Abstract
Tumor necrosis factor (TNF) α and mitogen-activated protein kinase/c-Jun N-terminal kinase (MAPK/JNK) pathways are both implicated in Alzheimer's disease (AD) pathogenesis. Increased expression of several members of the TNF pathway and JNK activation of c-Jun ultimately result in neuronal apoptosis. DENN/MADD, a multifunctional domain protein expressed in neurons, interacts with both the p55 TNF receptor (TNFR) type 1 and JNK3, placing it at a critical juncture in regulating signaling of neurodegeneration. We examined expression and interactions of the TNFR1 binding proteins, DENN/MADD, and TNFR-associated death domain (TRADD) protein in AD-affected tissues and cell cultures. We found reduced DENN/MADD and increased TRADD expression immunohistochemically in the hippocampus in areas of AD pathology compared to normal controls but little intraneuronal colocalization. In brain homogenates, DENN/MADD protein and mRNA expression was significantly reduced in AD compared to controls. Conversely, TRADD, TNFR1, and activated JNK were increased. Murine neuroblastoma and rat hippocampal cultures stressed with Aβ1–42 and the cortices of AD transgenic mice (Tg2576Swe) each showed decreased DENN/MADD expression and TRADD up-regulation in the mice, compared to controls. DENN/MADD antisense treatment of cultured rat hippocampal neurons reduced endogenous DENN/MADD and promoted neuronal cell death. DENN/MADD and TRADD competitively bound to TNFR1 when overexpressed in N2A cells, with DENN/MADD abrogating TNFR1 binding to TRADD. DENN/MADD may therefore be protective by inhibiting TRADD-induced apoptotic cell death. Reduction of DENN/MADD may affect long-term neuronal viability in AD by allowing TRADD mediation of TNFR1 signaling in response to oxidative or cytokine-promoted stresses.
Keywords: amyloid, apoptosis
Alzheimer's disease (AD) is characterized by gradual deposition of beta amyloid (Aβ), neurofibrillary tangle (NFT) pathology, loss of synaptic terminals, progressive dementia, and neuronal cell death (1–3). Multiple signaling pathways have been implicated in AD progression. Cross-talk between the tumor necrosis factor (TNF) α and mitogen-activated protein kinase/c-Jun N-terminal kinase (MAPK/JNK) pathways potentially provides a cell death mechanism in the chronic setting of AD.
While the TNF receptor (TNFR) pathway contributes to the progression of neuronal cell death in AD, Parkinson's disease, and stroke (4–7), Aβ fibril formation in AD brains induces microglial/monocytic activation and TNF-α production (8). TNF-α signals through either of two receptors, p55 TNFR1 or p75 TNFR2 (9, 10). TNF-mediated signaling involving recruitment or inhibition of adapter components may ultimately affect neuronal fate. Binding of TNFR-associated death domain (TRADD) protein and DENN (differentially expressed in normal versus neoplastic)/MADD (MAPK activating death domain) protein to TNFR1 occurs through death domain (DD) interactions (10, 11). TNFR1 activation promotes binding of TRADD and subsequent recruitment of Fas-associated death domain (FADD) protein, receptor-interacting protein (RIP), or TNFR-associated factor (TRAF2) to promote apoptosis, JNK activation, and NF-κB activation. The resulting death-inducing signaling complex (DISC) eventually determines apoptotic transduction or cell survival. TNFR1 triggers the NF-κB signaling pathway via recruitment of the IKK complex (12), whereas JNK is activated via TRAF2-mediated activation of apoptosis signaling kinase (ASK1) (13).
Expression of various components of TNF, JNK, and caspase pathways have been determined in the AD-affected brain and in neuronal cultures (14–16). TNF-α is up-regulated in the AD brain and proinflammatory cytokines, such as TNF-α with IFN, induce production of Aβ1–42 in neurons (17). Aβ activates JNK and induces apoptosis in neuronal cultures (18–20), and is up-regulated in AD (3). Activation of caspase-8 and caspase-3 also occurs in the AD brain and in neuronal cultures incubated with Aβ (14, 21).
Down-regulation of TNF pathway proteins is observed in the AD-affected brain, including FADD-like IL-1β-converting enzyme (FLICE) inhibitor protein (FLIP) and TNFR2 (14, 15). These factors potentially promote neuroprotective mechanisms, involving the TNFR pathway. FLIP interacts with TRADD and blocks caspase-8 recruitment (22), and TNFR2 plays a trophic or protective role in neuronal survival (23, 24). Recently, Micheau and Tschopp (25) reported that a complex involving TNFR1, TRADD, RIP, and TRAF2 dissociates rapidly on posttranslational modification and binds FADD and caspase-8 to initiate apoptosis (Fig. 2 A). Furthermore, Aβ induces cell death via a FADD-mediated pathway potentially linking Aβ-induced neuronal apoptosis to death receptors of the Fas/TNFR family (21).
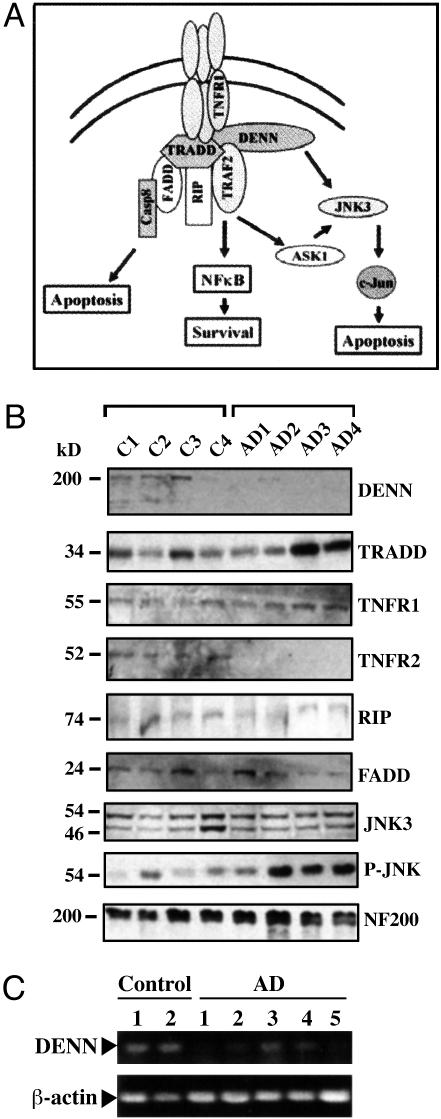
Fig. 2.
