Abstract
Freeman-Sheldon syndrome (FSS), as first described by Freeman and Sheldon in 1938, is a morphologically well-defined syndrome that results in a dysmorphic status combining bone anomalies and joint contractures with characteristic facies. It is part of the nosologic group of pathologies currently known as distal arthrogryposis as reported by Hall et al. (Am J Med Genet 11:185–239, 1982 [1]). It is a rare disorder and its exact prevalence is unknown. Our objective is to report a case of FSS presenting with microstomia and add a brief review of the literature for similar cases.
Keywords: Freeman-Sheldon syndrome, Multiple congenital contracture, Distal arthrogryposis type 2A, Microstomia
Introduction
Freeman-Sheldon syndrome (FSS), also termed distal arthrogryposis type 2A (DA2A), craniocarpotarsal dysplasia (or dystrophy), Cranio-carpo-tarsal syndrome, Windmill-Vane-Hand syndrome, or Whistling-face syndrome, was originally described by Freeman and Sheldon in 1938. [2, 3] It is a rare form of multiple congenital contracture (MCC) syndromes (arthrogryposes) and is the most severe form of distal arthrogryposis (DA) [4–6].
Freeman-Sheldon syndrome is a condition that primarily affects the face, hands, and feet. People with this disorder have a distinctive facial appearance including a small mouth (microstomia) with pursed lips, giving the appearance of a whistling face. For this reason, the condition is sometimes called “whistling face syndrome.”
Epidemiological data for the prevalence of FSS are not available, but less than 100 cases have been reported in the literature.
Most reported cases of FSS occur sporadically with no family history of the disease, though there are reports of a specific pattern of autosomal dominant inheritance in several families [7, 8]. Temtany and McKusick [9] observed that FSS affected two generations of three different families. However, there are also reports of the syndrome suggesting an autosomal recessive inheritance; in other words, of affected children born to parents who were clinically normal but possible carriers (heterozygotic) of the gene responsible for the syndrome [10–14].
Case Report
An 11-year-old girl was referred to our department with a complaint of microstomia (Fig. 1). She is the second child born to young, healthy but consanguineous parents. The mother is 32 years of age and the father 35 years presently. The mother had not taken any drugs, nor was exposed to X-rays during pregnancy.
Fig. 1.
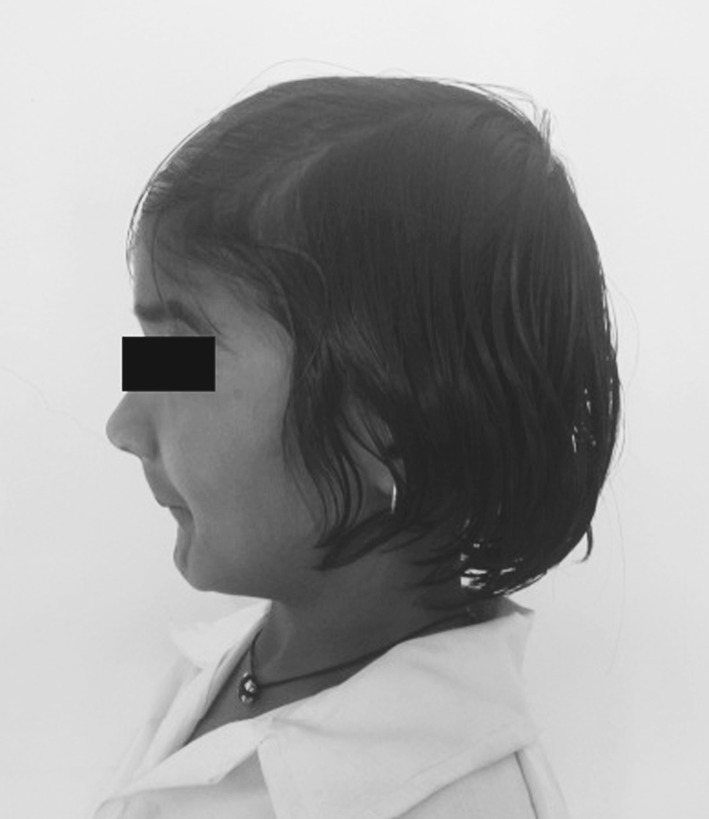
Lateral view
The pedigree analysis showed that no other family members had the syndrome. Other more distant relatives such as grandparents, uncles, and aunts from either the father’s or the mother’s side were not examined; however, they were reported to us as apparently normal.
