Abstract
Objective:
algae isolates obtained from fresh and marine resources could be one of the richest sources of novel bioactive secondary metabolites expected to have pharmaceutical significance for new drug development. This study was conducted to evaluate the antiangiogenic and antiproliferative activity of Chlorella pyrenoidosa in experimental models of angiogenesis and by MTT assay.
Materials and Methods:
lyophilized extract of C. pyrenoidosa was extracted using dichloromethane/methanol (2:1), concentrated and vacuum evaporated to obtain the dried extract. The crude extract was evaluated in the vascular endothelial growth factor (VEGF)-induced angiogenesis in in ovo chick chorioallantoic membrane assay (CAM) at various concentrations (n = 8) using thalidomide and normal saline as positive and untreated control groups, respectively. The crude extract was also subjected to the antiangiogenic activity in the silver nitrate/potassium nitrate cautery model of corneal neovascularization (CN) in rats where topical bevacizumab was used as a positive control. The vasculature was photographed and blood vessel density was quantified using Aphelion imaging software. The extract was also evaluated for its anti proliferative activity by microculture tetrazolium test (MTT) assay using HeLa cancer cell line (ATCC).
Results:
VEGF increased the blood vessel density by 220% as compared to normal and thalidomide treatment decreased it to 67.2% in in ovo assay. In the in-vivo CN model, the mean neovascular density in the control group, the C. pyrenoidosa extract and bevacizumab group were found to be 100%, 59.02%, and 32.20%, respectively. The Chlorella pyrenoidosa extract negatively affected the viability of HeLa cells. An IC50 value of the extract was 570 μg/ml, respectively.
Conclusion:
a significant antiangiogenic activity was observed against VEGF-induced neovascularization and antiproliferative activity by MTT assay. In this study, it could be attributed that the activity may be due to the presence of secondary metabolites in the C. pyrenoidosa extract.
KEY WORDS: Algae, angiogenesis, chorioallantoic membrane, corneal neovascularization, vascular endothelial growth factor
Introduction
The growth of blood vessels (a process known as angiogenesis) is essential for organ growth and repair. An imbalance in this process contributes to numerous malignant, inflammatory, ischaemic, infectious, and immune disorders.[1] Formation of new blood capillaries from existing vessels is considered as a complex process mediated by several stimulating and inhibiting factors on the endothelial cells that line blood vessels.[2] Interestingly, it is also an important mechanism for supplying nutrients to cells that are distant from existing blood vessels.[3]
Antiangiogenesis therapy is designed to arrest newly developing nutrient supply system rather than attacking tumor directly. This is accomplished through the use of angiogenesis inhibitors that interfere with the angiogenesis process. Antiangiogenic drugs are also reported to offer improvement over traditional cancer treatment in reducing side effects, inhibiting the formation of new blood vessels supplying the tumor and in this process the chances drug resistance are less likely.[4]
Chemical and biological diversity of the aquatic environment is inestimable and therefore is an extraordinary resource for the discovery of compounds having exotic structures with novel mechanism of actions. Algae are a diverse group of photo-autotrophic organisms that are found in terrestrial and aquatic environments. They are an essential component of the food chain in many ecosystems. However, they can often form dense scums or blooms that have been shown to be hazardous to humans and animals.[5] Cynobacteria and algae are reported to produce secondary metabolites that inhibit the transcriptional activation of vascular endothelial growth factor (VEGF), a critical angiogenic factor produced by tumor cells to promote new blood vessel formation and facilitate tumor growth.[6]
Microalgae are a major natural source for a vast array of novel compounds: nutrients, including proteins, vitamins, minerals, and fatty acids, as well as carotenoid pigments, such as xanthophylls and carotenes. Subspecies of Chlorella are known to have several bioactive secondary metabolites.[7] Therefore, we made an attempt to evaluate antiangiogenic and antiproliferative activity of dichloromethane/methanol extract of Chlorella pyrenoidosa by chorioallantoic membrane assay (CAM) and corneal neovascularization (CN) methods, and microculture tetrazolium test (MTT) assay.
