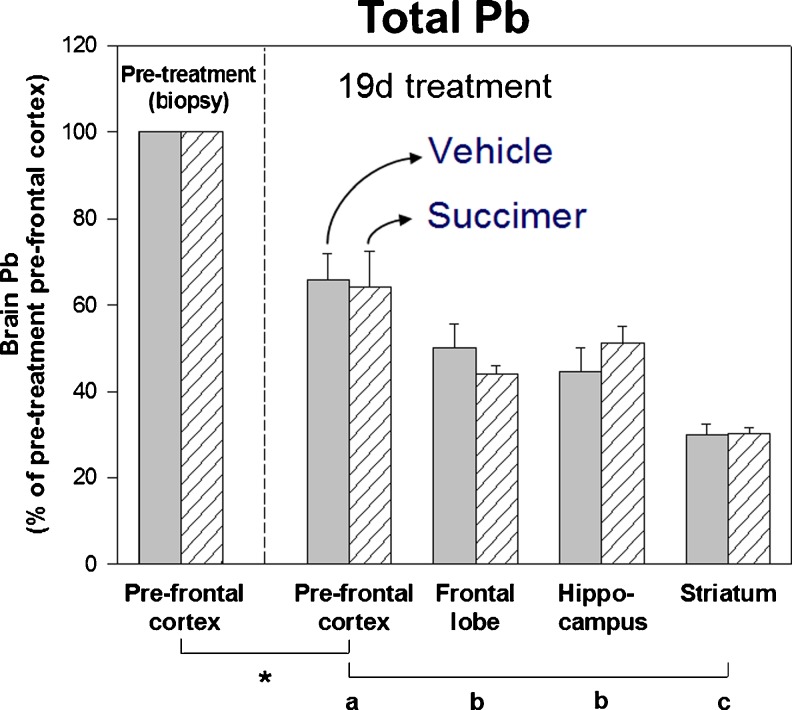
To best evaluate the extent that succimer treatment reduced brain lead levels, we normalized lead levels in various brain regions within each animal to the brain lead levels in each animal’s prefrontal cortex biopsy collected prior to starting succimer treatment. These data clearly show that there is no measurable reduction in brain lead levels as a result of the 19-day succimer treatment regimen, compared to the vehicle treatment (Fig. 10). Notably, there was a significant reduction in brain lead levels in all animals over the 19-day treatment period, as shown in the comparison of prefrontal cortex lead levels in the pre-treatment biopsy versus the post-treatment sample. There were also significant differences in lead levels across brain regions, consistent with results from prior studies. However, there were no differences in lead levels between the vehicle- and the placebo-treated groups, indicating that 19 days of succimer treatment was no more efficacious at reducing brain lead levels than the cessation of lead exposure alone.
Fig. 10.
Lead levels in brain prefrontal cortex (PFC, pre- and post-treatment), frontal lobe, hippocampus, and striatum of the vehicle- (solid bars, n = 5) and succimer- (hatched bars, n = 6) treated groups (lead levels expressed as a percentage of the pretreatment PFC level within each animal). Values are means (±SE). Mean values of lead concentrations in the pretreatment PFC biopsy for the vehicle- and succimer-treated groups were 1,980 and 1,410 ng/g dry wt., respectively. Symbols below the x-axis indicate: * = pre- and post-treatment mean values were significantly different; a, b, and c comparisons of post-treatment brain regions. Means with different letters were significantly different from one another. Data from Cremin et al. [13]. [Reprinted from Toxicology and Applied Pharmacology with permission from Elsevier]
Notably, results from the 204Pb tracer component of the study, which more specifically reflect elimination of lead exposure given a day or two prior to starting chelation treatment, show the same outcome as total lead levels. That is, there were significant reductions in brain 204Pb levels over the19-day treatment period, as well as significant differences in brain 204Pb levels across brain regions (Fig. 11). But, there was no difference between the vehicle- and succimer-treated groups in brain 204Pb levels across any brain region. One noteworthy caveat to this interpretation is shown in the post-treatment prefrontal cortex brain region data from the vehicle and succimer groups blocked out in red in Fig. 11; those data suggest there was an increase in 204Pb tracer in the prefrontal cortex of the vehicle group over the 19-day treatment period, which most likely reflects the continued uptake (i.e., enrichment) of the 204Pb tracer into this brain region of the vehicle-treated animals over that period.
Fig. 11.