The mother gave a history that the patient, as a child experienced feeding difficulties in the neonatal period due to a poor suckling, possibly because of an inability to form a tight seal around the nipple as a consequence of the microstomia and contractures of the circumoral muscles which is typical of such cases.
She had a history of recurrent chest infection on change of weather conditions. She manages her routine activities pretty well but was not involved in any sort of school sports activities as she could not run at a fast pace.
Cognitive development was appropriate for chronological age. She was calm and interacted well with the examiner. Vision and hearing were apparently normal.
Physical examination indicated weight as 18 kg, height of 3 ft, pulse at 82 bpm. Cardiac auscultation indicated that sounds were phonetically normal, without murmurs. The lungs were free of any problems. Patient did not have any visceromegaly. Spinal column had no pathological deviations which is an atypical finding in such cases. Ophthalmic evaluation revealed mild telecanthus and ptosis of the left eye (Fig. 2). Puckered lips (fused commissura labiorum) mimicking the act of whistling was noted with micrognathia (Fig. 3). “H”-shaped cutaneous dimpling on the chin which is a characteristic feature of cases of FSS was an obvious feature noted (Fig. 4).
Fig. 2.
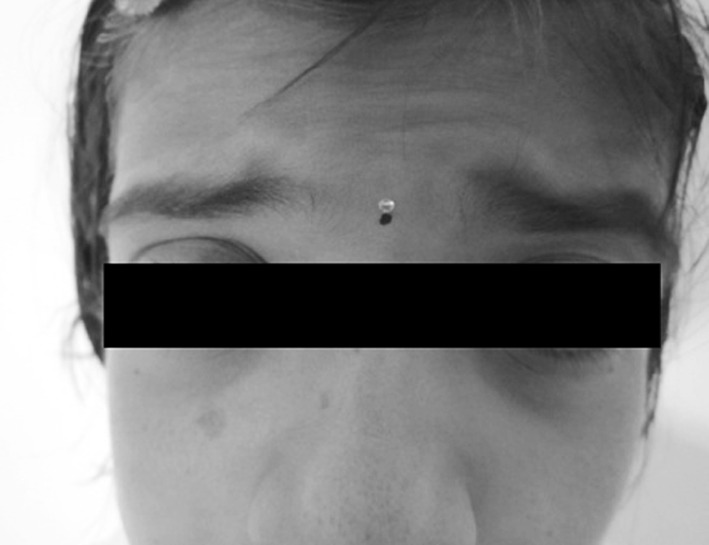
Ptosis
Fig. 3.

Micrognathia
Fig. 4.

H‐shaped dimpling
Intra oral examination revealed a small tongue, high-arched palate, mild crowding in both the arches and delayed eruption of permanent teeth (Fig. 5). All teeth were caries free and had no obvious periodontal pathology (Fig. 6).
Fig. 5.
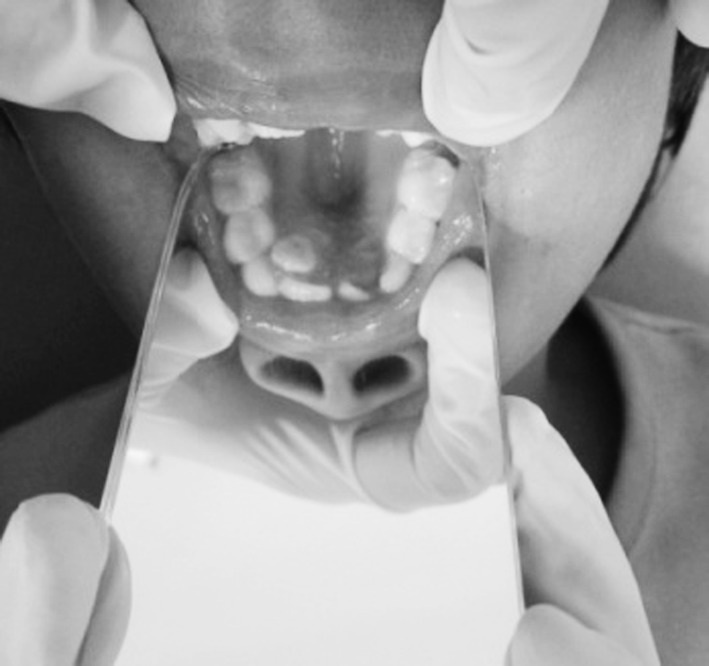
High arched palate
Fig. 6.
