Abstract
In this study, we investigated the relationship between the expression levels of self-antigen and the function of self-reactive T cells in the periphery. To this end, we used two rat insulin promoter-ovalbumin (RIP-OVA) transgenic mice (RIP-OVAhigh, RIP-OVAlow) in which was produced only in pancreatic β-islet cells. The OVA-producing transgenic mice were crossed to DO.11.10 (DO) mice expressing a T cell antigen receptor specific for OVA323–339. The responsiveness of peripheral CD4+ T cells in the double transgenic mice was examined. We demonstrated that hyporesponsive but highly IL-10-producing T cells were developed in DO × OVAhigh mice only, not in DO × OVAlow mice. These IL-10-producing T cells exhibited regulatory activity both in in vitro and in vivo experiments. Moreover, these IL-10-producing regulatory T (Tr) cells expressed high levels of inducible costimulator (ICOS) before in vitro stimulation. Blockade of ICOS-signaling inhibited the production of IL-10 and abrogated the inhibitory function of these Tr cells. Thus, these results suggested that the development of IL-10-producing Tr cells depends on the expression levels of self-antigen in vivo and that ICOS signal plays a critical role in immune regulation by IL-10-producing Tr cells in self-tolerance.
Self-tolerance is mediated by central and peripheral mechanisms. Clonal deletion in the thymus and the induction of unresponsiveness (anergy) are well characterized mechanisms for the establishment and maintenance of tolerance (1). However, it is now clear that these processes are imperfect. In addition to these mechanisms, active suppression by regulatory T (Tr) cells has been proposed for tolerance to both self and foreign antigens. Various subsets of Tr cells have been described, and much effort has been focused on understanding their ontogeny, function, and mechanisms of action. Within the CD4+ T cell subsets, at least three different types of cells with suppressive function may exist: CD4+ CD25+ T cells (2–4), T helper type 3 cells (5), and type 1 Tr (Tr1) cells (6, 7). These T cell subsets appear to be distinguishable based on their cytokine production profiles and their ability to suppress immune responses through direct cell-to-cell interaction. CD4+ CD25+ T cells are well characterized Tr cells among these subsets. Depletion of CD4+ CD25+ T cells results in the development of severe autoimmunity, which can be prevented by the injection of CD4+ CD25+ T cells (2, 8, 9). In addition to CD25 expression, they express cytotoxic T lymphocyte-associated antigen 4 and glucocorticoid-induced tumor necrosis factor receptor at higher levels (10, 11), and antibody against glucocorticoid-induced tumor necrosis factor receptor abolishes the suppressive activity (12). Moreover, the transcription factor Foxp3 is highly expressed, and this is associated with the suppressive ability and phenotype of these cells (13–15). T helper type 3 cells were identified in studies of oral tolerance. These cells secrete transforming growth factor type β (TGF-β), and their suppressive ability is mediated through a TGF-β-dependent mechanism (16, 17). Tr1 cells were initially defined in studies of CD4+ T cells, which were activated in the presence of IL-10 and rendered anergic (6). The Tr1 cells produce high levels of IL-10 and TGF-β, moderate amounts of IFN-γ and IL-5, but little or no IL-2 or IL-4. Importantly, Tr1 cells were shown to be involved in the down-regulation of immune responses in vitro and in vivo through the production of the immunosuppressive cytokines IL-10 and TGF-β (6). Similarly, IL-10-producing Tr cells were also induced in vitro by culturing T cells with immature dendritic cells (18, 19) by using immunosuppressive drugs (20) or by stimulation with CD2 (21). The importance of suppression by IL-10-producing Tr cells is noteworthy; however, specific cell markers or instances of specific gene expression have not been clarified.
The importance of costimulatory molecules, such as CD28, cytotoxic T lymphocyte-associated antigen 4, or PD-1 (programmed death 1), for the activation of T cells is well known (22). A new member of the CD28 family, inducible costimulator (ICOS) is a T cell-specific cell-surface molecule structurally related to CD28 and cytotoxic T lymphocyte-associated antigen 4 (23, 24). Both CD28 and ICOS molecules are able to amplify the secretion of several cytokines, but only CD28 induces substantial amounts of IL-2, whereas ICOS shows a certain preference for the induction of IL-10 (23). Recently, Tr cells with the ability to produce IL-10 were reported in the respiratory tolerance system (25). In that report, IL-10-producing Tr cells developed by means of the ICOS signaling pathway. More recently, Lohning et al. (26) suggested a correlation between stable ICOS expression and T cell effector capacity; they showed that the expression of ICOS in vivo was strongly biased to CD4+ T cells for IL-10 production but did not provide any direct information on the regulatory function of these cells.
Another important problem to be solved is the relationship between expression levels of self-antigens and the mechanisms of self-tolerance. It was reported that the distribution and amount of self-antigen influenced the induction and mechanisms of tolerance in self-antigen-specific CD4+ T cells (27). Depending on the pattern of self-antigen expression, deletion of double-positive thymocytes ranged from minimal to complete, and peripheral CD4+ T cells exhibited graded reduction in T cell antigen receptor (TCR) expression and in vitro proliferation. However, the relationship between the amount of self-antigen and the function of these CD4+ T cells in the periphery remains to be clarified.