Relative enrichment of 204Pb tracer levels in brain prefrontal cortex (PFC, pre- and post-treatment), frontal lobe (FL), hippocampus (H), and striatum (S) of the vehicle- (solid bars, n = 4) and succimer-(hatched bars, n = 6) treated groups (204Pb tracer relative enrichment levels expressed as a percentage of the pretreatment PFC level within each animal). Values are means (±SE). Mean (relative enrichment levels are expressed as a percentage of the pretreatment PFC level within each animal. Mean relative enrichment values of the pretreatment PFC were 0.291 and 0.317 % for the vehicle- and succimer-treated groups, respectively. Symbols below the x-axis indicate: NS pre- and post-treatment mean values were not significantly different; a, b comparisons of post-treatment brain regions; means with different letters were significantly different from one another. # trend (p < 0.10) towards a significant difference between the vehicle and succimer groups. Data from Cremin et al. [13]. [Reprinted from Toxicology and Applied Pharmacology with permission from Elsevier]
We then conducted a rodent study to follow up the adult primate study to evaluate the efficacy of repeated chelation regimens to reduce blood and brain lead levels [14]. The rodent study utilized a 3 × 3 factorial design, with three levels of lead exposure (none, low, or high) and three levels of succimer treatment (none, one, or two succimer regimens). Animals were exposed to lead orally from the first day of life until postnatal day 40, followed by either one regimen of succimer chelation (PND 40–61) or two regimens, with the second regimen from PND 68 to 89. In each of these succimer regimens, the daily oral succimer dose over treatment days 1–7 was 50 mg/kg/day, followed by 25 mg/kg/day over treatment days 8–21, divided into two oral doses per day.
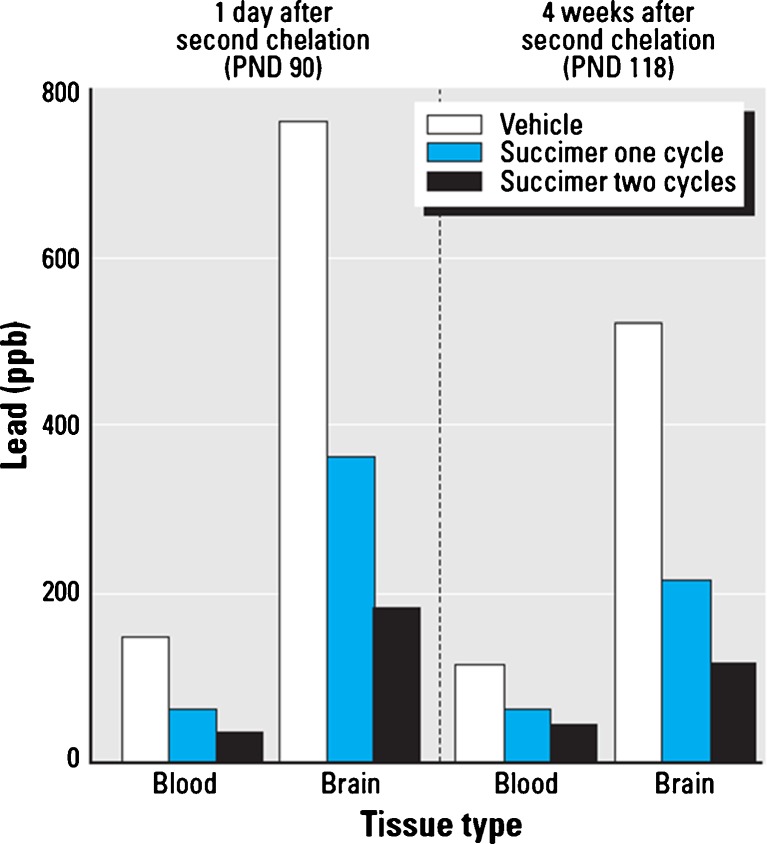
The results show that there were significant reductions in both blood and brain lead levels with succimer treatment, compared to vehicle, in this rodent model (Fig. 12). Specifically, blood and brain lead levels in animals treated with vehicle, one cycle of succimer or two cycles of succimer, and then evaluated either 1 day or 4 weeks after the second regimen ended show that two successive chelation regimens are significantly more efficacious at reducing brain lead levels than a single regimen. By comparison, the additional benefit of two versus one chelation regimen for reducing blood lead levels was not nearly as striking (Fig. 12).
Fig. 12.
Relative benefit of none, one, or two succimer chelation regimens for reducing blood and brain lead levels in rats. Data highlight the additional benefit of a second regimen of succimer treatment (vs. one regimen and vehicle treatments) in reducing blood and brain lead levels in rats, as a function of time since the second regimen ended. Data from Stangle et al. [14]. [Reproduced with permission from Environmental Health Perspectives]
Overall, these non-human primate and rodent study results indicate that blood lead levels may not accurately reflect changes in brain lead with chelation, and that reductions in brain lead levels lag significantly behind reductions in blood lead levels. They also indicate that brain lead levels may not be readily reduced with succimer chelation treatment when compared to the cessation of lead exposure alone, unless a sufficiently prolonged or aggressive chelation regimen(s) is used.
Can succimer treatment alleviate the neurobehavioral impacts of lead poisoning?
