Abstract
The limitations of currently available therapies in addressing the non motor symptoms of Parkinson's disease (PD) have egged on the search for newer options. Zonisamide has been in use for epilepsy and it was serendipitously found to improve the symptoms of PD in a patient who had both epilepsy and PD. Thereafter, various trials were designed to assess the use of zonisamide in PD. The present article investigates the evidence for use of zonisamide in PD from the various clinical trials that were designed to address this issue. Furthermore, the article also summarizes the various mechanisms of its use in PD as described in various animal experiments. A search protocol was designed with predefined inclusion and exclusion criteria. The databases searched were Pubmed, Ovid medline, Cochrane and clinicaltrials.gov. The data thus generated, was fed into a predesigned format. Most of the clinical trials on zonisamide in PD have come from Japan. Most of these trials used the changes in the Unified Parkinson's Disease Rating Scale (UPDRS) score as the endpoints and the most conclusive evidence is for a dose of 25-50 mg, which caused a change in UPDRS part III (motor symptoms). These patients were on levodopa and other drugs used for PD during the trials. One of the clinical trials conducted in Spain investigates the use of zonisamide in impulse control disorders among 15 patients of PD. Among the many mechanisms postulated, a reduction in levodopa induced quinone formation, protection against mitochondrial impairment and an increase in astroglial cysteine transport, an inhibition of microglial activation, monoamine oxidase-B (MAO-B) inhibition, an increased dopamine release and blockade of calcium channels are the most cited. There is evidence for use of zonisamide in PD in addition to levodopa and other therapies for control of motor symptoms. For now, the evidence for its use in control of non motor symptoms in PD is not enough and needs to be investigated further.
KEY WORDS: Neuroprotection, Parkinson's Disease, Zonisamide
Introduction
Parkinson's disease (PD) is a neurodegenerative disorder, the exact pathophysiology of which still eludes the scientific mind. Factors like genetic predisposition, environmental toxins, oxidative stress or an amalgamation of these are usually implicated.[1] To further complicate the situation, the symptoms are varied. Conventional drug therapies like levodopa, address only the motor symptoms. The cognitive and psychiatric manifestations that cripple the patient and affect the quality of life of the patient adversely are not altered by any of the currently used drugs for PD.[2,3] Relief with levodopa itself comes at the price of disabling dyskinesia and worsening of symptoms after a few years. The other commonly used drugs for PD are dopaminergic agonists and catechol-O-methyl transferase (COMT) inhibitors. Dopaminergic agonists like ropinirole and pramipexole, though better tolerated than the earlier drugs in this group, have been associated with sudden sleep attacks and impulse control disorders.[3,4] In order to overcome the limitations of the currently used drugs and to provide a better quality of life for the patients, various treatment modalities with varied mechanisms of action are being tried in the treatment of PD.
Zonisamide is an antiepileptic drug that was discovered in Japan. The antiepileptic action of zonisamide was first attributed to its Na+ channel blocking activity.[5] In due course of time, other facets of its pharmacological actions were discovered. It was found to reduce the transient inward T-type calcium current (Suzuki 1992), enhance GABA mediated neuronal inhibition (Ueda 2003) and reduce glutamate release (Okada 1998).[6,7,8] A biphasic, dose dependent effect of zonisamide on serotonin and dopamine release was found by various authors (Okada 1995;Okada 1999; Kawata 1999).[9,10,11] It has been approved in Japan for use as an anti-Parkinson's disease agent in 2009.[12] The present review has been written with the intention of weighing the clinical and experimental evidence systematically for the use of zonisamide in PD.
Research Question
What is the clinical evidence for the use of zonisamide in PD in terms of relief of motor symptoms and non-motor symptoms?
What is the suggested mechanism of action in PD from various experimental studies carried out?
Search Strategy
A search protocol was designed with predefined inclusion and exclusion criteria for the references or studies that were found. The following databases were searched.
Pubmed
Ovid medline
Cochrane
clinicaltrials.gov
Inclusion Criteria
The search was limited to studies dating from year 2001 onwards. Randomized clinical trials (RCTs), open trials, observational studies, case reports and animal experiments demonstrating the mechanism of action of zonisamide in PD were included in the search.
Exclusion Criteria
All references pertaining to uses of zonisamide other than in PD were excluded. Studies pertaining to the use of zonisamide in the treatment of benign essential tremor not related to PD were also excluded. The search was conducted in the databases mentioned above and results were sorted into animal and clinical studies. The references found from these studies were also searched. The data on clinical studies thus generated was fed into a pre-designed format. The format is provided in the form of Table 1. The animal studies retrieved were used to arrive at the various mechanisms of action attributed to zonisamide in the treatment of PD.
Table 1.
The standard format used to extract information from the clinical trials of Zonisamide in PD
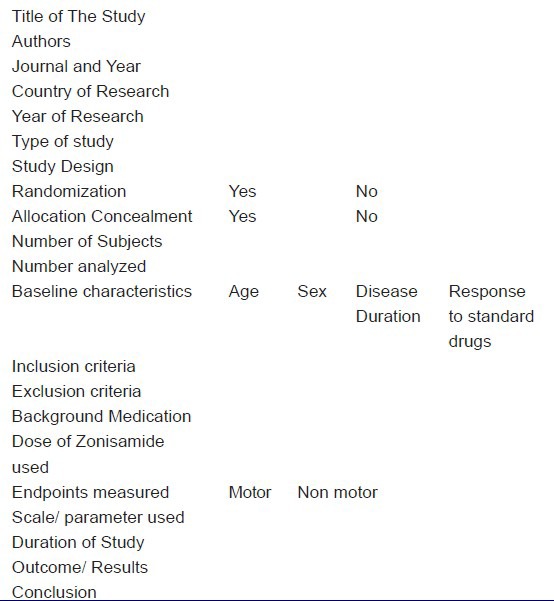
There were no Cochrane reviews on the use of zonisamide in PD. There was one RCT of zonisamide in PD registered with clinicaltrials.gov (NCT01766128), which was yet to start, hence not included.
Clinical Evidence
Most of the clinical trials in PD rest on the grading or staging provided by rating tools or scales. Unified Parkinson's Disease Rating Scale (UPDRS) is the most widely and universally used rating tool.[13] It has four domains, (I, II, III and IV), each designed to grade a different aspect of PD. Part I deals with mentation, behaviour and mood. Part II assesses the ‘activities of daily living’ for both the ‘on time’ as well as the ‘off time’. Part III deals with motor examination, whereas part IV addresses complications of therapy. UPDRS may be used along with other scales like the Hoehn and Yahr and the Modified Hoehn and Yahr, that grade the disability due to PD into stages from 0 to 5.[14] The clinical studies retrieved that satisfied the inclusion criteria of our study have been summarized in Table 2.