In this report, we investigated the relationship between the expression levels of self-antigens and the function of self-reactive CD4+ T cells in the periphery by using two rat insulin promoter-ovalbumin (RIP-OVA) mouse lines in which OVA is produced only in pancreatic β-islet cells as a self-antigen (28). We demonstrate that the development of IL-10-secreting cells depends on the expression levels of self-antigen and that the ICOS molecule is the critical factor for IL-10 production from self-antigen-specific Tr cells.
Materials and Methods
Mice. BALB/c mice were purchased from CLEA Japan (Tokyo). RIP-OVAhigh (hereafter referred to as OVAhigh) and RIP-OVAlow (hereafter referred to as OVAlow) transgenic (Tg) mice on a C57BL/6 background were donated by W. R. Heath (The Walter and Eliza Hall Institute of Medical Research, Melbourne) (28). Both OVA Tg mice on a BALB/c background were produced by crossing OVA Tg C57BL/6 mice to BALB/c mice for more than six generations. DO.11.10 (DO) Tg mice carrying a TCR specific for OVA323–339 (OVAp) were kindly provided by M. Kubo (Research Institute for Biological Sciences, Tokyo University of Science). DO × OVA double-Tg mice were bred in our animal facility.
Preparation of CD4+ T Cells. CD4+ T cells were purified with CD4 microbeads (Miltenyi Biotec, Bergish Gladbach, Germany) as described (29). The purity of CD4+ T cells was routinely estimated to be ≈94–98%.
Flow Cytometry Analysis for Cell-Surface Molecules. Biotin-conjugated anti-ICOS mAb (15F9) was purchased from eBioscience (San Diego). Phycoerythrin- or FITC-labeled anti-CD4 mAbs, biotin-conjugated-CD45RB (RA3–6B2), CD44 (IM7), CD25 (7D4), and CD69 (H1.2F3) mAbs were purchased from BD Pharmingen. Clonotype-specific KJ1.26 mAb was purified from ascites and conjugated with FITC in our laboratory. Anti-B7h mAb (HK5.3, rat IgG2a) was prepared as described (30).
T Cell Proliferation Assay. The T cell proliferation assay was performed in 96-well flat-bottom plates. CD4+ T cells (5 × 104 per well) in a total volume of 200 μl were stimulated with ≈0–5 μM OVAp in the presence of irradiated syngeneic spleen cells (2 × 105 per well). The cells were cultured for 54 h. Proliferation was assessed by measuring the incorporation of [3H]thymidine (1 μCi per well) added for the final 18 h of culture.
Cytokine ELISA. CD4+ T cells (1 × 106) in a total volume of 1 ml were cultured with 0.5 μM OVAp in the presence of antigen-presenting cells (2 × 106) in 24-well plates. The cultured supernatants were recovered 24 h later for measurement of IL-2 or 72 h later for measurement of IFN-γ, IL-10, and IL-4. The cytokine content was determined by means of a two-site ELISA. All Abs used in ELISA were purchased from BD Pharmingen.
In Vitro Assay of Suppressive Activity. Target CD4+ T cells (5 × 104 cells per well) from DO mice and effector CD4+ T cells (1 × 105 cells per well) from DO × OVAhigh were cultured with irradiated syngeneic spleen cells (3 × 105 cells per well) in the presence of 5 μM OVAp for 54 h with or without anti-B7h mAb. Proliferation was assessed as described above.
In Vivo Assay of Suppressive Activity. CD4+ T cells from DO mice were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes) at a final concentration of 0.1 μM. A total of 4 × 106 CFSE-labeled CD4+ T cells with 4 × 106 CD4+ KJ1.26+ T cells from DO × OVAhigh mice were transferred into the tail vein of BALB/c mice. Some mice received 0.5 mg of anti-B7h mAb or isotype control IgG. Two days after the transfer, mice received one i.p treatment of OVA in complete Freund's adjuvant. Division of CFSE-labeled CD4+ T cells in the spleen was monitored 2 d later.