The question of whether succimer chelation has the potential to alleviate the neurobehavioral impacts of lead poisoning is of tremendous public health interest, since deficits in IQ and cognitive function are among the most sensitive and well-described effects of lead exposure in children [15–17]. There have been a limited number of published studies in animal models, as well as the pediatric double-blinded clinical study [18, 19] that have addressed this issue.
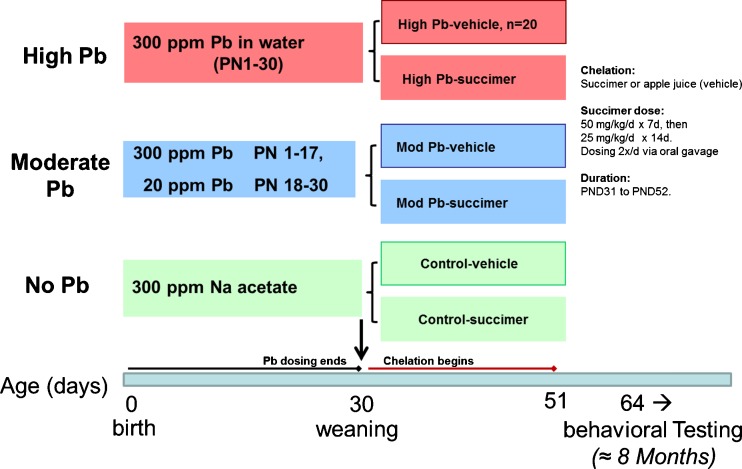
We present results from our published rodent studies to address this question [20, 21]. This study used a 3 × 2 factorial design with three levels of lead exposure (no lead, low lead, and high lead), crossed with two levels of succimer treatment (no succimer, i.e., the apple juice vehicle, or one 3-week succimer regimen; Fig. 13). The animals were lead exposed from birth until postnatal day 30 (pre-weaning exposure was via the lactating dam, post-weaning was via drinking water), followed by chelation, and then behavioral testing.
Fig. 13.
Study design and timing of testing in the rodent succimer chelation study (Stangle et al. [20]; Beaudin et al. [21]) [Stangle reproduced with permission from Environmental Health Perspectives; Beaudin reprinted from Neurotoxicology and Teratology with permission from Elsevier]
An extensive battery of behavioral tests, requiring approximately 8 months to complete, was administered. The tasks, which tapped a broad range of cognitive and affective functions, were similar in design and concept to tasks used with human subjects, to facilitate extrapolating the results to the target human population. We will focus mainly on a subset of the findings, specifically, the results from a series of visual discrimination and attention tasks.
Table 1 shows the blood lead levels of these rats immediately after the 3-week course of succimer chelation. The blood lead levels are quite low, within the range expected in current clinical studies of humans that are treated for lead exposure.
Table 1.
Mean ± SE blood and brain lead levels in rodents following succimer chelation treatment
| Group | Blood Pb (mcg/dL) | Brain Pb (ng/g dw)a |
|---|---|---|
| Control | 1.5 ± 0.1 | 41 ± 9 |
| Mod-Pb | 12.6 ± 0.8* | 1,040 ± 49* |
| High-Pb | 31.0 ± 0.8** | 3,690 ± 260** |
| Mod-Pb–succimer | 2.8 ± 0.2# | 196 ± 14.2# |
| High-Pb–succimer | 8.5 ± 0.7## | 1,370 ± 150## |
Data from Stangle et al. [20]. [Reproduced with permission from Environmental Health Perspectives.] n = 7–11 animals per group
*p < 0.005; **p < 0.0001, Pb-exposed (non-chelated) groups vs. control; # p < 0.003 for both blood and brain, Mod-Pb–succimer vs. Mod-Pb; ## p < 0.0001 for both blood and brain, high-Pb–succimer vs. high-Pb
ang Pb/g dry tissue
Behavioral testing was conducted in 12 automated Plexiglas chambers, each operated by a personal computer. Briefly, each chamber consisted of a waiting area, and a small testing alcove recessed into one wall. The alcove was separated from the waiting area by a metal guillotine-type door. Each of the three walls of the alcove contained a funnel-shaped response port. A light-emitting diode (LED) was mounted above each port; the illumination of one of these LEDs served as the discriminative cue in all of the tasks described here. Each trial began with the opening of the alcove door. Immediately after the animal broke the infrared beam at the alcove entrance, one of the three LEDs was illuminated. The LED remained illuminated until the animal made a 1-s nose-poke into one of the three ports or 60 s elapsed, whichever came first. A 1-s nose-poke into the port under the illuminated LED constituted the correct response and was rewarded with delivery of a 45-mg food pellet into the alcove.
During the initial visual discrimination, the light was illuminated immediately at trial onset, and the animals learned that a nose-poke into the port under the illuminated LED is rewarded. In the first attention task (attention task 1), we imposed a variable delay between trial onset and cue presentation as well as reduced the duration of cue illumination. These changes increase the demands on inhibitory control and attention. In addition the animals must learn that the cue will be presented after a delay on some trials, thus tapping associative ability as well.