Table 2.
A summary of the clinical trials retrieved for use of zonisamide in PD
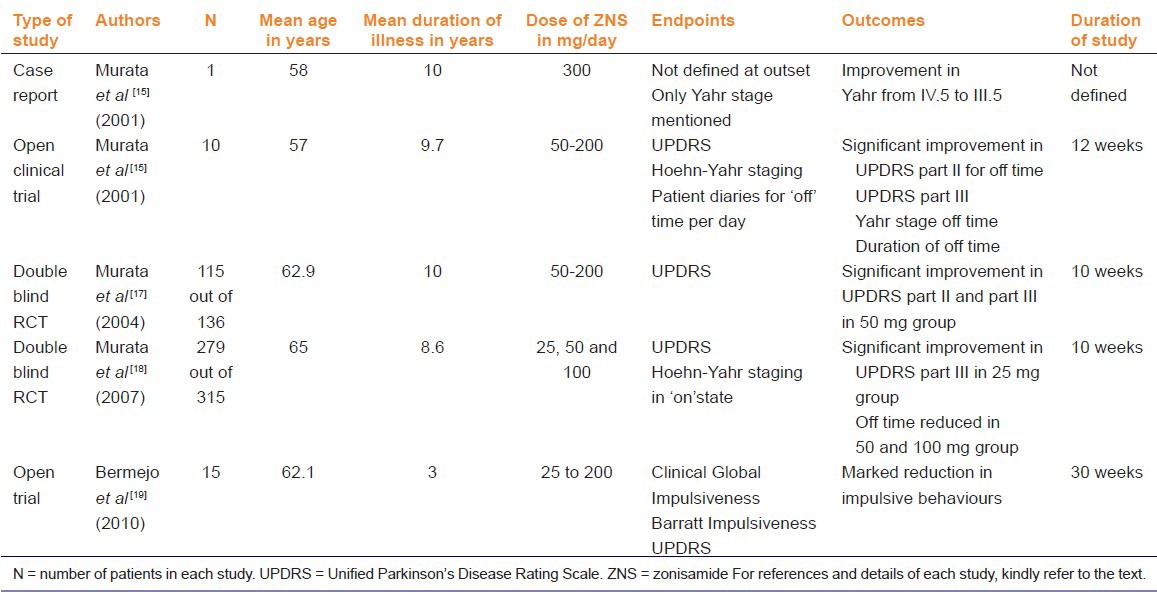
It was discovered in Japan in 2001, that when a 58 year old male patient who had both intractable epilepsy and PD was administered 300 mg of zonisamide, both the epilepsy and the PD came under control.[15] Based on this case report by Murata et al., an open trial was designed with 10 patients (eight men and two women) of PD.[15] Although the text in this particular article mentions nine patients, the observations are recorded for 10 patients. Hence, we have reported the number as ten. The average patient age was 57 years and the average duration of illness was 9.7 years. All the patients, except two, reported motor fluctuations. Those two patients showed a poor response to levodopa and had a positive family history. Zonisamide was added to the existing therapies in all these patients, in a dose of 50-200 mg/per day. The treatment was assessed using the UPDRS and Hoehn-Yahr stage and the ‘off time’ per day at the end of 12 weeks. The ‘off time’ per day was assessed on the basis of patient kept diaries. The UPDRS part II (assesses activities of daily living) of ‘off time’ improved (P < 0.001). The UPDRS part III (assesses motor symptoms) also improved significantly (P < 0.01). The Yahr stage ‘off time’ as well as the duration of ‘off time’ improved significantly (P < 0.001 and 0.001 respectively). These effects were seen within 3-7 days and the authors claim that although the study lasted only 12 weeks, the benefit has been maintained for one year. The changes in the UPDRS part I (deals with mentation, behaviour and mood) and the changes in UPDRS part IV (complications of therapy) have not been mentioned. It was a non comparative open trial. The patients had not been randomized to receive different doses. As a result, five of the patients were on 100 mg, two on 50 mg, two on 200 mg and one on 300 mg of zonisamide. It was also observed that the two patients who had shown poor response to levodopa showed poor response to zonisamide as well.[15]
Another open non comparison clinical trial, also in nine patients, was carried out by Nakanishi et al.[16] These patients were well controlled on standard anti PD medication but had unacceptable tremors at rest that interfered with daily activity. Zonisamide up to 100 mg per day reduced the tremors in seven patients. (Article in Japanese, only abstract available in English). Since we were unable to get the translation and the details of this article; it has not been included in the tabulated summary.
Murata et al., published the results of another clinical trial on zonisamide in PD carried out by the Japan Zonisamide Study Group.[17] This study has been published as a conference abstract. The study was a 23 centre, randomized, double blind, placebo controlled study. Patients experiencing ‘wearing off’ while on levodopa and peripheral decarboxylase inhibitor combination were included in this study. The study was of 10 weeks duration (8 weeks treatment and 2 weeks for dose reduction) excluding the 2 week run in period. The patients were randomized into 4 groups. The groups were: Zonisamide 50 mg, 100 mg, 200 mg and the placebo group. Concomitant medication for PD was kept constant. A total of 136 patients were randomized into these 4 groups out of which, 115 (84.5%) completed the study. Mean age of the patients was 62.9 and the mean duration of PD was 10 years. The sex of the patients was not specified. UPDRS was used to assess the response of the patients. There was significant improvement in the UPDRS part III (assesses motor symptoms) in the 50 mg group (P = 0.002). The responder rates for UPDRS part II (off time) were 53.6% (50 mg), 37.0% (100 mg), 34.8% (200 mg), 10.0% (placebo) and those for UPDRS part III were 60.7% (50 mg), 51.9% (100 mg), 65.2% (200 mg), 30.0% (placebo), respectively.