Results
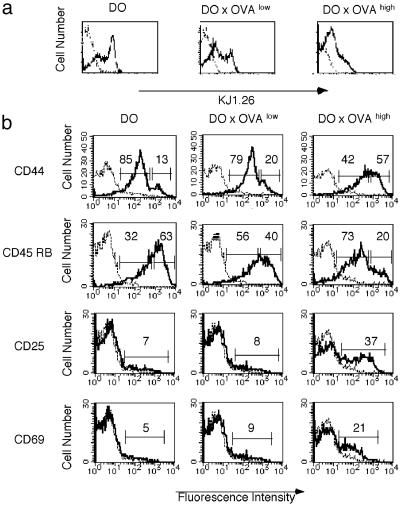
Altered Numbers and Phenotypes of Peripheral CD4+ T Cells in Tolerant Mice. Previously, for the study of reactivity of self-antigen specific CD8+ T cells, two RIP-OVA Tg mouse lines (OVAhigh mice and OVAlow mice) that expressed different amounts of OVA under the control of RIP were generated (28). OVAhigh mice expressed 1.0 ± 0.4 ng of OVA per μg of protein in the pancreatic β-islet cells, and OVAlow mice expressed a lower level of antigen that was <0.03 ng of OVA per μg of protein (28). Both CD8+ T cells (28) and CD4+ T cells (Fig. 6, which is published as supporting information on the PNAS web site) of these RIP-OVA mice were tolerant to OVA. To compare the fate of self-reactive CD4+ T cells recognizing OVA expressed in the pancreas, OVAhigh mice and OVAlow mice were crossed to DO mice. Numbers and phenotypes of peripheral CD4+ T cells were changed in DO × OVA double-Tg mice. In DO × OVAhigh mice, the number of spleen CD4+ T cells was reduced 3- to 5-fold compared with DO mice. However, in DO × OVAlow mice, the change was minimal, and the number of CD4+ T cells in the spleen was only reduced by 1.5- to 2-fold. Both DO × OVA double-Tg mice carried CD4 subsets which expressed low levels of KJ1.26 (Fig. 1). CD4+ T cells from DO × OVA double-Tg mice expressed increased levels of CD44 and decreased levels of CD45RB compared with DO mice, and the change was more dramatic in DO × OVAhigh mice (Fig. 1). These results indicated that peripheral CD4+ T cells from DO × OVA double-Tg mice displayed a phenotype of previously activated cells in proportion to the amount of self-antigen expression. Moreover, in DO × OVAhigh mice, some CD4+ T cells also expressed the early activation markers CD69 and CD25 (Fig. 1).
Fig. 1.
Changes in cell-surface molecules on CD4+ KJ1.26+ T cells of DO × OVA mice. (a) Decreased expression of transgenic KJ1.26+ TCR on spleen CD4+ T cells from DO × OVA double-Tg mice. (b) Expression of CD44, CD45RB, CD25, and CD69 on spleen CD4 and KJ1.26 double-positive T cells. One representative data set of five is shown.
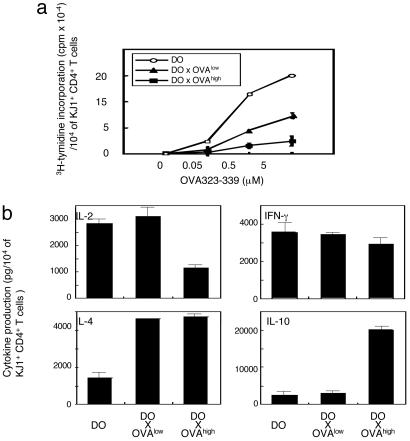
CD4+ T Cells from DO × OVAhigh Mice Produce High Levels of IL-10. To compare the functional ability of peripheral CD4+ T cells from DO × OVA double-Tg mice, the proliferation ability and cytokine production in response to OVAp were examined. Corresponding to the different degrees of TCR expression described above (Fig. 1), CD4+ T cells from the different DO × OVA double-Tg mice displayed reduced proliferative ability when stimulated with OVAp (Fig. 2a). To assess whether the observed hyporesponsiveness could be reversed by the addition of IL-2, as in the case with anergic T cells in vitro (31), recombinant IL-2 was added to the culture when stimulated with OVAp. However, proliferative capacity of CD4+ T cells from these DO × OVA double-Tg mice was not recovered (data not shown). To further characterize the functional difference of CD4+ T cells, purified CD4+ T cells were examined for cytokine production. To this end, CD4+ T cells from either DO or DO × OVA double-Tg mice were purified from spleen cells and stimulated with OVAp (Fig. 2b). IL-2, IFN-γ, IL-4, and IL-10 were detected in all mice, whereas TGF-β1 was not (data not shown). IL-4 production was increased in both of the DO × OVA double-Tg mice compared with DO mice. The most significant difference was observed in IL-2 and IL-10 production. CD4+ T cells from DO × OVAhigh mice produced low levels of IL-2 but very high levels of IL-10 (Fig. 2b). Thus, CD4+ T cells in DO × OVAhigh mice are hyporesponsive and are able to produce high amounts of IL-10.
Fig. 2.
Proliferation and cytokine production of CD4+ T cells from DO, DO × OVAlow, and DO × OVAhigh mice after antigenic stimulation in vitro. CD4+ T cells were purified from splenocytes of DO, DO × OVAlow, or DO × OVAhigh mice. (a) CD4+ T cells were stimulated with irradiated splenocytes in the presence of the indicated amounts of OVAp. Total cpm values were divided by the number of KJ1.26+ cells as determined by flow cytometry. (b) CD4+ T cells were stimulated with irradiated splenocytes in presence of 0.5 μM OVAp. Cytokines in the culture supernatant were measured by ELISA. The amount of cytokines was divided by the number of KJ1.26+ cells as determined by flow cytometry. Data are representative of five separate experiments with similar results.