Developing dentition
A short neck along with muscle contractures leading to an inability of completely extend the neck was noted. The patient could not raise her arms above the shoulder level.
Further treatment planning and investigations like full craniofacial radiographs was postponed as the patient insisted on delaying surgical intervention for correction of microstomia at a later date.
Discussion
Freeman-Sheldon syndrome is a type of distal arthrogryposis, related to distal arthrogryposis type 1 (DA1) [15]. In 1996, more strict criteria for the diagnosis of FSS were drawn up, assigning FSS as DA2A [6].
In March 2006, Stevenson et al. [16] published diagnostic criteria for DA2A. Antley et al. [17] also established certain diagnostic criteria for FSS. In an attempt to classify the distal arthrogryposis, Bamshad et al. [18] identified a total nine disorders related to FSS.
FSS is understood as part of a group of disorders that concur with congenital multiple contractures. A congenital contracture is a structural deformity that hinders normal flexion and/or extension of a specific area of the body. The presence of contractures in one or more areas of the body of newborn infants is usually called arthrogryposis. This term is, however, merely descriptive and not pathognomonic nor diagnostic. Interestingly, it is common for FSS children to be considered as simple cases of arthrogryposis before a final diagnosis of the syndrome. However, not all arthrogryposis children have FSS. Likewise, the presence of craniofacial deformities suggestive of FSS but not concomitant with arthrogryposis are a rare condition, and the deformities alone do not allow for diagnosis of FSS. Toydemir et al. [19] described the only case in the literature until the present time related to a child with whistling face phenotype without limb abnormalities, the patient being born to normal and nonconsanguineous parents.
Growth parameters during the prenatal and perinatal periods are usually within the range of normal. Gross and fine motor milestones may be achieved somewhat later than age-matched controls but virtually all affected individuals become ambulatory without assist devices. Speech and language are rarely delayed. Overall facial movement, however, may be diminished.
The intelligence of patients is usually reported normal, though there are occasional reports of association with mental retardation, especially in cases with combined important structural anomalies of the central nervous system. Approximately one-third have some degree of intellectual disability.
Affected individuals may have a number of abnormalities that affect the eyes. These may include hypertelorism, deep-set eyes, outside corners of the eyes that point downward (down-slanting palpebral fissures), a narrowing of the eye opening, ptosis and eyes that do not look in the same direction (strabismus).
Freeman-Sheldon syndrome is also characterized by joint deformities and contractures that restrict movement. People with this disorder typically have multiple contractures in the hands and feet at birth. These contractures lead to permanently bent fingers and toes (camptodactyly), a hand deformity in which all of the fingers are angled outward toward the fifth finger (ulnar deviation, also called “windmill vane hand”), and inward- and downward-turning feet (clubfoot). Affected individuals may also have a spine that curves to the side (scoliosis).
Pneumonitis and bronchitis often follow seemingly mild upper respiratory tract infections.
Freeman-Sheldon syndrome may be caused by mutations in the MYH3 gene. The MYH3 gene provides instructions for making a protein called embryonic skeletal muscle myosin heavy chain 3. Myosin and another protein called actin are the primary components of muscle fibers and are important for muscle contraction. Embryonic skeletal muscle myosin heavy chain 3 forms part of a myosin protein complex that is active before birth and is important for normal development of the muscles.
Some people with FSS do not have mutations in the MYH3 gene. In these individuals, the cause of the disorder is unknown.
Freeman-Sheldon syndrome can have different inheritance patterns. In some cases, the condition is inherited in an autosomal dominant pattern, which means one copy of the altered gene in each cell is sufficient to cause the disorder. The condition can also have an autosomal recessive inheritance pattern, which means both copies of the gene in each cell have mutations. The parents of an individual with an autosomal recessive condition each carry one copy of the mutated gene, but they typically do not show signs and symptoms of the condition.
Bekir et al. [20] are among those who described the case of two siblings affected by FSS and who were born to normal parents. Despite the syndrome being a result of autosomal recessive inheritance, the authors, nevertheless, indicated that this could be explained by genetic expression of the mutant gene in one of the parents.