The animals generally receive 150 to 200 trials in each daily testing session, in tasks that are administered for approximately 10 to 20 sessions each. Thus, in each of these tasks, we collect a wealth of information about how these animals learn these tasks, and how they perform as a function of manipulating the duration of the pre-cue delay and the duration of cue illumination. We also can assess performance as a function of the outcome of the previous trial (correct or incorrect), which provides very useful information concerning how the animals respond to making mistakes. This has proven very important in the area of lead toxicity, as we will discuss. In general, this approach enables us to assess many different cognitive and affective functions.
Before detailing specific task results, it may be useful to summarize the overall pattern of effects seen in this study. First, a short period of lead exposure during early development produced lasting cognitive and affective dysfunction. Second, the 3-week course of succimer chelation produced a significant benefit for the lead-exposed animals, although the degree of benefit varied as a function of both the intensity of the lead exposure and the specific area of dysfunction. These results are summarized in Table 2. Finally, this same 3-week course of succimer treatment, when administered to animals that were not exposed to lead, produced lasting cognitive and affective dysfunction that was as pervasive and large in magnitude as the dysfunction produced by the higher lead exposure regimen.
Table 2.
Summary of the specific functional domains affected by each of the two lead exposure regimens and the efficacy of succimer chelation in alleviating the dysfunction
| Domain of function | Mod Pb | High Pb | ||
|---|---|---|---|---|
| Pb effect? | Succ. benefit? | Pb effect? | Succ. benefit? | |
| Impaired learning ability | Yes | Yes | Yes | No |
| Impaired regulation of arousal and/or negative emotion | No | NA | Yes | Yes |
| Greater disruption following an error | ||||
| Attentional dysfunction | ||||
| Sustained attention/error reactivity | No | NA | Yes | Yes |
| Lapses in attention | No | NA | Yes | Intermediate |
| Impaired inhibitory control | No | NA | Yes | No |
| Impulsivity | ||||
Data from Stangle et al. [20]. [Reproduced with permission from Environmental Health Perspectives]
Pb effect? denotes whether a statistically significant effect of lead was detected, Succ. benefit? indicates whether succimer was efficacious at alleviating the lead induced dysfunction, NA not applicable
Let us start with the animals in the lower lead exposure group (referred to as the Mod-Pb group in these figures). The impairment seen in these animals was very specific, limited to learning ability. Figure 14 depicts the rate at which errors decreased across the first six sessions in the initial discrimination task (panel a), and the rate at which premature responses declined during the first attention task (panel b), as the rats learned to inhibit responses until presentation of the light cue. As seen in these two panels, the rats who received this lower exposure level without subsequent chelation learned both tasks significantly more slowly than the controls. These figures also illustrate that the rats given this same 30-day lead exposure regime but followed by succimer chelation performed like the controls. So succimer totally alleviated the learning deficit in these animals.
Fig. 14.
Succimer treatment significantly improved learning ability of the Mod-Pb rats. a Visual discrimination task (Mod-Pb–succimer vs. Mod-Pb; main effect contrast, p = 0.03). b Attention task 1. Data points are means ± SEs. *p = 0.056; **p ≤ 0.03; #p < 0.01, Mod-Pb vs. control. ##p = 0.03; †p = 0.006, Mod-Pb–succimer vs. Mod-Pb. Data from Stangle et al. [14]. [Reproduced with permission from Environmental Health Perspectives]
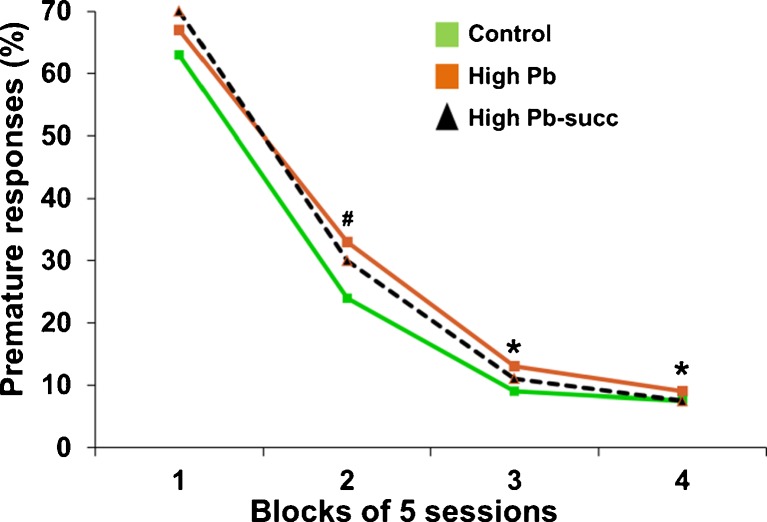
The results were a bit more complicated for the rats who received the higher lead exposure regimen (referred to as the high-Pb group in the tables and figures). These animals experienced a greater range of cognitive and affective dysfunction than those exposed to the lower lead exposure regimen, and the degree to which succimer was effective for these animals varied across these different functional domains, as summarized in Table 2. For example, the impaired learning of the high-Pb animals was in general not alleviated by succimer treatment; the high-Pb animals treated with succimer learned more slowly than the controls in the visual discrimination task and the attention task 1, and did not differ from their high-Pb-treated counterparts who had not been chelated. This pattern is depicted in Fig. 15, which illustrates the rate at which the animals learned to inhibit premature response in the first attention task that included long pre-cue delays on some trials. As seen in this figure, the high-Pb rats learned to wait for the cue more slowly than controls, and succimer chelation was only somewhat effective in normalizing learning rate (i.e., the succimer-chelated animals were not significantly different from controls or the high-Pb placebo-treated animals).