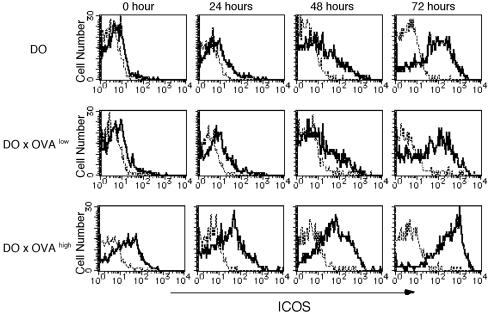
ICOS Expression Is Highly Correlated with IL-10 Production in CD4+ T Cells from DO × OVAhigh Mice. It has been suggested that stimulation of ICOS on CD4+ T cells preferentially promotes IL-10 production (23, 32, 33). Moreover, the importance of the ICOS molecule in IL-10-producing Tr cells was reported in respiratory tolerance (25). To clarify the relationship between IL-10 production and ICOS expression in our self-tolerance system, we analyzed ICOS expression in CD4+ T cells. CD4+ T cells isolated from DO mice and DO × OVAlow mice expressed low levels of ICOS, but from DO × OVAhigh mouse expressed a high level of ICOS (Fig. 3). When these cells were stimulated with OVAp in vitro, the expression of ICOS was enhanced in all mice after 24–72 h. Interestingly, at any time point after stimulation, induced ICOS expression was significantly higher in DO × OVAhigh mice compared with other Tg mice.
Fig. 3.
CD4+ KJ1.26+ T cells from DO × OVAhigh mice highly express ICOS. CD4+ T cells from the spleen of DO, DO × OVAlow, and DO × OVAhigh mice were stimulated with 0.5 μM OVAp, and the expression levels of ICOS were examined at the indicated times after stimulation in vitro. CD4+ T cells were stained with mAb against CD4, KJ1.26, and ICOS. ICOS expression was analyzed by gating on CD4 and KJ1.26 double-positive T cells. One representative experiment of three is shown
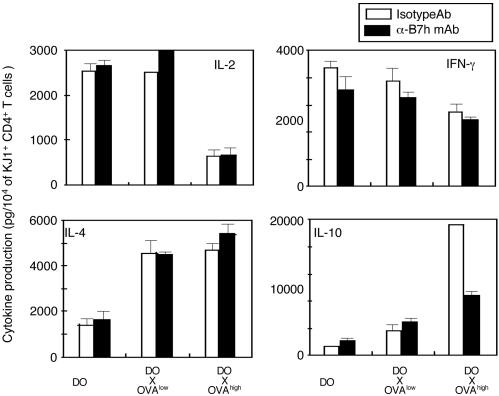
To determine the contribution of the ICOS signaling pathway to IL-10 production in our system, we examined whether blocking ICOS signaling reversed IL-10 production in CD4+ T cells from DO × OVAhigh mice. As shown in Fig. 4, the blocking of ICOS signaling by the mAb to B7h, a ligand of ICOS, reduced IL-10 production of CD4+ T cells from DO × OVAhigh mice but had no effect on CD4+ T cells from DO mice. In addition, IFN-γ production was decreased and IL-4 production was increased, but the effect was not as prominent. The blocking of the ICOS signaling pathway had no effect on proliferation (data not shown). These results suggest that the ICOS signaling pathway is specifically involved in IL-10 production of CD4+ T cells from DO × OVAhigh mice.
Fig. 4.
Blocking the ICOS signaling pathway inhibits IL-10 production of CD4+ T cells from DO × OVAhigh mice after antigenic stimulation in vitro. CD4+ T cells were purified from splenocytes of DO, DO × OVAlow,orDO × OVAhigh mice. CD4+ T cells were stimulated with irradiated splenocytes in the presence of 0.5 μM OVAp with or without α-B7h mAb. Cytokines in the culture supernatant were measured by ELISA. The amount of cytokines was divided by the number of KJ1.26+ cells as determined by flow cytometry. Data are representative of three separate experiments with similar results.