A multicentric, randomized, double blind, parallel treatment, placebo controlled trial was carried out by Murata et al in patients with PD in Japan.[18] This study was published in 2007. The trial included patients of PD, who showed inadequate response to levodopa. All patients received placebo for two weeks and then were randomized to receive 12 weeks of therapy with either zonisamide (25, 50 or 100 mg/day) or placebo. As a result, there were 4 parallel groups in this study. The placebo or zonisamide were used in addition to a background medication of levodopa. Fifty eight institutions in Japan recruited participants into this study. A total of 347 patients had been randomized into the study and 279 patients (80.4%) completed the study. The Intention To Treat (ITT) population consisted of 330 patients (95.1%). The ITT numbers were used for the safety assessment. The Full Analysis Set (FAS) consisted of 326 patients (181 men and 145 women, mean age 65 years) and was used for efficacy estimation. The mean duration of the disease was 8.6 years. Of the FAS (326 patients), 47 patients discontinued therapy prematurely. Twenty one of the 47 patients who discontinued therapy belonged to the 100 mg group. The treatment was followed by a 2 week dose reduction period. The duration of the study was 12 weeks, excluding the run in period and a dose reduction period. The primary endpoints selected were based on the UPDRS (Unified Parkinson's Disease Rating Scale). Change in the UPDRS part III score was taken as a primary endpoint. The responders were defined as >/ = 30% reduction in UPDRS part III. Significant improvement was observed on primary endpoints with 25 mg (P = 0.001) and 50 mg (P = 0.003) doses in comparison to the placebo group. The change was not significant with 100 mg dose (P = 0.066). Secondary endpoints were UPDRS I, II and IV and Modified Hoehn and Yahr score. No difference was observed in secondary endpoints. The duration of the ‘off time’ reduced significantly in 50 mg (P = 0.014) and 100 mg (P = 0.013) groups in comparison to placebo. The duration in the ‘off time ‘ was not significant in the 25 mg group (P = 0.0997) Adverse effects were highest in the 100 mg group and mainly consisted of drowsiness, weight loss, apathy and constipation. The main differences of this RCT from that conducted by the same authors earlier (Murata 2004) are; a larger sample size, longer duration of study and the inclusion of a 25 mg dose group instead of the 200 mg group.
Another single group, open, non-comparison study was undertaken in Spain on 15 patients.[19] The PD patients on levodopa and/or dopamine agonists with Impulse Control Disorders (ICD) were included in this study. These were the patients who did not respond to a reduction in the dose of levodopa and/or dopamine agonists. ICD's have been recognized as a known complication of dopaminergic therapy in PD patients and can disrupt the life of the patient and their families.[20,21] Fifteen patients (nine men and six women) at an average age of 62.1 years were included in the study. All these patients had been diagnosed with PD at least 3 years prior to the emergence of the ICDs. Five men had hypersexuality, 3 suffered from pathological gambling and one had compulsive shopping. Amongst the women, 4 had compulsive shopping, one had compulsive eating and one had compulsive shopping and eating. None of these patients had exhibited this behaviour before they developed PD. The compulsive behaviour had been present for at least one year in the patients. A dose of 25 mg titrated to 200 mg per day of zonisamide was used, as tolerated by the patients. The treatment was continued for at least 120 days, with the patients being on the maximum dosage for at least 60 days. There were no dosage adjustments made in the levodopa, carbidopa, rasagiline or the dopamine agonists that the patient was already having. Barratt Impulsiveness Scale for ICD, Clinical Global Impression for ICD and UPDRS for motor impairment were used to grade the response. Barratt's Impulsiveness Scale is a widely accepted scale to predict ICD in patients.[22] According to the authors, marked reduction in severity of impulsive behaviour (mean change from baseline-5.8) and global impulsiveness (mean change from baseline-4.8) was observed. The average UPDRS motor score changed only marginally from 25.8 to 24.9. There are no statistical tests mentioned and as the authors mention in their discussion, the sample size was very small. The dose of zonisamide used in this setting was much higher than that used in the trials discussed earlier. The earlier clinical trials by Murata et al., used changes in motor impairment as the primary endpoint whereas Bermejo et al., in their study used changes in ICD as a primary endpoint.
Most of the studies with zonisamide in PD have used the UPDRS part III (motor system examination) as the primary endpoint and it is this domain that shows a marked improvement in all but one of the studies. The one exception is the study by Bermejo et al., in which UPDRS motor score has been used as a secondary endpoint and there was only a marginal change in this, as mentioned earlier. The Movement Disorder Society (MDS) has published an evidence based review on the treatment of motor symptoms of PD in 2011.[23] Along with the other therapies for PD, zonisamide was also reviewed. The RCT by Murata et al., (2007) was given a quality score of 85% on the basis of which it was concluded that “Zonisamide is effective as a symptomatic adjunct to levodopa, with the practice implication that it is clinically useful.” Further, it was also concluded that use of zonisamide in PD for any other indication (apart from the use with levodopa for the control of motor symptoms) is investigational.[23] Since there has been only one open, randomized clinical trial with only 15 patients to assess the use of zonisamide in ICD in PD, more robust evidence is needed to make any claims in this issue.
Hence, zonisamide may be used in patients of PD as add on therapy in those who have insufficient response to standard therapy for the control of motor symptoms. There are no studies that assess the effect of zonisamide on the cognitive symptoms of PD. The doses showing most significant results seem to be in the range of 25-50 mg, lesser than that used for epilepsy.[15,17,18] In fact, the benefit seems to be lesser in doses higher than 50 mg and the adverse effects are more. However, keeping in mind the fact that most of the studies have been conducted in the Japanese population, it would be advisable to have more high quality RCTs in various ethnic populations with predefined endpoints before the results can be generalized.
Experimental Evidence
Before discussing the various mechanisms of action of zonisamide in PD, we would like to briefly mention the animal models used to study PD. Loss of dopaminergic neurons in the nigrostriatal pathway has been long held responsible for the symptoms of PD. Misfolding and abnormal aggregation of proteins, oxidative stress, mitochondrial dysfunction and gene mutation are the newer hypotheses that have been put forth to explain the neuropathology of PD.[24] Animal models of PD, though not replicating the exact pathophysiology of PD in humans are nevertheless useful for screening drugs that can prove useful in PD. Animal models of PD are broadly speaking those induced by neurotoxins (environmental or synthetic) or genetic models.[25,26] The various neurotoxins used are:1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), 6-hydroxydopamine (6-OHDA), rotenone and paraquat. These neurotoxins induce selective death of dopaminergic neurons in rodents and primates. The discovery of the PD genes has led to the development and use of genetic models of PD, based on defects/mutations in a-synuclein, Parkin, LRRK2, PINK1 and DJ-1 genes. The genetically modified animals are more susceptible to the toxins mentioned above and many a time, these models are combined. Rodent and non human primate models are used mainly. The use of primates though physiologically similar to humans, is wrought with ethical and cost constraints and as a result, most studies are done in rodents.[27] Apart from whole animal models, in vitro and ex vivo studies involving the use of brain slices, various cell lines and immunohistochemistry techniques have also been used.[28,29,30] Tyrosine hydroxylase (TH) is a rate limiting enzyme in the synthesis of DA. Antibodies to TH are used widely in immunocytochemistry to identify dopaminergic neurons.[31] Many of the studies have used TH identification of dopaminergic neurons to assess the effect of drugs in various models of PD.