Western analysis of TNF-α pathway components in control versus AD hippocampus. (A) Diagram of DENN interactions with the TNFR1 signaling pathway. DENN binds to TNFR1 and TRADD, whereas TRADD binds to TNFR1 and recruits TRAF2, RIP, and FADD. Further activation leads to apoptosis via caspase-8, survival via NF-kβ, or activation of JNK through apoptosis signaling kinase (ASK1). DENN also binds to JNK3 and may affect JNK-mediated apoptosis. (B) Western blotting of tissues from four age-matched controls and AD patients. Hippocampal homogenates (50 μg per lane) were analyzed by 4–15% gradient SDS/PAGE, transferred to a poly(vinylidene difluoride) membrane, and probed with antibodies specific for DENN, TRADD, TNFR1, TRAF2, RIP, JNK3, P-JNK, and NF200. Anti-DENN detects a specific band at 200 kDa, the predicted molecular weight of DENN, which is reduced in AD hippocampus. Increased expression of the 34-kDa TRADD protein and the 55-kDa TNFR1 protein in AD contrasts with expression of RIP, FADD, and JNK3, which remain constant in both control and AD tissues. TRAF2 expression is similarly decreased in AD, whereas activated JNK (P-JNK) is increased and NF200 expression remains constant, suggesting neuronal distribution is similar in both control and AD. (C) RT-PCR of total RNA pooled from the hippocampi of two age-matched controls and five AD patients were amplified by using primers specific for DENN or β-actin. The amplified product is a 452-bp region spanning the N-terminal region of DENN. DENN mRNA is significantly reduced in AD hippocampus, whereas β-actin levels are constant.
To translate acute responses of DENN/MADD and TNFR1-related proteins in experimental models to the disease state, we first examined the AD-affected CNS, which is chronically exposed to neuroinflammatory and oxidative stresses, such as TNF-α and Aβ. DENN/MADD and TRADD expression was correlated with AD pathology and compared with CNS of normal, age-matched controls. Endogenous DENN/MADD expression was next defined in neuroblastoma and primary hippocampal cultures. DENN/MADD interactions with either TNFR1 or TRADD were examined for regulation of apoptosis in transfected neurotypic cultures.
Materials and Methods
Reagents (antibodies) used in this study and certain methods [cell culture, transfections, western blotting, immunocytochemistry, and antisense (AS)] may be found in Supporting Methods, which is published as supporting information on the PNAS web site.
Tissue Sources. Human CNS tissues were obtained from the University of Southern California Alzheimer's Disease Research Center. Neuropathological examination confirmed AD, using modified criteria of the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) (27) and the National Institute of Aging Reagan Institute Consensus Conference (28), and Braak staging (1). Blocks (1 cm3) were obtained from hippocampi at autopsy and either immediately fixed in phosphate-buffered formalin or snap-frozen in liquid nitrogen chilled isopentane. Patients were cognitively assessed longitudinally. Neuropathological evaluation included nine cognitively normal age-matched controls by Clinical Dementia Rating (CDR) and two patients with CDRs of 0.5 evaluated every 6 months. Neuropathological evaluation of this group included Braak and Braak scores of 0–III and CERAD scores of 0–1 (absent to sparse) [mean ages, 86.6 ± 6.2; gender, eight females and three males; mean postmortem interval (PMI), 6.6 h]. There were 11 AD patients with CDRs of 2–3 evaluated annually, Braak and Braak scores of V–VI, and CERAD scores of 3–5 (moderate to severe) (mean age, 85.5 ± 7.2; gender, eight females and three males; mean PMI, 5.8 h). Agonal histories excluded terminal hypoxia. For Tg2576 mouse brains, piriform cortex was snap-frozen and stored at -80°C, and extracts were prepared as described (29).
Antisense Inhibition of DENN Expression. Rat hippocampal neurons were grown on poly(l)-lysine-coated wells for 6–8 d in vitro and were transfected with Lipofectin (GIBCO/BRL). The AS sequences for DENN are JNK binding domain (JBD)-AS 5′-CCAGTCTCAAGCTGTTGGGCC-3′ and DD-AS 5′-TGTAGGAGATGAGGTTGTG-3′ (31). The control-AS sequence is 5′-CCTTGGGAGCTAGCTCTGACC-3′.
Results
DENN Expression Is Reduced in AD-Affected Human Hippocampus. As schematically shown (Fig. 2A), DENN interactions with DD-containing proteins of the TNF-α pathway, including TNFR1 and TRADD, as well as JNK3, suggest a role in transducing cell death (11, 26, 32). Activation of the TNF and JNK pathways resulting from extracellular stress signals may result from differential expression in AD-affected regions of the human brain compared with normal controls. We examined DENN expression immunohistochemically in human hippocampus obtained from 11 AD and 11 age-matched control patients. In control tissues, pyramidal neurons revealed strong, diffuse staining of the cytoplasm and perinuclear region. Pyramidal neurons and the surrounding neuropil in AD-affected regions of the hippocampus, including CA1, consistently showed less staining than the same region in companion controls (Fig. 1A). Glia and vessels showed no immunostaining. Thus, DENN protein expression is reduced in AD-affected neurons.