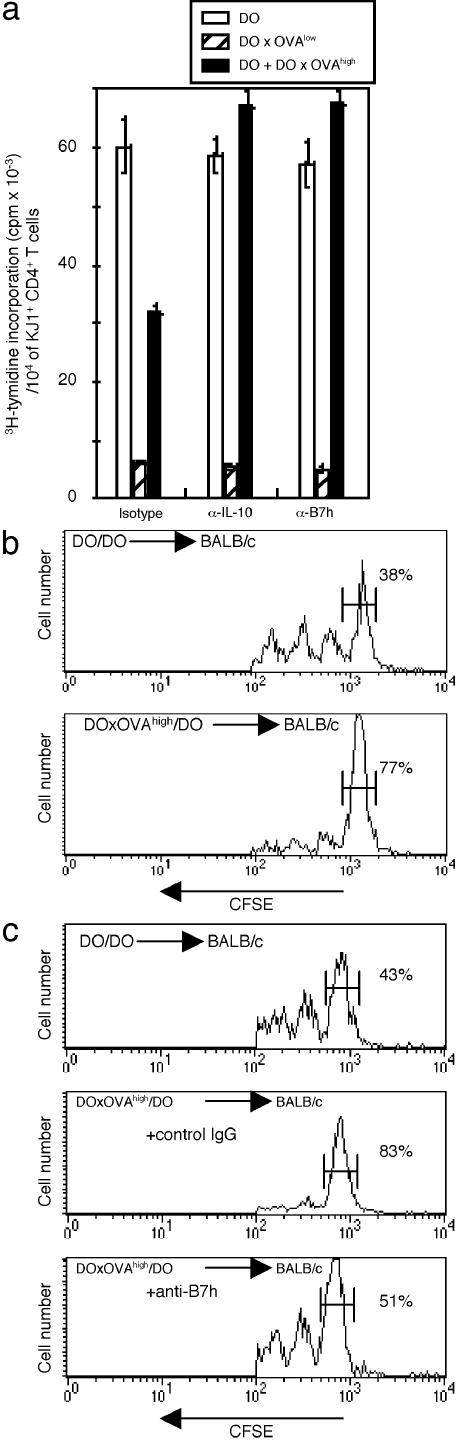
IL-10-Producing CD4+ T Cells from DO × OVAhigh Mice Inhibit T Cell Proliferation. We investigated whether IL-10-producing T cells in our double-Tg mouse system had the ability to suppress the activation of T cells in vitro. CD4+ T cells from DO × OVA high mice and DO mice were cocultured with irradiated syngeneic spleen cells in the presence of OVAp. CD4+ T cells from DO × OVA high mice inhibited the proliferation of CD4+ T cell from DO mice (Fig. 5a). The inhibitory ability of CD4+ T cells from DO × OVAhigh mice depended on IL-10 production, because the addition of anti-IL-10 mAb reversed the inhibitory effect. Moreover, the blockade of the ICOS signaling pathway abrogated the in vitro suppressive function. Next, we examined the in vivo suppressive ability. CFSE-labeled CD4+ T cells from DO mice were transferred into BALB/c mice with CD4+ T cells from DO × OVA high mice or DO mice by i.v. injection. Two days after cell transfer, the recipient mice were immunized with OVA/complete Freund's adjuvant. The splenic cells from these recipient mice were harvested 4 d after transfer. Fluorescence-activated cell sorter analysis of these populations was performed gaiting on the CD4, KJ1.26, and CFSE triple-positive cells. As shown in Fig. 5b, CFSE-labeled cells cotransferred with CD4+ T cells from DO mice divided readily in response to OVA, with only 38% remaining undivided. The same cells cotransferred with DO × OVA high mice also divided; however, a greater proportion of these cells (≈80%) did not undergo cell division. To investigate the role of ICOS in mediating this in vivo suppression, recipient mice transferred with CD4+ T cells from DO × OVA high mice were treated with anti-B7h mAb 1 h before antigen challenge. The suppression of proliferation was significantly reduced when the ICOS signal was blocked (Fig. 5c). These results suggested that CD4+ T cells from the DO × OVA high mice contained the Tr cells and that their suppressive activity on proliferation was mediated by ICOS-induced IL-10.
Fig. 5.
CD4+ T cells from DO × OVAhigh mice suppress the proliferation of CD4+ T cells from DO mice. (a) CD4+ T cells from DO × OVAhigh mice and DO mice were cultured with irradiated syngeneic spleen cells in the presence of 0.5 μM OVAp with or without both anti-IL-10 or anti-B7h mAb. One representative experiment of three is shown. (b and c) CD4+ T cells from DO were labeled with CFSE and transferred with CD4+ T cells from DO × OVAhigh mice or from DO into BALB/c mice. Some mice received anti-B7h mAb or control IgG before OVA injection. Two days after the transfer, the recipient mice were injected with OVA in complete Freund's adjuvant. Two days after immunization, spleen cells were collected from the recipients and stained with anti-CD4 and anti-KJ1.26 mAb. Fluorescence-activated cell sorter analysis was performed, gating on the CD4, KJ1.26, and CFSE triple-positive cells. The results are representative examples of experimental groups containing three mice. The experiments have been repeated three times with similar results.
Discussion
Here we demonstrate that the expression levels of self-antigen determine the function of self-reactive CD4+ T cells in the periphery and that IL-10-producing Tr cells developed in mice expressing high levels of self-antigen. Furthermore we show that IL-10 production and the inhibitory activity of these Tr cells are mediated by ICOS stimulation. Although IL-10-producing Tr cells have been reported in another self-tolerance system (34), we demonstrated that ICOS is crucial for IL-10 production by self-antigen specific Tr cells.