Based on its pharmacological profile, many hypotheses have been put forth to explain the mechanism of action of zonisamide in Parkinson's disease. These have been tested in various experimental settings and are summarized in Table 3. Only the studies dating from 2001 onwards have been summarized, since the research into the use of zonisamide in PD started. A schematic diagram illustrating the mechanisms of action of zonisamide in PD has been provided in Figure 1.
Table 3.
A summary of the animal studies demonstrating the different mechanisms of action of zonisamide in PD
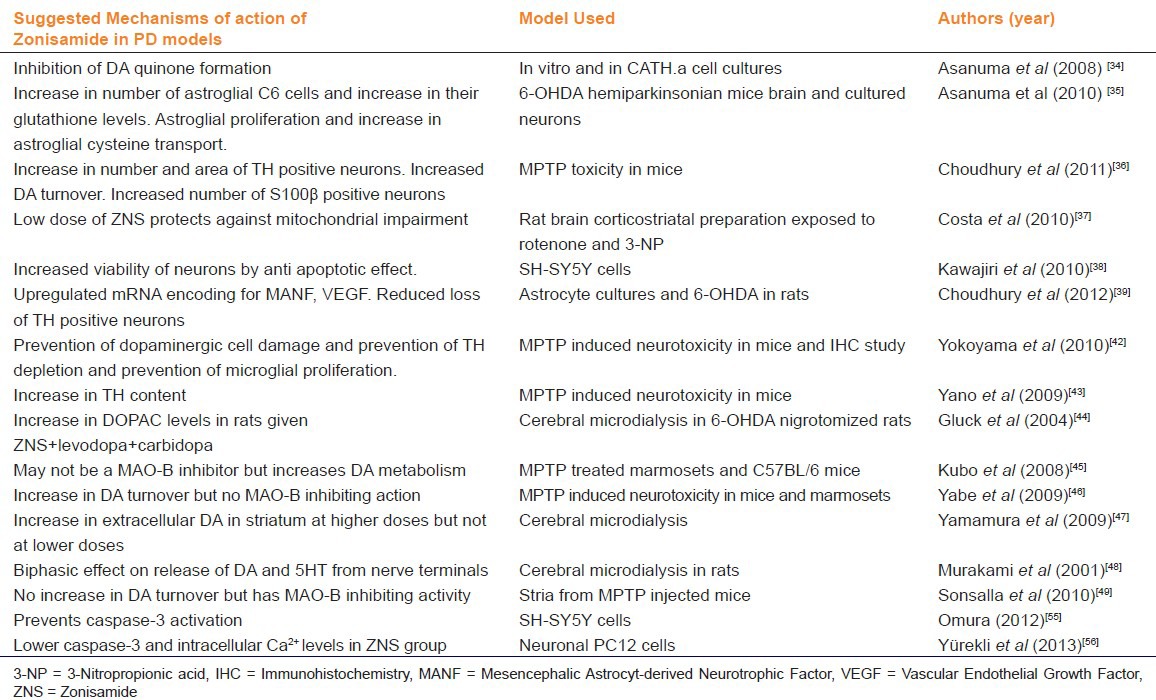
Figure 1.
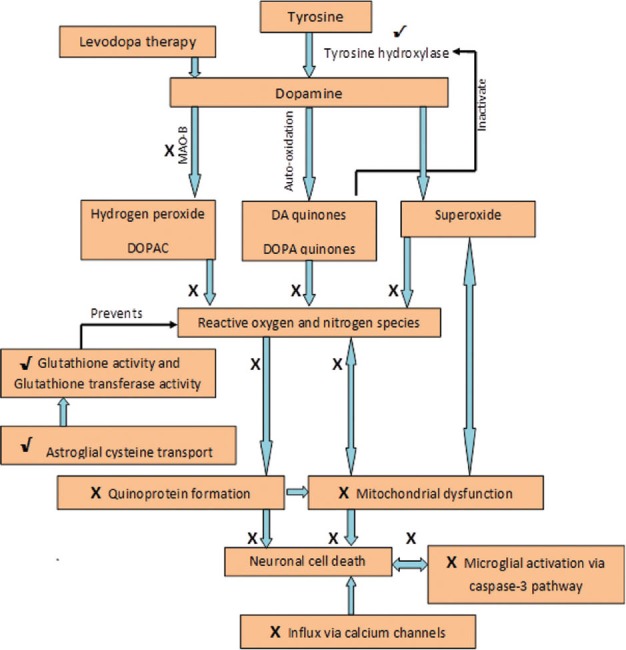
Mechanisms suggested in various studies, explaining the therapeutic effect of zonisamide in Parkinson's disease. X signifies inhibition of a process by zonisamide and √ signifies stimulation
Zonisamide and oxidative stress
Oxidative stress has been linked to the pathogenesis of PD since long time, especially in sporadic PD.[32] The toxic quinones generated by DA metabolism have been implicated in the pathogenesis of PD. Furthermore, quinones have been linked to mitochondrial complex I impairment, inactivation of TH and resultant oxidative stress.[32] Impairment of mitochondrial complex I has been implicated as a major factor in pathogenesis of PD.[33] Asanuma et al (2008) tested zonisamide in an in vitro setting as well as in CATH.a cell cultures.[34] They reported that a 5 day treatment with zonisamide prevented quinone formation induced by free cytosolic DA and also increased the DA/ DOPA chromes.
In another study, by Asanuma et al., (2010), 6-OHDA hemiparkinsonian mice brain and cultured neurons were used to the study the effect of zonisamide.[35] It was observed that zonisamide increased the cell number and caused proliferation of astroglial C6 cells and also increased their glutathione content. In addition to these findings, an increase in astroglial cysteine transport was seen. An increased astroglial cysteine transport and increased glutathione content are considered as neuroprotective. In keeping with the results of their earlier studies, zonisamide was found to inhibit DA quinone formation induced by levodopa. The authors conclude that these effects of astroglial proliferation were mediated via S100β secretion. S100β is a protein that is secreted by the astrocyes and it mediates communication between the glial cells and the neurons.