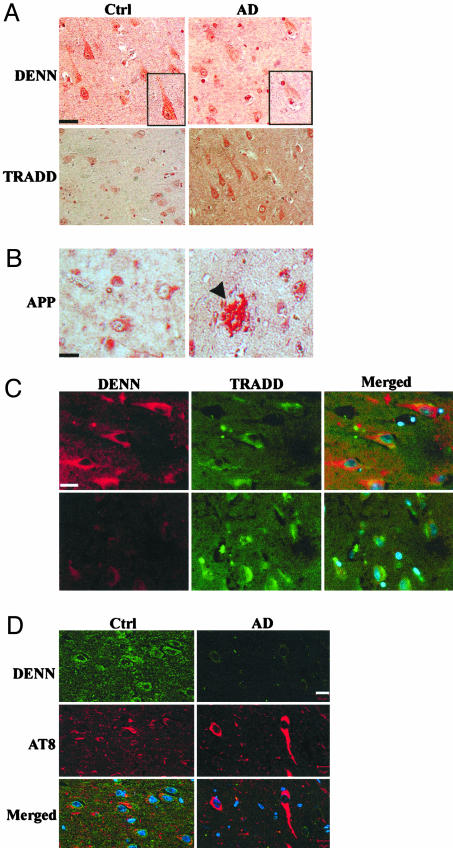
Fig. 1.
DENN expression is reduced in AD-affected human hippocampal neurons. (A) CA1 neurons from human hippocampus. Immunoperoxidase staining of DENN in control and AD hippocampus reveals cytoplasmic and membrane staining and faint cytoplasmic and nuclear staining, respectively. TRADD expression is increased in AD relative to the control. (Bar, 20 μm.) (B) Affected CA1 region of hippocampus shows Aβ plaque formation (arrowhead) and increased APP immunostaining. (C) Immunocytochemical colocalization of DENN and TRADD. DENN is stained with Alexa594 (red), and TRADD is stained with Alexa 488 (green). DENN is decreased and TRADD increased in AD relative to control. DENN and TRADD show minimal, focal overlap in the perinuclear cytoplasm of pyramidal neurons in controls but not in AD. (Bar, 20 μm.) (D) CA1 region of human hippocampus was stained with antibodies specific to DENN (Alexa 488, green) and hyperphosphorylated tau, mAb AT8 (Alexa 594, red). Normal, aged control shows DENN is localized to the cytoplasm of pyramidal neurons and surrounding neuropil. DENN expression is diminished in AD in merged, confocal images, compared to control neurons. Nuclei are labeled with Hoechst 33342 (blue). (Bar, 10 μm.) Filter settings were equivalent in both control and AD tissues.
In addition to DENN interaction with TNFR1, the adapter molecule TRADD binds to TNFR1 and activates the JNK pathway in various cell lines (33, 34). Confirming observations by Zhao et al. (14), we found increased TRADD immunostaining of CA1 neurons in AD-affected tissues compared to controls (Fig. 1A), especially in regions of Aβ deposition (Fig. 1B). There was little specific colocalization of DENN with TRADD in the neuronal cytoplasm of controls or AD tissues (Fig. 1C).
To determine effects of AD-related neurofibrillary pathology on DENN expression, pyramidal neurons of the human hippocampus were coimmunostained with polyclonal DENN antibody and mAb AT8, which identifies hyperphosphorylated tau. These sites were preselected by verification of Aβ deposits with thioflavin S fluorescence and Gallyas silver stains of adjacent diagnostic tissue blocks (data not shown). Confocal microscopy of the CA1 subregion of normal, age-matched hippocampi found DENN localized to the cytoplasm of pyramidal neurons and the surrounding neuropil (Fig. 1D). In AD-affected tissues, neurons contained mAb AT8-reactive neurofibrillary tangles (NFTs) in neuronal somata and adjacent neurites. Whereas controls showed minimal neurite staining, DENN expression was markedly diminished both in the neurons and neuropil and especially in NFT-bearing neurons.
DENN Protein Is Down-Regulated and TRADD Is Up-Regulated in AD Tissue Homogenates. Expression of DENN and other TNFR1-associated proteins are shown in Fig. 2A. Total homogenates of human hippocampus were immunoblotted with antibodies specific to each protein. Although AD pathology has a specific topographical distribution, pathologic changes are not uniformly distributed within each region. The variable expression of proteins from patient to patient is reflected on immunoblots. In Fig. 2B, protein expression was compared in hippocampi obtained from four representative age-matched controls and four AD patients. These are representative of hippocampal homogenates compared from 11 controls and 11 AD cases. Overall, 10 of 11 AD patients showed reduced DENN expression on Western blots compared with controls, and 8 of 11 AD patients had increased TRADD expression (data not shown). DENN showed a 15-fold decrease in protein expression in AD relative to controls. JNK3, a neuronal specific kinase, and the largest neurofilament protein, NF200, each revealed relatively stable expression in both AD and controls, suggesting that the decrease in DENN expression was not likely a result of neuronal loss.
We further probed immunoblots of hippocampal homogenates with antibodies specific to proteins of the TNF-α pathway: TRADD, TNFR1, RIP, TRAF2, and FADD. Densitometry indicated a 2.5-fold increase of TRADD expression, 2-fold increase of TNFR1, 4-fold decrease of TRAF2, and unchanged levels of RIP and FADD (Fig. 2B). Comparison of the densitometric averages of expression were statistically significant between AD and controls (P < 0.05, ANOVA). Tissue from two patients with the most enhanced TRADD expression, AD3 and AD4, also had the most histologically abundant neuritic plaques as assessed by CERAD criteria. Amyloid precursor protein (APP) expression was also up-regulated in AD brains (data not shown). The phosphorylated form of JNK was increased 10-fold in AD as reported by Zhu et al. (35). Moreover, we did not observe any significant changes in DENN expression in cerebellum (data not shown), a region free of AD pathology. These results suggest regulation of DENN and TRADD expression may govern the balance of downstream TNFR1 signaling events.
DENN mRNA Is Down-Regulated in AD. To examine endogenous DENN mRNA expression in an environment of long-term Aβ accumulation and oxidative stress, tissues from AD patients were compared to controls. RT-PCR of hippocampal tissues from two age-matched controls and five AD patients revealed dramatically reduced DENN expression in AD tissues, whereas β-actin remained at similar levels in both control and AD (Fig. 2C). In cerebellum, there was little difference in DENN mRNA expression in AD compared to controls (data not shown). These results further suggest reduced DENN expression correlates with AD pathogenesis.