Patients must have early consultation with craniofacial and orthopedic surgeons, when craniofacial [21–23], clubfoot [24], or hand correction[25–28] is indicated to improve function or aesthetics. Operative measures should be pursued cautiously, with avoidance of radical measures and careful consideration of the abnormal muscle physiology in FSS. Unfortunately, many surgical procedures have suboptimal outcomes, secondary to the myopathy of the syndrome.
When operative measures are to be undertaken, they should be planned for as early in life as is feasible, in consideration of the tendency for fragile health. Early interventions hold the possibility to minimize developmental delays and negate the necessity of relearning basic functions.
People with FSS also have an increased risk of developing a severe reaction to certain drugs like muscle relaxants and anesthetic gases used during surgery. This reaction is called malignant hyperthermia. Malignant hyperthermia presents with muscle rigidity, breakdown of muscle fibers (rhabdomyolysis), a high fever, increased acid levels in the blood and other tissues (acidosis), and a rapid heart rate. The complications of malignant hyperthermia can be life-threatening unless they are treated promptly.
Malignant hyperthermia and muscle rigidity after anesthesia has been reported previously in three children with FSS [29, 30].
Structural anomalies of the oropharynx and upper airways, which are commonly diagnosed in these patients, are a constant concern in cases of need for general anesthesia, not uncommon due to the various types of corrective surgery that are usually necessary in FSS. Among the several studies that approached this matter specifically, we would like to emphasize that of Munro et al. [31], which was directed to pediatric patients. Most often, tracheal intubation via direct laryngoscopy cannot be carried out in FSS pediatric patients. Robinson [32] reported a case of FSS combined with severe upper airway obstruction that required neonatal tracheostomy.
Due to the abnormal muscle physiology in FSS, therapeutic measures may have unfavorable outcomes [33]. Difficult endotracheal intubations and vein access complicate operative decisions in many DA2A patients. Cruickshanks et al. [34] reports uneventful use of non-MH-triggering agents. Reports have been published about spina bifida occulta in anesthesia management [35] and cervical kyphoscoliosis in intubations [36].
Conclusion
Freeman-Sheldon syndrome is heterogeneous not only in its clinical presentation but also in its genetic transmission. It is very important to be informed about the existence of more than one form of hereditary transmission of this syndrome, since genetic counseling should take into consideration all possibilities.
Until the gene for FSS is mapped, it is impossible to carry out prenatal diagnosis of the disorder through direct DNA analysis. However, Robbins-Furman et al. [37] were able to carry out prenatal diagnosis of FSS in a 20-week-old fetus using positive family history and ultrasonographic findings. The author’s findings were based on ultrasonographic features of abnormalities of the extremities of the fetus.
The follow-up of FSS pediatric patients requires support and special care including prolonged orthodontic and orthopedic treatment. Physical therapy can improve gait. Surgical correction of microstomia is important from both the aesthetic and functional points of view in relation to food intake and in this sense, good results have been reported [38].
There is no specific therapy for FSS. However, patients benefit from early intervention with occupational and physical therapy and/or surgery. Life expectancy and cognitive abilities are normal.
Though respiratory challenges and complications faced by a patient with FSS can be numerous, the syndrome’s primary involvement is limited to the musculoskeletal systems, and satisfactory quality and length of life can be expected with proper care.