The fact that zonisamide works as a neuroprotectant against MPTP induced dopaminergic neuronal degeneration and that this effect is mediated via an increase in S100β secreting astrocytes has also been discussed by Choudhary et al (2011).[36] They studied the effects of zonisamide in MPTP toxicity in mice. It was noted that treatment of mice with MPTP and zonisamide protected against the effect of MPTP toxicity one week after treatment.
Low doses of zonisamide have also been seen to protect against mitochondrial impairment.[37] In a study by Costa et al, the effects of zonisamide on the mitochondrial complexes was assessed by using rat corticostriatal slices. The rat corticostriatal slices were subjected to rotenone (mitochondrial complex I inhibitor) and 3-nitropropionic acid (mitochondrial complex II inhibitor). The investigators found that zonisamide protected against rotenone induced toxicity but did not protect against 3-nitropropionic induced toxicity. The authors concluded that zonisamide protects against mitochondrial complex I inhibition and this could be one of the mechanisms of its effectiveness in PD.
A few studies also report an increase in the Mn Superoxide Dismutase levels that point to an antioxidant property of zonisamide.[38,39]
Zonisamide and Microglial Activation
Microglia have been designated as the cells involved in the immune surveillance of the brain. Activation of the microglia has been seen in response to nigral cell death in PD and microglial activation and dopaminergic cell loss is seen in midbrain of early PD patients.[40] Furthermore, the microglial activation leads to the release of pro-inflammatory cytokines and neurotoxic substances, which can cause a vicious cycle of microglial activation and neuronal death.[41] It has been observed in a study by Yokoyama et al., that zonisamide causes an inhibition of microglial activation and hence may slow the progression of the disease.[42] They investigated the effect of zonisamide on MPTP induced neurotoxicity in mice. The study concluded that zonisamide prevented the depletion of TH and prevents the proliferation of microglia in the striatum and substantia nigra.
Zonisamide and Increased DA Synthesis and Release
Tyrosine hydroxylase, the rate limiting enzyme in DA synthesis, is one of the proteins inactivated by catechol quinones, as mentioned above.[32] Zonisamide has been shown to increase the DA synthesis by increasing the tyrosine hydroxylase activity in the striatum.[42,43] In the study by Gluck et al, zonisamide was tested in a cerebral microdialysis system in 6OHDA nigrotomized rats. DA and dopaminergic metabolites were increased when zonisamide was administered with levodopa and carbidopa.[44] Increase in TH content and an increase in DA turnover has also been documented with several other studies (Yokoyama 2010, Kubo 2008; Yabe 2009;Yamamura 2009;).[42,45,46,47] Murakami et al., had demonstrated in a study in 2001 that zonisamide has a biphasic, concentration dependent effect on the exocytosis of dopamine.[48] However, the dose at which this effect was seen was equivalent to antiepileptic doses of zonismaide and increasing the dose beyond that inhibits the release of dopamine. The authors have not correlated these effects to the use of the drug in PD. How this increased release of DA fits into the profile of zonisamide as a neuroprotective drug, is somewhat unclear, since dopamine itself can be a source of oxidative damage (as discussed above).
Zonisamide and Monoamine Oxidase -B (MAO-B) Inhibition
In their publication dated 2010, Sonsalla and colleagues demonstrated that zonisamide is a reversible inhibitor of MAO-B and this may be its mechanism of action in PD.[49] In their study they used MPTP model of PD in mice and measured the levels of dopamine, DOPAC, TH, glutathione and activity of MAO-A and MAO-B in presence and absence of zonisamide and selegiline. The result showed that zonisamide did not alter dopamine, DOPAC, TH or glutathione levels. This is in contrast to the earlier studies that revealed that zonisamide increases dopamine turnover and TH activity. In fact, in their study on mice and marmosets, Yabe et al., found that dopamine turnover increased with zonisamide and that it does not have appreciable MAO-B inhibiting action.[46] However, there were methodological differences in the two studies. Sonsalla et al used Swiss Webster mice and Yabe et al used C57BL/6J mice and marmosets. Whereas Sonsalla and colleagues used zonisamide in a dose of 1, 5, 10 and 20 mg/Kg dose, Yabe et al., used zonisamide in a dose of 160 mg/Kg. Sonsalla et al assayed the MAO-B levels whereas Yabe et al., did not. However, a recent publication by Binda et al seems to shed some light on the issue.[50] In this study, the authors have measured the MAO inhibiting activity of zonisamide on human MAO and rat MAO. They have reported that zonisamide does not have any MAO-A inhibiting activity but has MAO-B inhibiting activity. The Ki value of zonisamide for human MAO-B was found to be 3.1 ± 0.3 μM whereas that for rat MAO-B was 2.9 ± 0.4μM. The study reveals that zonisamide in a dose of 25 mg/Kg that is used in PD can lead to an effective MAO-B inhibition. But the question remains that in spite of having MAO-B inhibiting activity whether the action contributes to its efficacy in PD.
Zonisamide and Calcium Channels
Another activity that has been ascribed to zonisamde is the blockade of the neuronal calcium channels.[6] It is not clear, however, which type of calcium channel blockade is involved in the protective role in PD. According to Suzuki et al, zonisamide has a T-type calcium channel blocking activity. Other authors have advocated that blockade of L type calcium channels can translate into a neuroprotective effect.[51,52] In fact, isradipine, an L-type of calcium channel blocker (CCB), is in Phase II clinical trials for use in PD.[53] It has been suggested, that if mitochondrial complex I gets inhibited, then the generation of mitochondrial ATP's goes down, leading to neuronal depolarization and Ca2+ mediated excitotoxicity via the NMDA receptors. It has been shown that a special L-type of calcium channel is present in the substantia nigra pars compacta (SNpc) DA neurons, that remains open for a longer time, thus requiring a higher ATP usage to keep the cytosolic Ca2+ level under control.[54] Even though this explains the selective vulnerability of SNpc DA neurons to oxidative stress, the role of T-type Ca2+ channels in the pathogenesis of PD and the role of zonisamide in this context remains elusive.