Fig. 15.
The impaired learning ability of the high-Pb rats was only somewhat alleviated by succimer chelation. Data show percent premature responses in attention task 1 as a function of the stage of testing (stages divided into blocks of sessions). Superscripts above symbols indicate level of significance for the contrasts between the high-Pb group and controls, #p < 0.07, *p < 0.05. Data from Stangle et al. [14]. [Reproduced with permission from Environmental Health Perspectives]
In contrast, for some other areas of dysfunction in the high-Pb animals, succimer chelation was completely effective; one example was the aberrant reaction of the high-Pb rats to committing an error on the prior trial. In these tasks, there was evidence that committing an error on the prior trial was disruptive—to all groups of animals, including the controls. Specifically, the percentage of all types of errors was significantly higher on trials that followed an error than on trials that followed a correct response. Similarly, the latency to enter the testing alcove and the latency to respond following cue presentation were both longer on trials that followed an error than on trials that followed a correct response. Notably, the performance of the high-Pb animals was significantly more disrupted by a prior error than that of the controls, suggesting an impaired ability to regulate the negative emotions produced by committing an error and/or not receiving an expected reward. This area of dysfunction, however, was totally alleviated by succimer treatment, as seen in Fig. 16.
Fig. 16.
Heightened reactivity to errors of the high-Pb rats was completely normalized by succimer treatment. Left panel shows percent omission errors on the attention task 2 across session blocks for trials that followed a correct response on the prior trial (left panel), and for trials that followed an error on the prior trial (right panel). Note that no significant lead or succimer effects were evident for trials follow a correct response (left panel), but there was a significant lead effect on trials following an error on the prior trial, and succimer alleviated this effect (right panel). **p < 0.01, high-Pb vs. control. #p < 0.01, high-Pb + succimer vs. high-Pb. Data from Stangle et al. [20]. [Reproduced with permission from Environmental Health Perspectives]
This figure depicts the percentage of omission errors committed across the three blocks of trials in each daily session of the sustained attention task (attention task 2), for trials that either followed a correct response trial (left panel) or trials that followed an error trial (right panel). In this task, the pre-cue delay was quite long on some trials to intensify demands on attention. There are several interesting aspects of the findings from this task. First, the percentage of omission errors goes up towards the end of each testing session, reflecting the waning of sustained attention across these long sessions. Second, the incidence of omission errors is much higher on trials that follow an error than on trials that follow a correct response, supporting the inference that committing an error on the prior trial disrupts the ability of the animals to pay attention on the next trial. Moreover, it is striking that the high-Pb-exposed animals are impaired, relative to controls, only on trials that follow an error. This pattern is very helpful for localizing the nature of the deficit. The fact that the groups did not differ for trials that followed a correct response demonstrates that they understood the rules of the game, that they were as motivated as controls, and that they did not differ from controls in the sensory or motor skills required for performance in this task. The selective deficit seen for trials that followed an error suggests that the non-chelated high-Pb animals were less able than controls to regulate the emotional or affective reaction to committing an error. Note too that the incidence of omission errors for the chelated high-Pb group was indistinguishable from the controls, indicating that succimer treatment totally alleviated this area of dysfunction. We evaluated error reactivity in several other tasks, and all corroborated this conclusion. It was a very solid finding.
A comment may be warranted about why succimer treatment was effective in alleviating certain areas of dysfunction but not others. One possibility is that it relates to the sensitivity of particular functions to elevated brain lead levels, coupled with the efficacy with which a 3-week course of succimer chelation reduced brain lead. In this study, the fact that the lower lead (Mod-Pb) animals experienced only learning dysfunction, whereas the high-Pb animals experienced learning, attentional, and affective dysfunction suggests that learning ability is sensitive to even slightly elevated brain lead levels whereas these other areas of functioning are not (or less so). One hypothesis is that the 3-week course of succimer reduced brain lead levels in the lower lead group to a level at which brain dysfunction is not seen. In contrast, this same succimer regimen reduced brain lead of the high-Pb group only to the level seen in the Mod-Pb animals. It seems plausible that a more prolonged succimer regimen or multiple regimens would have further reduced brain lead in the high-Pb group and consequently, their dysfunction. In sum, these data demonstrate that under conditions in which succimer significantly reduces brain lead levels, it is efficacious at reducing cognitive or affective deficits produced by lead exposure.