Aβ Peptide Decreases DENN Expression in Neuronal Cultures. Aβ is selectively neurotoxic to a variety of neuronal subpopulations, including cells of the neocortex and hippocampus, as well as clonal neuronal cell lines (20, 36, 37). To extrapolate our findings from the human CNS, the impact of Aβ exposure on endogenous DENN expression was investigated in vitro, in primary CNS neuronal cultures and in neuroblastoma cells. In Fig. 3A, primary rat hippocampal cultures were exposed to 25 μM fibrillar Aβ1–42 for 24 h (19) and stained with antibodies specific to DENN and phosphorylated JNK (P-JNK). Confirming the observation of Coffey et al. (38), Aβ induced translocation of P-JNK to the nucleus. DENN colocalized with P-JNK in the cytoplasm and processes of control neurons but was diminished in the processes after Aβ exposure. Western immunoblotting of extracts prepared from hippocampal neurons exposed to Aβ for 0, 1, 2, 3, and 4 d showed decreased expression of DENN as early as day 1 (Fig. 3B).
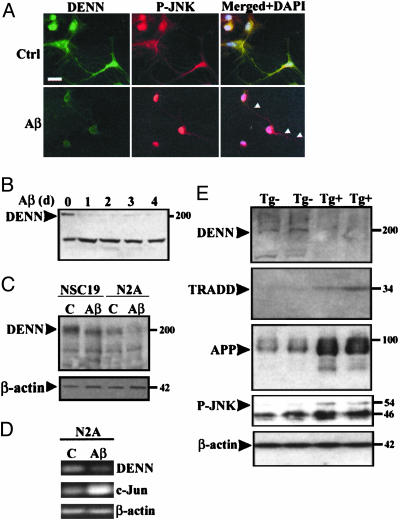
Fig. 3.
Endogenous DENN is reduced in neuronal cultures. (A) Primary rat hippocampal neurons (DIV8) were exposed to either DMSO (Ctrl) or 25 μM fibrillar Aβ1–42 for 24 h, fixed in 4% paraformaldehyde, and stained with antibodies specific for DENN and P-JNK. Nuclei were counterstained with DAPI. Merged image shows overlap of DENN (green) and phosphorylated-JNK (red) with decreased expression of DENN and nuclear translocation of phosphorylated-JNK in Aβ-exposed cultures. (Bar, 10 μm.) Processes remained intact. (B) Rat neurons were exposed to Aβ1–42 for 0, 1, 2, 3, and 4 d. DENN expression decreases by 70% after 1 d and remains reduced up to 4 d. Nonspecific band at 140 kDa remains unchanged under stressed conditions. (C) NSC19 and N2A neuroblastoma cells were exposed to DMSO (C) or 25μM fibrillar Aβ1–40 for 48 h. Cell extracts (100 μg) were immunoblotted with DENN antibody and reprobed with β-actin. DENN-reactive band at 200 kDa is decreased after Aβ exposure. NSC19 expression of DENN is greater than in N2A cells. Actin control is unchanged. Similar results were obtained in three independent experiments. (D) Total RNA from control (C) and 25 μM Aβ1–42 exposed N2A cultures was extracted, followed by RT-PCR using either DENN or primers specificto β-actin or c-Jun. RNA levels of DENN are reduced in Aβ-exposed cultures, whereas actin levels are comparable to controls and c-Jun is increased. Results are representative of three independent experiments. (E) Piriform cortical protein extracts were prepared from Tg2576Swe mice (aged 22 months, n = 2, wild type) Tg-. Samples (50 μg) were immunoblotted with antibodies specific for DENN, TRADD, APP, P-JNK, or β-actin. DENN expression was decreased and TRADD expression increased in transgenic positive (Tg+) mice along with increased APP and P-JNK expression. Note that equal protein applied per sample is indicated by constant β-actin.
Immunoblots of NSC19 and N2A cultures indicated that expression of endogenous DENN protein was significantly decreased by Aβ exposure (Fig. 3C). In untreated cultures, NSC19 cells expressed 3-fold more DENN relative to N2A, but β-actin was expressed in equivalent amounts in both cell lines regardless of Aβ exposure. Interestingly, TRADD expression in NSC19 and N2A cultures was close to threshold and did not change significantly with Aβ exposure (data not shown).
RNA expression was compared in Aβ-exposed and untreated N2A cells (Fig. 3D). Using DENN gene-specific primers, RT-PCR produced a 452-bp product. In representative samples, a 4-fold reduction in DENN expression compared to controls was confirmed by densitometry. RT-PCR of β-actin remained constant and c-Jun increased, indicating that the reduction in DENN RNA was not a result of a general decrease of transcriptional activity in the presence of JNK activation (39).
DENN Expression Is Reduced in Tg2576 APP-Overexpressing Mice. To relate our findings in neuronal cultures to an animal model of AD, we examined mouse cortical extracts from 22-month-old controls and APP-overexpressing mice (n = 2, each set). Antibodies specific to DENN and TRADD revealed down- and up-regulation, respectively (Fig. 3E). Up-regulation of cytokines, including TNF-α, in Tg2576 mouse brains (40) may lead to increased TRADD expression. In the context of DENN down-regulation, Tg+ mice showed increased APP and P-JNK expression, whereas β-actin remained unchanged (Fig. 3E).
Antisense Inhibition of DENN Induces Cell Death. With endogenous DENN expression reduced under conditions of Aβ-induced stress, we sought to determine the functional consequence of diminishing DENN expression in primary hippocampal neurons by using AS oligodeoxynucleotides. Sequences unique to the JBD and death domain (DD) regions of DENN were added to hippocampal cultures. In Fig. 4A, cultures stained with the neuronal marker MAP2 and DENN revealed inhibition of DENN expression by DENN-AS. Surviving neurons treated with DENN-AS maintained a low level of DENN expression. Western blots further confirmed reduced DENN expression by DENN-AS treatment (Fig. 4B).