Recent research on the mechanism of action of zonisamide in PD is revolving around the modification of the action of caspase-3 (a protease involved in apoptosis) and growth factors like the mesencephalic astrocyte-derived neurotrophic factor (MANF), vascular endothelial growth factor (VGEF).[39,55,56] A schematic diagram of the suggested mechanisms of action of zonisamide in PD is provided in Figure 1.
Parkinson's Disease in India
Muthane et al have reviewed the epidemiology of PD in India.[57] They have reviewed the previous studies done in the various regions of the country and in people of different ethnic origins. The prevalence of PD in the Indian population seems to be lesser than that in the west except in the Parsi community, which has prevalence comparable to that of the Caucasians, thus underlining the contribution of genetics as one of the factors involved. The authors advocate the need for larger studies, considering the public health importance of the disorder. Even though the overall prevalence of PD in India seems lesser than that of the west, the population of PD patients in India has been estimated to be around 7 million (publication dated 2002).[58] With such a large population demanding treatment for PD, it might be worthwhile investigating a drug that might be of use in the disease.
Current Status of Zonisamide in PD
Zonisamide has been launched in a 25 mg tablet form under the brand name Trerief by Dainippon Sumitomo Pharma in 2009. The PMDA (the Japanese drug regulatory authority) has allowed the marketing of this drug for the treatment of patients of PD who have been treated with other antiparkinson's drugs in combination with levodopa but have shown inadequate response. The Movement Disorders Society has labelled zonisamide as being effective as an addition to levodopa for controlling the motor symptoms but with insufficient evidence for its use in any other indication in PD patients. The use of zonisamide in non-motor indications in PD will remain investigational till enough evidence is provided by well designed randomized controlled clinical trials that are powered to study these specific endpoints. Moreover, some issues are still not addressed. There are insufficient studies to demonstrate the value of long term use of zonisamide in PD. It has been observed that the beneficial effects of zonisamide on the wearing-off phenomenon, gradually reduced within about 1.5 years, although a 30% improvement in the URPDS total score was maintained for up to 3 years.[59] Since, most of the data regarding the use of zonisamide in PD has come from Japan; it would be desirable that the drug be explored further in different populations in order to have a higher level of evidence regarding its various uses in PD.
Footnotes
Source of Support: Nil
Conflict of Interest: No
References
- 1.Fahn S, Przedbroski S. Parkinson's Disease. In: Rowland LP, Pedley TA, editors. Chapter in Merritt's Neurology. 12th ed. Philadelphia: Wolters Kluwer-Lippincott Williams and Wilkins; 2010. pp. 751–69. [Google Scholar]
- 2.Standaert DG, Roberson ED. Treatment of central nervous system degenerative disorders. In: Brunton LL, editor. Chapter in Goodman and Gilman's The Pharmacological Basis of Therapeutics. 12th ed. New York: McGraw Hill; 2011. pp. 609–28. [Google Scholar]
- 3.Schapira AV. Treatment options in modern management of PD. Arch Neurol. 2007;64:1083–8. doi: 10.1001/archneur.64.8.1083. [DOI] [PubMed] [Google Scholar]
- 4.Weintraub D, Comella CL, Horn S. Parkinson's Disease- Part 2: Treatment of motor symptoms. Am J Manag Care. 2008;14:S49–58. [PubMed] [Google Scholar]
- 5.Rogawski MA, Porter RJ. Antiepileptic drugs: Pharmacological mechanisms and clinical efficacy with consideration of promising developmental stage compounds. Pharmacol Rev. 1990;42:223–86. [PubMed] [Google Scholar]
- 6.Suzuki S, Kawakami K, Nishimura S, Watanabe Y, Yagi K, Seino M, et al. Zonisamide blocks T-type calcium channel in cultured neurons of rat cerebral cortex. Epilepsy Res. 1992;12:21–7. doi: 10.1016/0920-1211(92)90087-a. [DOI] [PubMed] [Google Scholar]
- 7.Ueda Y, Doi T, Tokumaru J, Willmore LJ. Effect of zonisamide on molecular regulation of glutamate and GABA transporter proteins during epileptogenesis in rats with hippocampal seizures. Brain Res Mol Brain Res. 2003;116:1–6. doi: 10.1016/s0169-328x(03)00183-9. [DOI] [PubMed] [Google Scholar]
- 8.Okada M, Kawata Y, Mizuno K, Kondo T, Otani K, Fukushima Y. Interaction between Ca2+, K+, carbamazepine and zonisamide on hippocampal extracellular glutamate monitored with a microdialysis electrode. Br J Pharmacol. 1998;124:1277–85. doi: 10.1038/sj.bjp.0701941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okada M, Kaneko S, Hirano T, Mizuno K, Kondo T, Otani K, et al. Effects of zonisamide on dopaminergic system. Epilepsy Res. 1995;22:193–205. doi: 10.1016/0920-1211(95)00078-x. [DOI] [PubMed] [Google Scholar]
- 10.Okada M, Hirano T, Kawata Y, Murakami T, Wada K, Mizuno K, et al. Biphasic effects of zonisamide on serotonergic system in rat hippocampus. Epilepsy Res. 1999;34:187–97. doi: 10.1016/s0920-1211(98)00109-0. [DOI] [PubMed] [Google Scholar]
- 11.Kawata Y, Okada M, Murakami T, Mizuno K, Wada K, Kondo T, et al. effects of zonisamide on K+ and Ca2+ evoked release of monoamine as well as K+ evoked intracellular Ca2+ mobilization in rat hippocampus. Epilepsy Res. 1999;35:173–82. doi: 10.1016/s0920-1211(99)00010-8. [DOI] [PubMed] [Google Scholar]
- 12.Pharmaceuticals and Medical Devices Agency Japan. Tablet Trerief 25 mg, approved by PMDA, Japan on Jan 21st. 2009. [Last accessed in 2012 Jun]. Accessed from the official website of PMDA. Available from: http://www.pmda.go.jp/english/service/pdf/list/NewdrugsFY2008.pdf .