These findings from the rodent study are consistent with previous animal studies but contrast with the results of the Treatment of Lead-Exposed Children (TLC) study, the one clinical trial in lead-exposed children that included cognitive outcomes [18, 19]. We have given these contrasting results quite a bit of thought and can propose several possible reasons for the different outcomes. First, it is possible that the succimer treatment protocol used in the TLC trial may not have achieved a sufficient reduction in brain lead levels to improve cognitive functioning. In the TLC study, succimer treatment was discontinued when the blood lead levels of the children reached 15 mcg/dL; in light of rodent and primate studies showing that succimer-induced reductions in blood lead greatly overestimate reductions in brain lead levels [19, 22], it is plausible that brain lead levels may not have been sufficiently reduced with succimer (versus controls) in the TLC subjects. This suggestion is consistent with the relatively modest 4.5 mcg/dL difference in blood lead levels between the succimer- and placebo-treated children in the TLC study over the 6 months following treatment [18], which would be expected to yield only a modest difference in the lead concentration gradient between brain and blood, the likely mechanism underlying succimer's ability to lower brain lead levels [22]. Second, the disparate outcomes of the TLC trial and the rodent study presented here may also reflect differences in the nature of the cognitive tasks that were used. In particular, the tasks used here (relative to those in the TLC trial) may have provided more specific indices of the two functional domains most improved by succimer in the present study: associative learning ability and regulation of arousal and/or emotion (indexed by reactivity to errors).
Does succimer treatment, in the absence of lead poisoning, produce negative effects on neurobehavioral measures?
One other very interesting and important aspect of the findings from this study [20] pertain to the effects of succimer chelation in the animals that were not exposed to lead. We included this group to gauge the safety of prolonged succimer regimens, which might continue in some cases past the point at which brain lead levels are elevated. Obviously in clinical practice, the physician does not have information about brain lead levels, and we know from our animal model studies that reductions in blood lead levels may not accurately predict reductions in brain lead. Also, succimer (and other chelating agents) is at times used off label for the treatment of other conditions, such as autism, when there is no indication of lead poisoning.
Figure 17 shows the performance of the control and succimer-treated groups, neither with a history of lead exposure. Panel a depicts the slower learning of the succimer-chelated animals (called the “succimer-only group”) in the initial visual discrimination task. As seen in panel b, these animals also performed more poorly than controls in the first attention task, committing a higher percentage of inaccurate responses throughout the 20 sessions on the task, indicative of attentional dysfunction. Panel c illustrates the higher incidence of omission errors of this group in the sustained attention task particularly for trials with the longer cues, indicative of lapses in attention; i.e., this group benefited less than controls by the lengthening of the cue.
Fig. 17.
Succimer treatment of the non-lead-exposed rats impaired performance in a visual discrimination task (main effect contrast, p = 0.04), b attention task 1, and c the sustained attention task (treatment × cue duration, p = 0.004). Data points are means ± SEs. *p = 0.07; **p < 0.05, succimer-only vs. controls. Data from Stangle et al. [20]. [Reproduced with permission from Environmental Health Perspectives]
Finally, Fig. 18 depicts the performance of these two groups in a selective attention task, in which an olfactory distractor was presented on some trials. In this task, too, the animals who received succimer in the absence of lead exposure were impaired relative to controls (panels a and b), with the largest impairment seen for trials that both included a distractor and followed an error on the prior trail (panel b), indicating both impaired selective attention and an impaired ability to regulate the affective response to an error.
Fig. 18.
This figure depicts the percent inaccurate responses in the selective attention task, as a function of whether or not a distractor was presented on the current trial and whether the prior trial was correct (a) or incorrect (b). The rats treated with succimer in the absence of lead exposure performed significantly worse than controls, with the largest impairment seen for trials that both included a distractor and followed an error. Data are means ± SEs. *p = 0.02; **p < 0.01, succimer-only vs. controls. Data from Stangle et al. [20]. [Reproduced with permission from Environmental Health Perspectives]
Summary
In summary, several conclusions about succimer chelation can be drawn from the presented animal studies:
The primate study showed that succimer treatment is effective at accelerating the elimination of lead from the body, but it was only marginally better than the complete cessation of lead exposure alone, at least with respect to the conditions of this study. This is particularly true if one looks beyond the period after succimer treatment ended, when often there was some rebound in blood lead levels in the succimer group that diminished differences in blood lead levels between the chelated and vehicle groups.