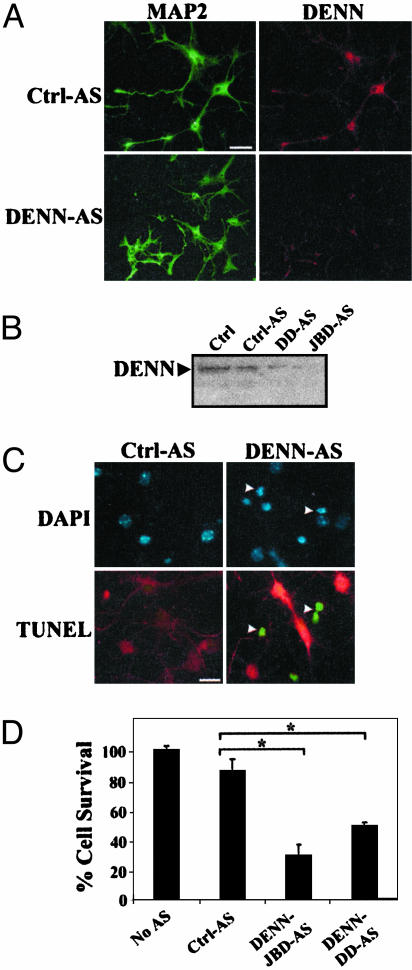
Fig. 4.
AS inhibition of endogenous DENN expression leads to cell death in primary cultures. (A) Control-antisense (Ctrl-AS) and DENN-AS were transfected into (DIV8) rat hippocampal neurons. Neurons were exposed to AS for 2 d, fixed in 4% paraformaldehyde, and stained with antibodies specific to MAP2 and DENN. The merged image indicates greatly diminished expression of DENN in the DENN-AS-treated cultures. (Bar, 20 μm.) Similar results were obtained in three separate experiments repeated in duplicate. (B) Western analysis of AS-treated hippocampal neurons. DENN expression is reduced in cultures exposed to two different DENN-AS oligonucleotides (DD-AS and JBD-AS), which correspond to regions spanning the DENN death domain (DD) and JBD. With both AS oligonucleotide sets, DENN expression is reduced 10-fold relative to Ctrl cultures. (C) Cell death induced by DENN-AS treatment. DAPI staining and TUNEL both demonstrate effects of Ctrl-AS or DENN-AS treatment in hippocampal neurons. DAPI reveals pyknotic nuclei (arrowheads). TUNEL (green) occurs in neurons (arrowheads) with reduced DENN expression (red), whereas TUNEL is absent in neurons exposed to Ctrl-AS. (D) Graphical representation of cell survival (%) of hippocampal neurons stained by TUNEL. DENN-AS-JBD and DENN-DD-AS resulted in reduced survival by ≈25% and 40%, respectively (*, P < 0.01), by ANOVA.
DENN-AS treatment resulted in increased cell death in hippocampal neurons. DAPI staining revealed pyknotic nuclei and condensed chromatin (Fig. 4C). Terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) further showed selectively increased cell death in neurons with depleted DENN expression, whereas remaining neurons expressing normal levels of DENN were not TUNEL positive (Fig. 4C). Quantification of TUNEL-positive neurons showed DENN-AS treatment resulted in 25% and 40% survival with DENN-JBD and DENN-DD AS, respectively (Fig. 4D). Thus, DENN expression is essential for cell survival of hippocampal neurons.
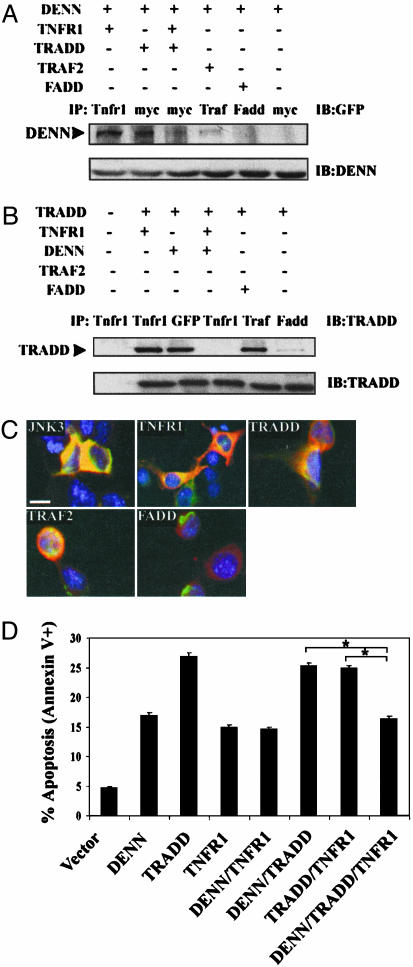
Competition Between DENN and TRADD May Alter Neuronal Cell Death. To determine the consequence of relative levels of either TRADD or TNFR1 on interactions with DENN, we coexpressed TNFR1, myc-tagged TRADD, and GFP-tagged DENN, in N2A cells, as well as either TRAF2 or FADD. Extracts were immunoprecipitated with antibodies specific to TNFR1, myc, TRAF2, or FADD and immunoblotted with anti-GFP to determine binding of DENN. In Fig. 5A, DENN is immunoprecipitated by TNFR1, TRADD, and TRAF2 but not FADD. When TNFR1, DENN, and TRADD are coexpressed, there was a 3-fold reduction in DENN binding to TRADD. DENN expression was equivalent in all samples when probed with anti-DENN (Fig. 5A, lower blot).
Fig. 5.
DENN blocks interaction between TRADD and TNFR1 in neuroblastoma. (A) GFP-tagged DENN, myc-tagged TRADD and TNFR1, TRAF2, and FADD were cotransfected in indicated combinations and immunoprecipitated (IP) with antibodies specific to TNFR1, myc, TRAF2, or FADD, and immunoblotted with GFP antibody. DENN binds to TNFR1, TRADD, and TRAF2 but not FADD. In the presence of TNFR1, DENN interaction with TRADD is decreased. (B)N2A cells were cotransfected as in A, immunoprecipitated, and immunoblotted with TRADD antibody. TRADD binds to TNFR1, DENN, TRAF2, and FADD. In the presence of DENN, TRADD binding to TNFR1 is abrogated. (C) In transfected N2A cells, DENN colocalizes with JNK3, TNFR1, TRADD, and TRAF2 but not FADD. (Bar, 10 μm.) (D) Overexpression of TRADD, TNFR1, and DENN in N2A cells, followed by flow cytometric analysis of Annexin V+ cells reveals TRADD-induced apoptosis is blocked by DENN coexpression. Data represents mean of triplicate cultures from three independent experiments. *, P < 0.05 by ANOVA.