References
- 1.Hall JG, Reed SD, Greene G. The distal arthrogryposes: delineation of new entities-review and nosologic discussion. Am J Med Genet. 1982;11:185–239. doi: 10.1002/ajmg.1320110208. [DOI] [PubMed] [Google Scholar]
- 2.Freeman EA, Sheldon JH. Cranio-carpo-tarsal dystrophy: undescribed congenital malformation. Arch Dis Child. 1938;13:277–283. doi: 10.1136/adc.13.75.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.James W, Berger T, Elston D. Andrews’ diseases of the skin: clinical dermatology. 10. Saunders: Philadelphia; 2005. [Google Scholar]
- 4.Online ‘Mendelian Inheritance in Man’ (OMIM) 193700 [PubMed]
- 5.Stevenson DA, Carey JC, Palumbos J, Rutherford A, Dolcourt J, Bamshad MJ. Clinical characteristics and natural history of Freeman-Sheldon syndrome. Pediatrics. 2006;117(3):754–762. doi: 10.1542/peds.2005-1219. [DOI] [PubMed] [Google Scholar]
- 6.Bamshad M, Jorde LB, Carey JC. A revised and extended classification of the distal arthrogryposes. Am J Med Genet. 1996;65(4):277–281. doi: 10.1002/(SICI)1096-8628(19961111)65:4<277::AID-AJMG6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 7.Fraser FC, Pashayan H, Kadish ME. Cranio-carpo-tarsal dysplasia. Report of a case in father and son. JAMA. 1970;8:1374–1376. doi: 10.1001/jama.1970.03170080062018. [DOI] [PubMed] [Google Scholar]
- 8.Klemp P, Hall JG. Dominant distal arthrogryposis in a Maori family with marked variability of expression. Am J Med Genet. 1995;55:414–419. doi: 10.1002/ajmg.1320550406. [DOI] [PubMed] [Google Scholar]
- 9.Temtany S, McKusick V. Contracture deformities as a part of syndromes. Birth Defects Orig Artic Ser. 1978;3:447–449. [Google Scholar]
- 10.Alves AFP, Azevedo ES. Recessive form of Freeman-Sheldon’s syndrome or “whistling face”. J Med Genet. 1977;14:139–141. doi: 10.1136/jmg.14.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dallapiccola B, Giannotti A, Lembo A, Sagui L. Autosomal recessive form of whistling face syndrome in sibs. Am J Med Genet. 1989;22:542–544. doi: 10.1002/ajmg.1320330426. [DOI] [PubMed] [Google Scholar]
- 12.Fitzsimmons JS, Zaldua V, Chrispin AR. Genetic heterogeneity in the Freeman-Sheldon syndrome: two adults with probable autosomal recessive inheritance. J Med Genet. 1984;21:364–368. doi: 10.1136/jmg.21.5.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kousseff BG, McConnachie P, Hadro TA. Autosomal recessive type of whistling face syndrome in twins. Pediatrics. 1982;69:328–331. [PubMed] [Google Scholar]
- 14.Sanchez JM, Kaminker CP. New evidence for genetic heterogeneity of the Freeman-Sheldon syndrome. Am J Med Genet. 1986;25:507–511. doi: 10.1002/ajmg.1320250312. [DOI] [PubMed] [Google Scholar]
- 15.Hall JG, Reed SD, Greene G. The distal arthrogryposes: delineation of new entities—review and nosologic discussion. Am J Med Genet. 1982;11(2):185–239. doi: 10.1002/ajmg.1320110208. [DOI] [PubMed] [Google Scholar]
- 16.Stevenson DA, Carey JC, Palumbos J, Rutherford A, Dolcourt J, Bamshad MJ. Clinical characteristics and natural history of Freeman-Sheldon syndrome. Pediatrics. 2006;117:754–762. doi: 10.1542/peds.2005-1219. [DOI] [PubMed] [Google Scholar]
- 17.Antley RM, Uga N, Burzynski NJ, Baum RS, Bixler D. Diagnostic criteria for the whistling face syndrome. Birth Defects Orig Artic Ser. 1975;11:161–168. [PubMed] [Google Scholar]
- 18.Bamshad M, Jorde LB, Carey JC. A revised and extended classification of distal arthrogryposis. Am J Med Genet. 1996;65:277–281. doi: 10.1002/(SICI)1096-8628(19961111)65:4<277::AID-AJMG6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 19.Toydemir PB, Toydemir R, Bokesoy I. Whistling face phenotype without limb abnormalities. (Letter) Am J Med Genet. 1999;86:86–87. doi: 10.1002/(SICI)1096-8628(19990903)86:1<86::AID-AJMG17>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 20.Bekir N, Bayraktaroglu YC, Coskun Y, Karaaslan C. Whistling face (Freeman Sheldon) syndrome in two siblings. Turk J Pediatr. 1994;36:329–332. [PubMed] [Google Scholar]