Materials and Methods
C. pyrenoidosa was obtained from ‘Sun Chlorella’, Japan, in the form of tablets and was used in this study. Mass grade reagents were purchased from Merck, Germany, and all other reagents of analytical grade were purchased from their respective commercial resources. Recombinant VEGF was purchased from Sigma USA. The fertilized White Leghorn chicken eggs were purchased from local hatchery (Keggs farm Pvt Ltd. Gurgoan, India) and rats were obtained from the Central Animal Facility of All India Institute of Medical Sciences, New Delhi, India. Food and water were given ad libitum to all animals throughout each experiment.
Extraction of C. pyrenoidosa
C. pyrenoidosa lyophilized algal material of 100 g was homogenized in a solvent mixture containing dichloromethane and methanol in the ratio of 2:1 (v/v) using a Polytron high-speed homogenizer (Kinematica AG, Switzerland). The homogenate was subsequently subjected for ultrasonication for 3 min using a Sigma bath-type sonicator (Bendelin Sonorex, Berlin). The homogenized material was centrifuged at 5800 g for 5 min and passed through Whatman No.1 filter paper. This process was repeated three times and the extracts were pooled and subjected for vacuum rotary evaporation (Christ, RVC 2-18, Germany) to remove the solvents. The residual moisture (if any) was also removed by overnight lyophilization. This process yielded 1.2 g dark green residue. The extracts were stored at — –80°C till further studies.
Phytochemical screening
Phytochemical screening of the extract was done as per the standard method.[8]
Evaluation of C. pyrenoidosa extract in VEGF-induced angiogenesis in in ovo chick chorioallontoic membrane assay
The CAM assay was performed in an identical fashion with slight modification as described by Ribbati et al., (1997).[9] The fertilized eggs were incubated at 37°C and the incubator was humidified by keeping bowl of water inside. On the third day of incubation, 2-3 ml of albumin was removed to detach the developing CAM from the shell. On the eighth day, a square window was opened in the egg. VEGF (50 ng) containing ovalbumin pre-coated coverslips (12 mm diameter) were carefully placed over the developing chorionic allantoic membrane and served as untreated control. Similarly, only ovalbumin coated, ovalbumin, along with VEGF and thalidomide (10 μg) coated, ovalbumin, along with VEGF and CP extracts of various concentrations (25, 50 and 100 μg) coated cover slips were placed individually in every egg and considered as normal, positive control, and experimental, respectively.
Morphometric measurements of the expansion of the CAM vasculature
On 12th day, the cover slip area of the CAM was subjected for light photo microscopy and by using digital camera (Scout SCA1390-17½’’ CCD GigE color camera) under uniform magnification. The digital images in the defined area of the cover slip were analyzed by Aphelion imaging software (Version 2.8 SP1) which was customized and standardized to calculate the blood vessel density. The digital reconstructions were processed through a specific vascular algorithm to obtain total vascular density in terms of square millimeter area.
Evaluation of C. pyrenoidosa extract in in-vivo model of cautery-induced corneal neovascularization
Wistar rats weighing 200-225 g were used for this study, food water were given ad libitum to all animals throughout the experiments. For each group six eyes of six rats were used. This study was conducted in accordance with the guidelines for the usage of animals in vision research by Association of Research for Vision and Ophthalmology (ARVO). The protocol was approved by the standing Institutional Animal Ethics Committee (IAEC) of All India Institute of Medical Sciences, New Delhi. After anesthetizing the rats using sodium pentobarbitone (35 mg/kg intraperitoneally), right cornea of each rat eye was cauterized by gently pressing applicator sticks coated with 75% silver nitrate and 25% potassium nitrate to the surface of the cornea eccentrically at a point approximately 2.5 mm from the corneoscleral limbus for 5 sec. After the application, the eye was washed with sterile saline. Following cauterization, the rats were randomized in to three groups to eliminate potential bias in the degree of injury within the different groups. Sham-operated group received only sterile phosphate buffered saline (PBS) three times a day topically. Positive control received bevacizumab at the dose of 4mg/ml, diluted in sterile PBS having the pH of 7.2 with the osmolarity of 290 mOsM/L. Extract of C. pyrenoidosa was dissolved in 1% ethanol and later diluted to get the concentration of 1 mg/ml (0.1%w/v) with PBS and was filtered through 0.22 μ filters (Milex filters, Millipore, USA). Each rat received three drops of respective treatments (saline/bevacizumab/0.1% CP extract) in a gap of 30 s intervals by topical instillation for each period in their respective groups. All treatments were given three times per day (8:00 AM, 2.00 PM, 8:00 PM) for 5 days. The first treatment with each medication was initiated approximately 30 min after cauterization.