Previously, the effect of distribution and expression levels of self-antigen to tolerance induction of self-reactive CD4+ T cells has been examined by using several types of hen egg lysozyme (HEL) expressing Tg mouse lines: e.g., expression on the thyroid epithelium, the pancreatic B-islet cell, or systemically (27). These HEL Tg mice were crossed to TCR Tg mice specific for HEL. The analysis of the double-Tg mice has revealed that the deletion of double-positive thymocytes, reduction of TCR expression, and proliferative response of peripheral CD4+ T cells were influenced by the distribution and amount of the self-antigen. However, the cytokine production profile and regulatory function of the T cells involved in tolerance were not examined. In this study, we clearly demonstrated that the proliferative response of T cells from both DO × OVAhigh and DO × OVAlow mice was suppressed in proportion to the expression levels of the self-antigen (Fig. 2a), which is in accordance with a previous report (27). These hypoproliferative T cells from the double-Tg mice produce high amounts of IL-4 in response to in vitro antigenic stimulation. However, enhanced IL-10 production was detected only in CD4+ T cells from DO × OVAhigh mice, which show a greater suppression of proliferation. These results led us to hypothesize that self-specific T cells in the periphery become hypoproliferative irrespective of the expression level of self-antigen, but they gain the ability to produce the immunosuppressive cytokine (IL-10) and regulatory function when the self-antigen is expressed at a level beyond a certain threshold, and, as a result, chronic antigen stimulation occurs. This hypothesis is in agreement with Buer's work (34) showing that deeply anergic, but IL-10-producing, T cells were generated in the double-Tg tolerance mouse model in which a specific antigen was expressed in hematopoietic cells. Furthermore, IL-10 production by anergic T cells has also been induced by the repeated administration of superantigen (35, 36) or repeated intranasal administration of a high-affinity MHC-binding analog (37).
Our results clearly demonstrate the specific role of the ICOS signal in IL-10 production and immune regulation by Tr cells. Blocking of the ICOS signal with anti-B7h mAb inhibited production of IL-10 but not of IL-4 and IFN-γ from Tr cells in DO × OVAhigh mice (Fig. 4). Furthermore, the treatment abrogated the inhibition of proliferation by Tr cells in both in vitro and in vivo experiments (Fig. 5). These data are in agreement with recent studies showing that the regulatory function of IL-10-producing T cells in intranasal tolerance completely depends on ICOS (25). Thus, the ICOS signal seems to be critical for Tr cell-mediated self and nonself tolerance. ICOS knockout mice showed enhanced susceptibility to experimental autoimmune encephalomyelitis, suggesting that ICOS signaling is involved in protection from autoimmune disease (38). CD4+ T cells from the ICOS knockout mice, however, have produced comparable levels of IL-10 to those from WT mice. The role of ICOS in IL-10 production may depend on the nature of the T cells: i.e., T helper or Tr, and this point should be clarified in future experiments.
A recent study has also demonstrated a clear correlation between the ability of T cells to produce IL-10 and the levels of their ICOS expression. Lohning et al. (26) have shown that ≈1% of splenic CD4+ T cells from nonimmunized mice express ICOS. These T cells show an activated/memory phenotype and that a proportion of their ICOS expression is linked to their IL-10 production ability; i.e., IL-10 is the dominant cytokine produced by T cells with high levels of ICOS. These findings are consistent with our present study in that CD4+ T cells in DO × OVAhigh mice express high levels of ICOS before in vitro stimulation (Fig. 3). Thus, ICOS expression could be one of the molecular markers of T cells with IL-10-producing ability. It remains unclear why IL-10-producing T cells express high levels of ICOS and how these high levels are maintained. Taking into account that ICOS is one of the activation markers of T cells, continuous stimulation of self-antigens in vivo may sustain ICOS expression.
IL-10-producing Tr cells have been generated by culturing T cells in the presence of large quantities of exogenous IL-10 (6), culturing T cells with immature IL-10-producing dendritic cells (19), or by using immunosuppressive drugs (20). These studies suggested that the presence of IL-10 was critical for the development of IL-10-producing cells. From these observations, the differentiation of IL-10-producing Tr cells in our double-Tg mouse system might be also induced by IL-10-producing dendritic cells presenting OVA as the self-antigen. Induced IL-10 production from T cells lends credence to the finding that, after exposure to intranasally applied OVA pulmonary, dendritic cells produced IL-10 transiently (25). On the other hand, this study has also demonstrated the involvement of ICOS itself in the development of IL-10-producing T cells. In future studies, it will be important to clarify whether a signal by means of ICOS is also required for the development of self-specific Tr cells with IL-10-producing ability.
Usually, IL-10 production from normal mouse T cells can be observed 48–72 h after antigenic stimulation, but CD4+ T cells producing IL-10 in the DO × OVAhigh mouse were detected immediately, and significant levels of IL-10 were observed only 24 h after stimulation (Fig. 7, which is published as supporting information on the PNAS web site). The characteristics of rapid IL-10 production and the dependency of suppressive ability on IL-10 are reminiscent of Tr 1 cells (39). However CD4+ T cells in the DO × OVAhigh mouse differed from conventional Tr1 cells in other cytokine production profiles. Tr1 cells produced high levels of IL-10, TGF-β1, and moderate amounts of IFN-γ, but little or no IL-2 or IL-4 (6). On the other hand, Tr cells in DO × OVAhigh mice produce high levels of IL-4 in addition to IL-10 (Fig. 3b). It may be possible that Tr cells from DO × OVAhigh mice consist of several types of cells, such as Tr cells producing IL-10 alone, IL-10 plus IL-4-producing T helper type 2 cells, and T cells producing IL-4 alone. Nonetheless, it is important that the regulatory activity of the Tr cells depends on IL-10 production. This point should be clarified by future experiments.