- 21.Vaitiekaitis AS, Hornstein L, Neale HW. A new surgical procedure for correction of lip deformity in cranio-carpo-tarsal dysplasia (whistling face syndrome) J Oral Surg. 1979;37(9):669–672. [PubMed] [Google Scholar]
- 22.Nara T. Reconstruction of an upper lip and the coloboma in the nasal ala accompanying with Freeman-Sheldon syndrome. Nippon Geka Hokan. 1981;50(4):626–632. [PubMed] [Google Scholar]
- 23.Ferreira LM, Minami E, Andrews Jde M. Freeman-Sheldon syndrome: surgical correction of microstomia. Br J Plast Surg. 1994;47(3):201–202. doi: 10.1016/0007-1226(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 24.Malkawi H, Tarawneh M. The whistling face syndrome or craniocarpotarsal dysplasia. Report of two cases in a father and son and review of the literature. J Pediatr Orthop. 1983;3(3):364–369. doi: 10.1097/01241398-198307000-00017. [DOI] [PubMed] [Google Scholar]
- 25.Call WH, Strickland JW. Functional hand reconstruction in the whistling-face syndrome. J Hand Surg [Am] 1981;6(2):148–151. doi: 10.1016/S0363-5023(81)80168-2. [DOI] [PubMed] [Google Scholar]
- 26.Martini AK, Banniza von Bazan U. Hand deformities in Freeman-Sheldon syndrome and their surgical treatment (in German) Z Orthop Ihre Grenzgeb. 1983;121(5):623–629. doi: 10.1055/s-2008-1053288. [DOI] [PubMed] [Google Scholar]
- 27.Martini AK, Banniza von Bazan U. Surgical treatment of the hand deformity in Freeman-Sheldon syndrome (in German) Handchir Mikrochir Plast Chir. 1982;14(4):210–212. [PubMed] [Google Scholar]
- 28.Wenner SM, Shalvoy RM. Two-stage correction of thumb adduction contracture in Freeman-Sheldon syndrome (craniocarpotarsal dysplasia) J Hand Surg [Am] 1989;14(6):937–940. doi: 10.1016/S0363-5023(89)80040-1. [DOI] [PubMed] [Google Scholar]
- 29.Sobrado CG, Ribera M, Marti M, Erdocia J, Rodriguez R. Freeman-Sheldon syndrome: generalized muscular rigidity after anesthetic induction [in Spanish] Rev Esp Anestesiol Reanim. 1994;41:182–184. [PubMed] [Google Scholar]
- 30.Aldinger G, Eulert J. The Freeman-Sheldon syndrome (in German) Z Orthop Ihre Grenzgeb. 1983;121(5):630–633. doi: 10.1055/s-2008-1053289. [DOI] [PubMed] [Google Scholar]
- 31.Munro HM, Butler PJ, Washington EJ. Freeman-Sheldon (whistling face syndrome). Anesthetic and airway management. Pediatr Anaesth. 1997;4:345–348. doi: 10.1046/j.1460-9592.1997.d01-90.x. [DOI] [PubMed] [Google Scholar]
- 32.Robinson PJ. Freeman-Sheldon syndrome: severe upper airway obstruction requiring neonatal tracheostomy. Pediatric Pulm. 1997;6:457–459. doi: 10.1002/(SICI)1099-0496(199706)23:6<457::AID-PPUL10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 33.Jones R, Dolcourt JL. Muscle rigidity following halothane anesthesia in two patients with Freeman-Sheldon syndrome. Anesthesiology. 1992;77:599–600. doi: 10.1097/00000542-199209000-00031. [DOI] [PubMed] [Google Scholar]
- 34.Cruickshanks GF, Brown S, Chitayat D. Anesthesia for Freeman-Sheldon syndrome using a laryngeal mask airway. Can J Anaesth. 1999;46(8):783–787. doi: 10.1007/BF03013916. [DOI] [PubMed] [Google Scholar]
- 35.Namiki M, Kawamata T, Yamakage M, Matsuno A, Namiki A. Anesthetic management of a patient with Freeman-Sheldon syndrome (in Japanese) Masui. 2000;49(8):901–902. [PubMed] [Google Scholar]
- 36.Vas L, Naregal P. Anaesthetic management of a patient with Freeman Sheldon syndrome. Paediatr Anaesth. 1998;8(2):175–177. doi: 10.1046/j.1460-9592.1998.00676.x. [DOI] [PubMed] [Google Scholar]
- 37.Robbins-Furman P, Hecht JT, Rocklin M, Maklad N, Greenhaw G, Wilkins I. Prenatal diagnosis of Freeman-Sheldon syndrome (whistling face) Pren Diagn. 1995;15:179–182. doi: 10.1002/pd.1970150212. [DOI] [PubMed] [Google Scholar]
- 38.Ohyama K, Susami T, Kato Y, Amano H, Kuroda T. Freeman-Sheldon syndrome. Case management from age 6 to 16 years. Cleft Palate Craniofac J. 1997;34:151–153. doi: 10.1597/1545-1569(1997)034<0151:FSSCMF>2.3.CO;2. [DOI] [PubMed] [Google Scholar]