- 13.Movement Disorder Society Task Force on Rating Scales for Parkinson's Disease. The unified Parkinson's disease rating scale: status and recommendations. Mov Disord. 2003;18:738–750. doi: 10.1002/mds.10473. [DOI] [PubMed] [Google Scholar]
- 14.Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT, Counsell C, et al. Movement Disorder Society Task Force Report on the Hoehn and Yahr Staging Scale: Status and Recommendations. Mov Disord. 2004;19:1020–8. doi: 10.1002/mds.20213. [DOI] [PubMed] [Google Scholar]
- 15.Murata M, Horiuchi E, Kanazawa I. Zonisamide has beneficial effects on Parkinson's disease patients. Neurosci Res. 2001;41:397–9. doi: 10.1016/s0168-0102(01)00298-x. [DOI] [PubMed] [Google Scholar]
- 16.Nakanishi I, Kohomoto J, Miwa H, Kondo T. Effect of zonisamide on resting tremor resistant to antiparkinsonian medication. No To Shinkei. 2003;55:685–9. [PubMed] [Google Scholar]
- 17.Murata M, Hasegawa K, Kanazawa I The Japan Zonisamide Study Group. Randomized, double-blind study of zonisamide with placebo in advanced Parkinson's disease. Mov Disord. 2004;19:P555. [Google Scholar]
- 18.Murata M, Hasegawa K, Kanazawa I. Zonisamide improves motor function in Parkinson's disease: A randomized, double- blind study. Neurology. 2007;68:45–50. doi: 10.1212/01.wnl.0000250236.75053.16. [DOI] [PubMed] [Google Scholar]
- 19.Bermejo PE, Ruiz-Huete C, Anciones B. Zonisamide in managing impulse control disorders in Parkinson's disease. J Neurol. 2010;257:1682–5. doi: 10.1007/s00415-010-5603-7. [DOI] [PubMed] [Google Scholar]
- 20.Weintraub D. Dopamine and impulse control disorders in Parkinson's disease. Ann Neurol. 2008;64:S93–100. doi: 10.1002/ana.21454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weintraub D, Koester J, Potenza MN, Siderowf AD, Stacy M, Voon V, et al. Impulse control disorders in Parkinson's disease: A cross-sectional study of 3090 patients. Arch Neurol. 2010;67:589–95. doi: 10.1001/archneurol.2010.65. [DOI] [PubMed] [Google Scholar]
- 22.Stanford MS, Mathias CW, Dougherty DM, Lake SL, Anderson NE, Patton JH. Fifty years of the Barratt Impulsiveness Scale: An update and review. Pers Individ Dif. 2009;47:385–95. [Google Scholar]
- 23.Fox SH, Katzenschlager R, Lim SY, Ravina B, Seppi K, Coelho M, et al. The movement disorder society evidence based medicine review update: Treatments for the motor symptoms of Parkinson's disease. Mov Disord. 2011;26:2–41. doi: 10.1002/mds.23829. [DOI] [PubMed] [Google Scholar]
- 24.Olanow WC. The Pathogenesis of Cell Death in Parkinson's Disease–2007. Mov Disord. 2007;22:S335–42. doi: 10.1002/mds.21675. [DOI] [PubMed] [Google Scholar]
- 25.Blesa J, Phani S, Jackson-Lewis V, Przedborski S. Classic and new animal models of Parkinson's disease. J Biomed Biotechnol 2012. 2012 doi: 10.1155/2012/845618. 845618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hisahara S, Shimohama S. Toxin-induced and genetic models of Parkinson's disease. Parkinsons Dis 2010. 2011 doi: 10.4061/2011/951709. 951709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Potashkin JA, Blume SR, Runkle NK. Limitations of animal models of Parkinson's disease. Parkinsons Dis 2010. 2011 doi: 10.4061/2011/658083. 658083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho S, Wood A, Bowlby MR. Brain slices as models for neurodegenerative disease and screening platforms to identify novel therapeutics. Curr Neuropharmacol. 2007;5:19–33. doi: 10.2174/157015907780077105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasegawa T. Tyrosinase-expressing neuronal cell line as in vitro model of Parkinson's disease. Int J Mol Sci. 2010;11:1082–9. doi: 10.3390/ijms11031082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sherer TB, Betarbet R, Stout AK, Lund S, Baptista M, Panov AV, et al. An in vitro model of Parkinson's disease: Linking mitochondrial Iimpairment to altered α synuclein metabolism and oxidative damage. J Neurosci. 2002;22:7006–15. doi: 10.1523/JNEUROSCI.22-16-07006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Margolis EB, Coker AR, Driscoll JR, Lemiatre AI, Fields HL. Reliability in the identification of midbrain dopamine neurons. PLoS One. 2010;5:e15222. doi: 10.1371/journal.pone.0015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asanuma M, Miyazaki I, Diaz-Corrales F, Ogawa N. Quinone formation as dopaminergic neuron –specific oxidative stress in the pathogenesis of sporadic Parkinson's disease and neurotoxin- induced Parkinsonism. Acta Med Okayama. 2004;58:221–33. doi: 10.18926/AMO/32105. [DOI] [PubMed] [Google Scholar]
- 33.Schapira AV, Gegg M. Mitochondrial contribution to Parkinson's disease. Parkinsons Dis 2011. 2011:1–7. doi: 10.4061/2011/159160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asanuma M, Miyazaki I, Diaz-Corrales FJ, Miyoshi K, Ogawa N, Murata M. Preventing effects of a novel anti-parkinsonian agent zonisamide on dopamine quinone formation. Neurosci Res. 2008;60:106–13. doi: 10.1016/j.neures.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Asanuma M, Miyazaki I, Diaz-Corrales FJ, Kimoto N, Kikkawa Y, Takeshima M, et al. Neuroprotective effects of zonisamide target astrocyte. Ann Neurol. 2010;67:239–49. doi: 10.1002/ana.21885. [DOI] [PubMed] [Google Scholar]
- 36.Choudhury ME, Moritoyo T, Kubo M, Kyaw WT, Yabe H, Nishikawa N, et al. Zonosamide induced long lasting recovery of dopaminergic neurons form MPTP-toxicity. Brain Res. 2011;1384:170–8. doi: 10.1016/j.brainres.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 37.Costa C, Tozzi A, Luchetti E, Siliquini S, Belcastro V, Tantucci M, et al. Electrophysiological actions of zonisamide on striatal neurons: selective neuroprotection against complex I mitochondrial dysfunction. Exp Neurol. 2010;221:217–24. doi: 10.1016/j.expneurol.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Kawajiri S, Machida Y, Saiki S, Sato S, Hattori N. Zonisamide reduces cell death in SH-SY5Y cells via an anti-apoptotic effect and by upregulating MnSOD. Neurosci Lett. 2010;481:88–91. doi: 10.1016/j.neulet.2010.06.058. [DOI] [PubMed] [Google Scholar]