Furthermore, TRADD was immunoprecipitated by TNFR1, DENN, and TRAF2 and weakly with FADD (Fig. 5B). However, coexpression of DENN with TNFR1 and TRADD resulted in abrogation of TNFR1 and TRADD interactions (Fig. 5B). Immunocytochemically, DENN colocalized in N2A cells with the TNF-related factors, TNFR1, TRADD, and TRAF2, as well as with JNK3, but not with FADD (Fig. 5C). In Fig. 5D, DENN coexpression with TRADD and TNFR1 partially blocked TRADD-induced apoptosis compared to TRADD coexpression with either TNFR1 or DENN (P < 0.05, ANOVA). Taken together with the inhibition of binding of TRADD with TNFR1 demonstrated in Fig. 5B, DENN complex formation with TNFR1, TRADD, and TRAF2 suggests DENN interferes with TRADD signaling and thereby may protect neuronal cells from cell death.
Discussion
The interplay of TNF-α and MAPK signaling pathways in neuronal degeneration is reflected in the expression and regulation of key signaling proteins. DENN, a DD-containing protein that binds to TNFR1 and JNK3, showed significant down-regulation in AD tissues, but not in normal, age-matched controls. In the AD-affected CA1 region of the hippocampus, DENN expression was diffusely diminished immunohistochemically, and also by western analysis and RT-PCR, especially in areas of Aβ and tau pathology. In CA1 pyramidal neurons adjacent to neurofibrillary tangle (NFT)-bearing cells in the AD hippocampus, DENN accumulated in the perinuclear cytoplasm. These results suggest that neuropathological effects of AD are correlated with DENN expression.
Down-regulation of DENN in response to Aβ exposure of neurotypic and hippocampal cultures suggests that effects observed in AD hippocampus are reproducible in vitro and involve an Aβ-mediated pathway. Translocation of residual endogenous DENN expression from neurites to the cytoplasm was coupled with nuclear localization of activated JNK. Protein and RNA expression of DENN was decreased in neuronal cultures. Similarly, in the AD transgenic mouse model Tg2576 (41), DENN expression was also decreased in extracts of piriform cortices, where APP expression was increased and Aβ plaques numerous (29).
Several other TNFR1 binding factors revealed altered expression in regions with AD histopathology. TRAF2, which binds TRADD and promotes JNK activation via apoptosis signaling kinase (ASK1), was also decreased in AD tissue. Schievella et al. (11) report that DENN overexpression in nonneuronal culture systems results in activation of both extracellular-regulated kinase (ERK) and JNK. However, because MAPKs are activated in AD (42), and DENN and TRAF2 are down-regulated, it is not likely that these proteins contribute to JNK- or ERK-mediated cell death or survival pathways.
Interestingly, there was increased expression of other members of the TNF signaling pathway, including TRADD and TNFR1, both in AD tissues and Tg2576 mouse extracts. TRADD overexpression leads to increased cell death of nonneuronal cells (33), and we confirmed enhanced apoptosis by TRADD overexpression in neuronal N2A cultures. TNFR1 expression was also increased in AD tissues, in agreement with Zhao et al. (14), suggesting receptor involvement in promoting cell death. However, not all TNF signaling factors are affected, because FADD and RIP expression levels remained unchanged in AD-affected tissues.
Aβ effects on protein expression may result in reduced inhibition of cell death signals. Based on results of our in vivo competition assay coexpressing DENN, TRADD, and TNFR1 in N2A cells, the strong TNFR1–DENN interaction points to the potential to block TNFR1–TRADD interactions. This mechanism is also suggested by the immunocytochemical overlap of DENN and TRADD overexpressed in N2A cells and functionally, with DENN inhibition of TRADD-induced apoptosis. These results provide a significant rationale for DENN reduction leading to cell death in the setting of sustained activation by TNF-α.
Apoptosis was augmented by anti-sense depletion of DENN expression. DENN expression is therefore required for neuronal survival. Lim and Chow (30) report enhancement of cell death by exposure of mammalian tumor cell lines to DENN-AS, arguing that constitutive threshold levels of DENN are required for cell proliferation. Higher constitutive DENN expression in malignant cells relative to nontransformed cells suggests alteration of DENN activity may disrupt normal cellular proliferation.
Increasing evidence supports an essential function of DENN in Ca2+-mediated exocytosis and neurotransmitter release (43, 44). DENN plays a physiologic role as a guanine nucleotide exchange protein (GEP) for Rab3A in synaptic vesicle release in humans, as it does in rodent and Caenorhabditis elegans (43, 45). DENN up-regulates a postdocking step of synaptic exocytosis. Therefore, the stress-induced relocation of DENN may contribute to reduced neurotransmitter function in neurites, including release of acetylcholine, a known feature in AD (46).
DENN promotes ERK-mediated cell proliferation in Kaposi's sarcoma (KS) cells (47). TNF-α-mediated cell proliferation in KS cells couples TNFR1 with the ERK1/2 signaling pathway via DENN. In HeLa cells stably expressing DENN or its isoforms, ERK1/2 and NF-κB are activated in response to TNF-α exposure, suggesting a defined protective or proliferative function for DENN (31). Overexpression of DENN provides partial resistance to TNF-α-induced apoptosis and activation of caspase-3 and -8.
DENN reduction in AD correlates with a growing family of proteins involved in the neuroprotective mechanisms of TNF signaling, including FADD-like IL-1β-converting enzyme (FLICE) inhibitor protein (FLIP) and TNFR2. FLIP binds to FADD and blocks subsequent caspase-8 recruitment and activation. Down-regulation thus allows TRADD to induce apoptosis. TNFR2 does not contain a DD and therefore serves a more neuroprotective role in response to TNF-α (23).