With respect to the question of whether brain lead levels are reduced with succimer chelation, we think that the answer is yes, but only if the succimer treatment regimen is sufficiently aggressive. In the adult primate study, there was no evidence of a reduction in brain lead with a single 19-day succimer treatment regimen. But in the rodent studies, we found that succimer treatment significantly reduced brain lead levels. In comparing these results it should be considered that a 21-day treatment regimen in rodents would extrapolate to a much longer treatment regimen in a non-human primates or humans, based on allometric scaling between rodents and primates.
It was clear in both the rodent and in the primate studies that blood lead levels were a relatively poor surrogate of brain lead levels, and that the reduction in blood lead levels with treatment was a poor predictor of, and over-estimated, reductions in brain lead levels in those animals.
The rodent study demonstrated that it is possible for succimer chelation therapy to alleviate certain types of lead-induced behavioral/cognitive dysfunction. This is an important demonstration because lead-induced behavioral dysfunction has generally been considered to be irreversible [15]. The present findings thus suggest that if a succimer treatment protocol that produced a substantial removal of lead from the brain could be identified for humans, a functional benefit might be derived.
The finding that succimer produced lasting adverse effects when administered to non-lead-exposed rats highlights the potential risks of administering succimer or other metal-chelating agents to children who do not have elevated tissue lead levels. It is of significant concern that this type of therapy is being widely advocated as safe and effective for treating autism.
Acknowledgments
We are grateful to the many individuals who contributed to the research that provided the foundation for much of what we know about the efficacy and underlying mechanisms of lead chelation therapy in humans and animals models. In particular, we thank L. Bayer, S. Beaudin, J. Cremin, M. Kuypers, N. Laughlin, M. Luck, D. Stangle, M. Strawderman, and D. Woolard for their significant contributions to the studies presented here. Funding for the original studies presented here was provided by the National Institutes of Health (R01 ES06918, ES07535, ES07457, ES07457, ES05950, and DK0715827, and F32 ES05870).
ATSDR Disclaimer
This publication was supported by the cooperative agreement award number 1U61TS000117-04 from the Agency for Toxic Substances and Disease Registry (ATSDR). Its contents are the responsibility of the authors and do not necessarily represent the official views of the Agency for Toxic Substances and Disease Registry (ATSDR).
Sources of funding for project
n/a.
Conflict delineations
For the work under consideration for publication, Dr. Smith received an honorarium and reimbursement for travel through the ACMT/ATSDR Cooperative Agreement. Dr. Strupp has no conflicts.
References
- 1.Graziano JH, Siris ES, LoIacono N, Silverberg SJ, Turgeon L. 2,3-Dimercaptosuccinic acid as an antidote for lead intoxication. Clin Pharmacol Ther. 1985;37:431–438. doi: 10.1038/clpt.1985.67. [DOI] [PubMed] [Google Scholar]
- 2.Graziano JH. Role of 2,3-dimercaptosuccinic acid in the treatment of heavy metal poisoning. Med Toxicol. 1986;1:155–162. doi: 10.1007/BF03259834. [DOI] [PubMed] [Google Scholar]
- 3.Graziano JH, Lolacono NJ, Meyer P. Dose–response study of oral 2,3-dimercaptosuccinic acid in children with elevated blood lead concentrations. J Pediatr. 1988;113(4):751–757. doi: 10.1016/S0022-3476(88)80396-2. [DOI] [PubMed] [Google Scholar]
- 4.Graziano JH, Lolacono NJ, Moulton T, Mitchell ME, Slavkovich V, Zarate C. Controlled study of meso-2,3-dimercaptosuccinic acid for the management of childhood lead intoxication. J Pediatr. 1992;120(1):133–139. doi: 10.1016/S0022-3476(05)80618-3. [DOI] [PubMed] [Google Scholar]
- 5.Aposhian HV, Maiorino RM, Dart RC, Perry DF. Urinary excretion of meso-2,3-dimercaptosuccinic acid in human subjects. Clin Pharmacol Ther. 1989;45:520–526. doi: 10.1038/clpt.1989.67. [DOI] [PubMed] [Google Scholar]
- 6.Aposhian HV, Aposhian MM. Meso-2,3-dimercaptosuccinic acid: chemical, pharmacological and toxicological properties of an orally effective metal chelating agent. Annu Rev Pharmacol Toxicol. 1990;30:279–306. doi: 10.1146/annurev.pa.30.040190.001431. [DOI] [PubMed] [Google Scholar]