Sakaguchi et al. (2) reported that CD4+CD25+ T cells exhibit suppressive activity by cell-to-cell contact. These cells highly express Foxp3, and the introduction of the Foxp3 gene is able to convert naïve T cells toward Tr cells (13). Although the inhibitory activity of Tr cells in our double-Tg mice is mediated by IL-10 (Fig. 5), we found higher levels of Foxp3 mRNA expression in CD4+ T cells of DO × OVAhigh mice than those of DO mice (data not shown). Papiernik et al. (40) reported that CD4+CD25+ Tr cells secreted higher levels of IL-10 than did CD4-CD25+ T cells, and CD4+CD25+ Tr cells suppressed inflammatory bowel disease through an IL-10- and TGF-β-dependent mechanism (10, 41). From our findings and these previous reports, we are not able to rule out the possibility that at least a population of Tr cells in our system consist of so-called CD4+CD25+ Tr cells.
In conclusion, this study demonstrates that the development of self-specific IL-10-producing Tr cells depends on the amount of self-antigen expressed and the critical role of ICOS for the active function of these cells. The ICOS signaling pathway may be a novel target for the treatment of autoimmune disease, because ICOS costimulation seems to be specifically linked to IL-10 production of Tr cells.
Supplementary Material
Acknowledgments
We thank Susan Adlam for improving the English of the manuscript. This work was supported in part by grants from the Ministry of Education, Science, and Culture of Japan.
Abbreviations: ICOS, inducible costimulator; RIP, rat insulin promoter; OVA, ovalbumin; Tg, transgenic; Tr, regulatory T; DO, DO.11.10; TGF-β, transforming growth factor type β; TCR, T cell antigen receptor; OVAp, OVA323–339; CFSE, carboxyfluorescein diacetate succinimidyl ester.
References
- 1.Kisielow, P., Bluthmann, H., Staerz, U. D., Steinmetz, M. & von Boehmer, H. (1988) Nature 333, 742-746. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi, S., Sakaguchi, N., Asano, M., Itoh, M. & Toda, M. (1995) J. Immunol. 155, 1151-1164. [PubMed] [Google Scholar]
- 3.Thornton, A. M. & Shevach, E. M. (1998) J. Exp. Med. 188, 287-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakaguchi, S. (2003) Nat. Immunol. 4, 10-11. [DOI] [PubMed] [Google Scholar]
- 5.Fukaura, H., Kent, S. C., Pietrusewicz, M. J., Khoury, S. J., Weiner, H. L. & Hafler, D. A. (1996) J. Clin. Invest. 98, 70-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groux, H., O'Garra, A., Bigler, M., Rouleau, M., Antonenko, S., de Vries, J. E. & Roncarolo, M. G. (1997) Nature 389, 737-742. [DOI] [PubMed] [Google Scholar]
- 7.Cottrez, F., Hurst, S. D., Coffman, R. L. & Groux, H. (2000) J. Immunol. 165, 4848-4853. [DOI] [PubMed] [Google Scholar]
- 8.Sakaguchi, S., Sakaguchi, N., Shimizu, J., Yamazaki, S., Sakihama, T., Itoh, M., Kuniyasu, Y., Nomura, T., Toda, M. & Takahashi, T. (2001) Immunol. Rev. 182, 18-32. [DOI] [PubMed] [Google Scholar]
- 9.Shevach, E. M. (2002) Nat. Rev. Immunol. 2, 389-400. [DOI] [PubMed] [Google Scholar]
- 10.Read, S., Malmstrom, V. & Powrie, F. (2000) J. Exp. Med. 192, 295-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McHugh, R. S., Whitters, M. J., Piccirillo, C. A., Young, D. A., Shevach, E. M., Collins, M. & Byrne, M. C. (2002) Immunity 16, 311-323. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu, J., Yamazaki, S., Takahashi, T., Ishida, Y. & Sakaguchi, S. (2002) Nat. Immunol. 3, 135-142. [DOI] [PubMed] [Google Scholar]
- 13.Hori, S., Nomura, T. & Sakaguchi, S. (2003) Science 299, 1057-1061.12522256 [Google Scholar]
- 14.Fontenot, J. D., Gavin, M. A. & Rudensky, A. Y. (2003) Nat. Immunol. 4, 330-336. [DOI] [PubMed] [Google Scholar]
- 15.Khattri, R., Cox, T., Yasayko, S. A. & Ramsdell, F. (2003) Nat. Immunol. 4, 337-342. [DOI] [PubMed] [Google Scholar]
- 16.Chen, Y., Kuchroo, V. K., Inobe, J., Hafler, D. A. & Weiner, H. L. (1994) Science 265, 1237-1240. [DOI] [PubMed] [Google Scholar]
- 17.Weiner, H. L. (2001) Immunol. Rev. 182, 207-214. [DOI] [PubMed] [Google Scholar]