- 39.Choudhury ME, Sugimoto K, Kubo M, Iwaki H, Tsujii T, Kyaw WT, et al. Zonisamide up-regulated the mRNAs encoding astrocytic anti-oxidative and neurotrophic factors. Eur J Pharmacol. 2012;689:72–80. doi: 10.1016/j.ejphar.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 40.Ouchi Y, Yoshikawa E, Sekine Y, Futatsubashi M, Kanno T, Ogusu T, et al. Microglial activation and dopaminergic terminal loss in early Parkinson's disease. Ann Neurol. 2005;57:168–75. doi: 10.1002/ana.20338. [DOI] [PubMed] [Google Scholar]
- 41.Halliday GM, Stevens CH. Glia: Initiators and progressors of pathology in Parkinson's disease. Mov Disord. 2011;26:6–17. doi: 10.1002/mds.23455. [DOI] [PubMed] [Google Scholar]
- 42.Yokoyama H, Yano R, Kuroiwa H, Tsukada T, Uchida H, Kato H, et al. Therapeutic effect of a novel anti-parkinsonian agent zonisamide against MPTP(1-methyl-4-phenyl-1,2,3,6-terahydropyridine) neurotoxicity in mice. Metab Brain Dis. 2010;25:305–13. doi: 10.1007/s11011-010-9212-z. [DOI] [PubMed] [Google Scholar]
- 43.Yano R, Yokoyama H, Kuroiwa H, Kato H, Araki T. A novel anti-Parkinsonian agent, zonisamide, attenuates MPTP- induced neurotoxicity in mice. J Mol Neurosci. 2009;39:211–9. doi: 10.1007/s12031-009-9181-z. [DOI] [PubMed] [Google Scholar]
- 44.Gluck MR, Santana LA, Granson H, Yahr Md. Novel dopamine releasing response of an anti-convulsant agent with possible anti-Parkinson's activity. J Neural Transm. 2004;111:713–24. doi: 10.1007/s00702-004-0107-1. [DOI] [PubMed] [Google Scholar]
- 45.Kubo M, Nishikawa N, Yabe H, Nagai M, Moritoyo H, Moritoyo T, et al. Zonisamide increased metabolism of dopamine neurons in MPTP-treated C57BL/6 and common marmosets. Mov Disord. 2008;23:S311. [Google Scholar]
- 46.Yabe H, Choudhury ME, Kubo M, Nishikawa N, Nagai M, Nomoto M. Zonisamide increases dopamine turnover in the striatum of mice and common marmosets treated with MPTP. J Pharmacol Sci. 2009;110:64–8. doi: 10.1254/jphs.09019fp. [DOI] [PubMed] [Google Scholar]
- 47.Yamamura S, Ohoyama K, Nagase H, Okada M. Zonisamide enhances delta receptor-associated neurotransmitter release in striato-pallidal pathway. Neuropharmacology. 2009;57:322–31. doi: 10.1016/j.neuropharm.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 48.Murakami T, Okada M, Kawata Y, Zhu G, Kamata A, Kaneko S. Determination of effects of antiepileptic drugs on SNAREs-mediated hippocampal monoamine release using in vivo microdialysis. Br J Pharmacol. 2001;134:507–20. doi: 10.1038/sj.bjp.0704285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sonsalla PK, Wong LY, Winnik B, Buckley B. The antiepileptic drug zonisamide inhibits MAO-B and attenuates MPTP toxicity in mice: Clinical relevance. Exp Neurol. 2010;221:329–34. doi: 10.1016/j.expneurol.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Binda C, Aldeco M, Mattevi A, Edmondson DE. Interactions of monoamine oxidases with the antiepileptic drug zonisamide: Specificity of inhibition and structure of the human monoamine oxidase B complex. J Med Chem. 2011;54:909–12. doi: 10.1021/jm101359c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chan CS, Gertler TS, Surmeier DJ. A molecular basis for the increased vulnerability of substantia nigra dopamine neurons in aging and Parkinson's disease. Mov Disord. 2010;25(Suppl 1):S63–70. doi: 10.1002/mds.22801. [DOI] [PubMed] [Google Scholar]
- 52.Surmeier DJ, Guzman JN, Sanchez-Padilla J, Schumacker PT. The role of calcium and mitochondrial oxidant stress in the loss of substantia nigra pars compacta dopaminergic neurons in Parkinson's disease. Neuroscience. 2011;198:221–31. doi: 10.1016/j.neuroscience.2011.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Phase II safety and tolerability of isradipine (a potential neuroprotective agent) in patients with Parkinson's disease-Stage II. Clinical Trials Identifier NCT00753636. [Last accessed on 2012 Aug 06]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00753636?term = isradipineandrank = 1 .
- 54.Striessnig J, Koschak A, Sinnegger-Brauns MJ, Hetzenauer A, Nguyen NK, Busquet P, et al. Role of voltage gated L-type Ca2+ channel isoforms for brain function. Biochem Soc Trans. 2006;34:903–9. doi: 10.1042/BST0340903. [DOI] [PubMed] [Google Scholar]
- 55.Omura T, Asari M, Yamamoto J, Kamiyama N, Oka K, Hoshina C, et al. HRD1 levels increased by zonisamide prevented cell death and caspase-3 activation caused by endoplasmic reticulum stress in SH-SY5Y cells. J Mol Neurosci. 2012;46:527–35. doi: 10.1007/s12031-011-9638-8. [DOI] [PubMed] [Google Scholar]
- 56.Yürekli VA, Gürler S, Nazıroğlu M, Uğuz AC, Koyuncuoğlu HR. Zonisamide attenuates MPP+-induced oxidative toxicity through modulation of Ca2+ signaling and caspase-3 activity in neuronal PC12 cells. Cell Mol Neurobiol. 2013;33:205–12. doi: 10.1007/s10571-012-9886-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muthane UB, Ragothaman M, Gururaj G. Epidemiology of Parkinson's disease and movement disorders in India: problems and possibilities. JAPI. 2007;55:719–24. [PubMed] [Google Scholar]
- 58.Behari M. Experiences of Parkinson's disease in India. Lancet Neurol. 2002;1:258–62. doi: 10.1016/s1474-4422(02)00105-9. [DOI] [PubMed] [Google Scholar]
- 59.Murata M. Novel therapeutic effects of the anti-convulsant, zonisamide, on Parkinson's disease. Curr Pharm Des. 2004;10:687–93. doi: 10.2174/1381612043453180. [DOI] [PubMed] [Google Scholar]