The identification of key TNFR-related signaling components with altered expression in AD suggests Aβ-mediated regulation may affect neuronal survival. Although DENN expression was decreased in the AD hippocampus, TRAF2 also showed diminished expression and in vitro binding to DENN. Speculatively, with TRADD expression increased in the AD-affected CNS and Tg2576 mouse cortex, it is available to bind to TNFR1, suggesting neuronal cell death may be mediated by Aβ-induced TNF-α induction. In agreement with Micheau and Tschopp (25), multiprotein complexes containing TRADD, TRAF2, RIP, and additional protein factors are released from TNFR1 after TNF-α activation and promote downstream apoptotic events. DENN may be a critical factor in these complexes and reduce the potentiation of neuronal cell death.
Supplementary Material
Acknowledgments
We thank the Microsequencing Core Facility at the University of Southern California and Ernesto Barron and Anthony Rodriguez for confocal microscopy assistance, Dr. Li Zhou and Celia Williams for technical support, Jeanette Espinosa for expert secretarial assistance, Drs. Greg Cole and Giselle Lim for providing aged Tg2576 mouse cortical tissue, and Drs. David Goeddel, David Wallach, and V. M. Dixit for plasmid reagents. This work was supported in part by grants to C.A.M. from the Alzheimer's Disease Research Center (AG05142), the National Institute of Mental Health (5R37MH39145), the National Institute on Aging (5R01AG18879), and the Sankyo Corp., a grant to K.D.V. from the National Institute of Neurological Disorders and Stroke (5T32NS07149), the John Douglas French Foundation (C.A.M. and K.D.V.), and William and Laura Siart (K.D.V.).
Abbreviations: AD, Alzheimer's disease; APP, amyloid precursor protein; AS, antisense; FADD, Fas-associated death domain; JNK, c-Jun N-terminal kinase; JBD, JNK binding domain; MAPK, mitogen-activated protein kinase; P-JNK, phosphorylated JNK; TNF, tumor necrosis factor; TNFR, TNF receptor; TRADD, TNFR-associated death domain; TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling.
References
- 1.Braak, H. & Braak, E. (1997) Neurobiol. Aging 18, S82-S85. [DOI] [PubMed] [Google Scholar]
- 2.Selkoe, D. J. (1999) Nature 399, A23-A31. [DOI] [PubMed] [Google Scholar]
- 3.Zhu, X., Lee, H. G., Raina, A. K., Perry, G. & Smith, M. A. (2002) Neurosignals 11, 270-281. [DOI] [PubMed] [Google Scholar]
- 4.Fillit, H., Ding, W. H., Buee, L., Kalman, J., Altstiel, L., Lawlor, B. & Wolf-Klein, G. (1991) Neurosci. Lett. 129, 318-320. [DOI] [PubMed] [Google Scholar]
- 5.Gary, D. S., Bruce-Keller, A. J., Kindy, M. S. & Mattson, M. P. (1998) J. Cereb. Blood Flow Metab. 18, 1283-1287. [DOI] [PubMed] [Google Scholar]
- 6.Nagatsu, T., Mogi, M., Ichinose, H. & Togari, A. (2000) J. Neural Transm. Suppl. 60, 277-290. [DOI] [PubMed] [Google Scholar]
- 7.Lue, L. F., Rydel, R., Brigham, E. F., Yang, L. B., Hampel, H., Murphy, G. M., Jr., Brachova, L., Yan, S. D., Walker, D. G., Shen, Y. & Rogers, J. (2001) Glia 35, 72-79. [DOI] [PubMed] [Google Scholar]
- 8.Combs, C. K., Johnson, D. E., Cannady, S. B., Lehman, T. M. & Landreth, G. E. (1999) J Neurosci. 19, 928-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tartaglia, L. A., Ayres, T. M., Wong, G. H. & Goeddel, D. V. (1993) Cell 74, 845-853. [DOI] [PubMed] [Google Scholar]
- 10.Wallach, D., Varfolomeev, E. E., Malinin, N. L., Goltsev, Y. V., Kovalenko, A. V. & Boldin, M. P. (1999) Annu. Rev. Immunol. 17, 331-367. [DOI] [PubMed] [Google Scholar]
- 11.Schievella, A. R., Chen, J. H., Graham, J. R. & Lin, L. L. (1997) J. Biol. Chem. 272, 12069-12075. [DOI] [PubMed] [Google Scholar]
- 12.Zhang, S. Q., Kovalenko, A., Cantarella, G. & Wallach, D. (2000) Immunity 12, 301-311. [DOI] [PubMed] [Google Scholar]
- 13.Chen, G. & Goeddel, D. V. (2002) Science 296, 1634-1635. [DOI] [PubMed] [Google Scholar]
- 14.Zhao, M., Cribbs, D. H., Anderson, A. J., Cummings, B. J., Su, J. H., Wasserman, A. J. & Cotman, C. W. (2003) Neurochem. Res. 28, 307-318. [DOI] [PubMed] [Google Scholar]
- 15.Engidawork, E., Gulesserian, T., Yoo, B. C., Cairns, N. & Lubec, G. (2001) Biochem. Biophys. Res. Commun. 281, 84-93. [DOI] [PubMed] [Google Scholar]
- 16.Gervais, F. G., Xu, D., Robertson, G. S., Vaillancourt, J. P., Zhu, Y., Huang, J., LeBlanc, A., Smith, D., Rigby, M., Shearman, M. S., et al. (1999) Cell 97, 395-406. [DOI] [PubMed] [Google Scholar]
- 17.Blasko, I., Apochal, A., Boeck, G., Hartmann, T., Grubeck-Loebenstein, B. & Ransmayr, G. (2001) Neurobiol. Dis. 8, 1094-1101. [DOI] [PubMed] [Google Scholar]