- 18.Wakkach, A., Fournier, N., Brun, V., Breittmayer, J. P., Cottrez, F. & Groux, H. (2003) Immunity 18, 605-617. [DOI] [PubMed] [Google Scholar]
- 19.Jonuleit, H., Schmitt, E., Schuler, G., Knop, J. & Enk, A. H. (2000) J. Exp. Med. 192, 1213-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrat, F. J., Cua, D. J., Boonstra, A., Richards, D. F., Crain, C., Savelkoul, H. F., de Waal-Malefyt, R., Coffman, R. L., Hawrylowicz, C. M. & O'Garra, A. (2002) J. Exp. Med. 195, 603-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wakkach, A., Cottrez, F. & Groux, H. (2001) J. Immunol. 167, 3107-3113. [DOI] [PubMed] [Google Scholar]
- 22.Rudd, O. & Schneider, H. (2003) Nat. Rev. Immunol. 3, 544-556. [DOI] [PubMed] [Google Scholar]
- 23.Hutloff, A., Dittrich, A. M., Beier, K. C., Eljaschewitsch, B., Kraft, R., Anagnostopoulos, I. & Kroczek, R. A. (1999) Nature 397, 263-266. [DOI] [PubMed] [Google Scholar]
- 24.Yoshinaga, S. K., Whoriskey, J. S., Khare, S. D., Sarmiento, U., Guo, J., Horan, T., Shih, G., Zhang, M., Coccia, M. A., Kohno, T., et al. (1999) Nature 402, 827-832. [DOI] [PubMed] [Google Scholar]
- 25.Akbari, O., Freeman, G. J., Meyer, E. H., Greenfield, E. A., Chang, T. T., Sharpe, A. H., Berry, G., DeKruyff, R. H. & Umetsu, D. T. (2002) Nat. Med. 8, 1024-1032. [DOI] [PubMed] [Google Scholar]
- 26.Lohning, M., Hutloff, A., Kallinich, T., Mages, H. W., Bonhagen, K., Radbruch, A., Hamelmann, E. & Kroczek, R. A. (2003) J. Exp. Med. 197, 181-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akkaraju, S., Ho, W. Y., Leong, D., Canaan, K., Davis, M. M. & Goodnow, C. C. (1997) Immunity 7, 255-271. [DOI] [PubMed] [Google Scholar]
- 28.Kurts, C., Sutherland, R. M., Davey, G., Li, M., Lew, A. M., Blanas, E., Carbone, F. R., Miller, J. F. & Heath, W. R. (1999) Proc. Natl. Acad. Sci. USA 96, 12703-12707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohyama, M., Sugahara, D., Hosokawa, H., Kubo, M. & Hozumi, N. (2001) Eur. J. Immunol. 31, 3659-3666. [DOI] [PubMed] [Google Scholar]
- 30.Iwai, H., Kozono, Y., Hirose, S., Akiba, H., Yagita, H., Okumura, K., Kohsaka, H., Miyasaka, N. & Azuma, M. (2002) J. Immunol. 169, 4332-4339. [DOI] [PubMed] [Google Scholar]
- 31.Mueller, D. L. & Jenkins, M. K. (1995) Curr. Opin. Immunol. 7, 375-381. [DOI] [PubMed] [Google Scholar]
- 32.McAdam, A. J., Chang, T. T., Lumelsky, A. E., Greenfield, E. A., Boussiotis, V. A., Duke-Cohan, J. S., Chernova, T., Malenkovich, N., Jabs, C., Kuchroo, V. K., et al. (2000) J. Immunol. 165, 5035-5040. [DOI] [PubMed] [Google Scholar]
- 33.Beier, K. C., Hutloff, A., Dittrich, A. M., Heuck, C., Rauch, A., Buchner, K., Ludewig, B., Ochs, H. D., Mages, H. W. & Kroczek, R. A. (2000) Eur. J. Immunol. 30, 3707-3717. [DOI] [PubMed] [Google Scholar]
- 34.Buer, J., Lanoue, A., Franzke, A., Garcia, C., von Boehmer, H. & Sarukhan, A. (1998) J. Exp. Med. 187, 177-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sundstedt, A., Hoiden, I., Rosendahl, A., Kalland, T., van Rooijen, N. & Dohlsten, M. (1997) J. Immunol. 158, 180-186. [PubMed] [Google Scholar]
- 36.Miller, C., Ragheb, J. A. & Schwartz, R. H. (1999) J. Exp. Med. 190, 53-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sundstedt, A., O'Neill, E. J., Nicolson, K. S. & Wraith, D. C. (2003) J. Immunol. 170, 1240-1248. [DOI] [PubMed] [Google Scholar]
- 38.Dong, C., Juedes, A. E., Temann, U. A., Shresta, S., Allison, J. P., Ruddle, N. H. & Flavell, R. A. (2001) Nature 409, 97-101. [DOI] [PubMed] [Google Scholar]
- 39.Bacchetta, R., Bigler, M., Touraine, J. L., Parkman, R., Tovo, P. A., Abrams, J., de Waal Malefyt, R., de Vries, J. E. & Roncarolo, M. G. (1994) J. Exp. Med. 179, 493-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Papiernik, M., do Carmo Leite-de-Moraes, M., Pontoux, C., Joret, A. M., Rocha, B., Penit, C. & Dy, M. (1997) J. Immunol. 158, 4642-4653. [PubMed] [Google Scholar]
- 41.Asseman, C., Mauze, S., Leach, M. W., Coffman, R. L. & Powrie, F. (1999) J. Exp. Med. 190, 995-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.