- 7.Rivera M, Zheng W, Aposhian HV, Fernando Q. Determination and metabolism of dithiol chelating agents VIII. Metal complexes of meso-dimercaptosuccinic acid. Toxicol Appl Pharmacol. 1989;100:96–106. doi: 10.1016/0041-008X(89)90094-X. [DOI] [PubMed] [Google Scholar]
- 8.Maiorino RM, Akins JM, Blaha K, Carter DE, Aposhian HV. Determination and metabolism of dithiol chelating agents: X. In humans, meso-2,3-dimercaptosuccinic acid is bound to plasma proteins via mixed disulfide formation. J Pharmacol Exp Ther. 1990;254(2):570–577. [PubMed] [Google Scholar]
- 9.Dart RC, Hurlbut KM, Maiorino RM, Mayersohn M, Aposhian HV, Hassen LV. Pharmacokinetics of meso-2,3-dimercaptosuccinic acid in patients with lead poisoning and in healthy adults. J Pediatr. 1994;125:309–316. doi: 10.1016/S0022-3476(94)70217-9. [DOI] [PubMed] [Google Scholar]
- 10.Asiedu P, Moulton T, Blum CB, Roldan E, Lolacono NJ, Graziano JH. Metabolism of meso-2,3-dimercaptosuccinic acid in lead-poisoned children and normal adults. Environ Health Perspect. 1995;103(7–8):734–739. doi: 10.1289/ehp.95103734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith DR, Woolard D, Luck M, Laughlin N. Succimer and the reduction of tissue lead in juvenile monkeys. Toxicol Appl Pharmacol. 2000;166:230–240. doi: 10.1006/taap.2000.8973. [DOI] [PubMed] [Google Scholar]
- 12.Cremin J, Luck M, Laughlin N, Smith DR. Oral succimer decreases the gastrointestinal absorption of lead in juvenile monkeys. Environ Health Perspect. 2001;109:613–620. doi: 10.1289/ehp.01109613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cremin J, Luck M, Laughlin N, Smith DR. Efficacy of succimer chelation for reducing brain lead in a primate model of human lead exposure. Toxicol Appl Pharmacol. 1999;161:283–293. doi: 10.1006/taap.1999.8807. [DOI] [PubMed] [Google Scholar]
- 14.Stangle D, Strawderman M, Smith D, Kuypers M, Strupp B. Reductions in blood lead overestimate reductions in brain lead after repeated succimer regimens in a rodent model of childhood lead exposure. Environ Health Perspect. 2004;112:302–308. doi: 10.1289/ehp.6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Needleman HL, Riess JA, Tobin MJ, Biesecker GE, Greenhouse JB. Bone lead levels and delinquent behavior. JAMA. 1996;275:363–369. doi: 10.1001/jama.1996.03530290033034. [DOI] [PubMed] [Google Scholar]
- 16.ACCLPP, Report of the Advisory Committee on Childhood Lead Poisoning Prevention of the Centers for Disease Control and Prevention. “Low Level Lead Exposure Harms Children: A Renewed Call for Primary Prevention”, January 4, 2012. http://www.cdc.gov/nceh/lead/ACCLPP/Final_Document_030712.pdf .
- 17.CDC, Response to Advisory Committee on Childhood Lead Poisoning Prevention Recommendations in “Low Level Lead Exposure Harms Children: A Renewed Call of Primary Prevention”. June 7, 2012. http://www.cdc.gov/nceh/lead/ACCLPP/CDC_Response_Lead_Exposure_Recs.pdf .
- 18.Rogan WJ, Dietrich KN, Ware JH, Dockery DW, Salganik M, Radcliffe J, et al. The effect of chelation therapy with succimer on neuropsychological development in children exposed to lead. N Engl J Med. 2001;344:1421–1426. doi: 10.1056/NEJM200105103441902. [DOI] [PubMed] [Google Scholar]
- 19.Dietrich KN, Ware JH, Salganik M, Radcliffe J, Rogan WJ, Rhoads GG, et al. Treatment of lead-exposed children clinical trial group: effect of chelation therapy on the neuropsychological and behavioral development of lead exposed children after school entry. Pediatrics. 2004;114:19–26. doi: 10.1542/peds.114.1.19. [DOI] [PubMed] [Google Scholar]
- 20.Stangle D, Smith DR, Beaudin S, Strawderman M, Levitsky D, Strupp B. Succimer chelation improves learning, attention and arousal regulation in lead-exposed rats but produces lasting cognitive impairment in the absence of lead exposure. Env Health Perspect. 2007;115(2):201–209. doi: 10.1289/ehp.9263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beaudin S, Stangle D, Smith DR, Levitsky D, Strupp B. Succimer chelation normalizes reactivity to reward omission and errors in lead-exposed rats. Neurotoxicol Teratol. 2007;29:188–202. doi: 10.1016/j.ntt.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Smith DR, Bayer LE, Strupp BJ. Efficacy of succimer chelation for reducing brain Pb in rodents. Env Res. 1998;78:168–176. doi: 10.1006/enrs.1998.3854. [DOI] [PubMed] [Google Scholar]