- 18.Bozyczko-Coyne, D., O'Kane, T. M., Wu, Z. L., Dobrzanski, P., Murthy, S., Vaught, J. L. & Scott, R. W. (2001) J. Neurochem. 77, 849-863. [DOI] [PubMed] [Google Scholar]
- 19.Morishima, Y., Gotoh, Y., Zieg, J., Barrett, T., Takano, H., Flavell, R., Davis, R. J., Shirasaki, Y. & Greenberg, M. E. (2001) J. Neurosci. 21, 7551-7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Troy, C. M., Rabacchi, S. A., Xu, Z., Maroney, A. C., Connors, T. J., Shelanski, M. L. & Greene, L. A. (2001) J. Neurochem. 77, 157-164. [DOI] [PubMed] [Google Scholar]
- 21.Ivins, K. J., Thornton, P. L., Rohn, T. T. & Cotman, C. W. (1999) Neurobiol. Dis. 6, 440-449. [DOI] [PubMed] [Google Scholar]
- 22.Tschopp, J., Irmler, M. & Thome, M. (1998) Curr. Opin. Immunol. 10, 552-558. [DOI] [PubMed] [Google Scholar]
- 23.Shen, Y., Li, R. & Shiosaki, K. (1997) J. Biol. Chem. 272, 3550-3553. [PubMed] [Google Scholar]
- 24.Yang, L., Lindholm, K., Konishi, Y., Li, R. & Shen, Y. (2002) J. Neurosci. 22, 3025-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Micheau, O. & Tschopp, J. (2003) Cell 114, 181-190. [DOI] [PubMed] [Google Scholar]
- 26.Zhang, Y., Zhou, L. & Miller, C. A. (1998) Proc. Natl. Acad. Sci. USA 95, 2586-2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mirra, S. S., Heyman, A., McKeel, D., Sumi, S. M., Crain, B. J., Brownlee, L. M., Vogel, F. S., Hughes, J. P., van Belle, G. & Berg, L. (1991) Neurology 41, 479-486. [DOI] [PubMed] [Google Scholar]
- 28.The NIA-Reagan Working Group (1997) Neurobiol. Aging 18, S1-S2. [PubMed] [Google Scholar]
- 29.Lim, G. P., Yang, F., Chu, T., Chen, P., Beech, W., Teter, B., Tran, T., Ubeda, O., Ashe, K. H., Frautschy, S. A. & Cole, G. M. (2000) J. Neurosci. 20, 5709-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim, K. M. & Chow, V. T. (2002) Mol. Carcinog. 35, 110-126. [DOI] [PubMed] [Google Scholar]
- 31.Al-Zoubi, A. M., Efimova, E. V., Kaithamana, S., Martinez, O., El-Idrissi Mel, A., Dogan, R. E. & Prabhakar, B. S. (2001) J. Biol. Chem. 276, 47202-47211. [DOI] [PubMed] [Google Scholar]
- 32.Hsu, H., Xiong, J. & Goeddel, D. V. (1995) Cell 81, 495-504. [DOI] [PubMed] [Google Scholar]
- 33.Hsu, H., Shu, H. B., Pan, M. G. & Goeddel, D. V. (1996) Cell 84, 299-308. [DOI] [PubMed] [Google Scholar]
- 34.Zhu, X., Castellani, R. J., Takeda, A., Nunomura, A., Atwood, C. S., Perry, G. & Smith, M. A. (2001) Mech. Ageing Dev. 123, 39-46. [DOI] [PubMed] [Google Scholar]
- 35.Zhu, X., Raina, A. K., Rottkamp, C. A., Aliev, G., Perry, G., Boux, H. & Smith, M. A. (2001) J. Neurochem. 76, 435-441. [DOI] [PubMed] [Google Scholar]
- 36.Li, Y. P., Bushnell, A. F., Lee, C. M., Perlmutter, L. S. & Wong, S. K. (1996) Brain Res. 738, 196-204. [DOI] [PubMed] [Google Scholar]
- 37.Kruman, I., Bruce-Keller, A. J., Bredesen, D., Waeg, G. & Mattson, M. P. (1997) J. Neurosci. 17, 5089-5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coffey, E. T., Hongisto, V., Dickens, M., Davis, R. J. & Courtney, M. J. (2000) J. Neurosci. 20, 7602-7613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Estus, S., Tucker, H. M., van Rooyen, C., Wright, S., Brigham, E. F., Wogulis, M. & Rydel, R. E. (1997) J. Neurosci. 17, 7736-7745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benzing, W. C., Wujek, J. R., Ward, E. K., Shaffer, D., Ashe, K. H., Younkin, S. G. & Brunden, K. R. (1999) Neurobiol. Aging 20, 581-589. [DOI] [PubMed] [Google Scholar]
- 41.Hsiao, K., Chapman, P., Nilsen, S., Eckman, C., Harigaya, Y., Younkin, S., Yang, F. & Cole, G. (1996) Science 274, 99-102. [DOI] [PubMed] [Google Scholar]
- 42.Ferrer, I., Blanco, R., Carmona, M. & Puig, B. (2001) J. Neural Transm. 108, 1397-1415. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka, M., Miyoshi, J., Ishizaki, H., Togawa, A., Ohnishi, K., Endo, K., Matsubara, K., Mizoguchi, A., Nagano, T., Sato, M., Sasaki, T. & Takai, Y. (2001) Mol. Biol. Cell 12, 1421-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamaguchi, K., Tanaka, M., Mizoguchi, A., Hirata, Y., Ishizaki, H., Kaneko, K., Miyoshi, J. & Takai, Y. (2002) Proc. Natl. Acad. Sci. USA 99, 14536-14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iwasaki, K., Staunton, J., Saifee, O., Nonet, M. & Thomas, J. H. (1997) Neuron 18, 613-622. [DOI] [PubMed] [Google Scholar]
- 46.Greenwald, B. S. & Davis, K. L. (1983) Adv. Neurol. 38, 87-102. [PubMed] [Google Scholar]
- 47.Murakami-Mori, K., Mori, S., Bonavida, B. & Nakamura, S. (1999) J. Immunol. 162, 3672-3679. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.