On the fifth day of the CN study, the eyes were subjected to photo microscopy. Vascular density was quantified using the aforesaid method using Aphelion imaging software (Version 2.8 SP1). The area of each cornea and its blood vessels were independently determined and the percentage of the corneal area occupied by blood vessels was computed for each photograph. The data are expressed in mean values of density of total blood vessels in the cornea of the individual group.
Evaluation of Chlorella pyrenoidosa extract for screening the anti proliferative activity microculture tetrazolium test assay
MTT assay gives a measure of the non-viable cells in a given population. The mitochondrial dehydrogenase enzyme of the viable cells reduces the yellow colored tetrazolium dye {MTT or [3-(4,5-Dimethylthiazolyl-2)-2,5-diphenyl[tetrazolium bromide]} into insoluble purple colored formazan crystals. The crystals are solubilized by the addition of a detergent and the color of the treatment and control group can be differentiated spectrophotometrically (560 nm) and percentage killing can be calculated. This is a sensitive method for screening cytotoxicity of test samples.
Cancer cell line developed from cervical cancer cell (HeLa), procured from the American-Type Culture Collection (ATCC; Rockville, MD), and was used to study the anticancer activity of different concentrations of algal extract. The cells were grown under aseptic conditions using Dulbecco's modified eagle's medium (DMEM) enriched with 10% fetal calf serum (FCS), at a pH of 7.2. To the medium, antibiotics [ciprofloxacin (10 μg/ml) and amphotericin B (1 μg/ml) were also added. The medium was prepared aseptically and filtered through 0.22 μm sterile filter membrane. The sterility of the medium was confirmed by incubating it for 48 h in the incubator at 37°C with 5% CO2. Cells grown between passage numbers 11 to 25 were used for all experiments.
For MTT assay, 106cells were plated in 25 cm2 flasks in 5 ml medium and incubated at 37°C with 5% CO2 in an incubator. The medium was periodically changed to provide ideal growing conditions for cells. The cells from confluent flasks were subcultured using the standard cultured protocol. Briefly, the medium was withdrawn and the cells given a wash with sterile phosphate buffer saline (PBS) to remove all traces of medium. PBS was discarded and about 800 μl of sterile trypsin-EDTA (0.5%) was added to the flask. The detached cells were counted using hemocytometer and the medium was adjusted to give a plating density of 106cells.
In order to perform the MTT assay, the confluent flask was processed as mentioned earlier and the medium adjusted to give a cell density of 7000-8000/100 μl. The cells were plated onto 96 well plates in a volume of 100 μl in each well. The cells were allowed to attach and incubated undisturbed for 24 h. The medium was removed after 24 h and the cells treated with different concentrations of test samples or vehicle in sterile medium. The control wells did not receive any treatment and were maintained in the medium.
The cells were subjected to test samples for 24 h. After the test sample treatment schedule, the medium from well plate was removed with simultaneous addition of yellow-colored tetrazolium solution. The tetrazolium solution was prepared aseptically in a concentration of 5 mg/ml in FCS free medium. The cells were exposed to the dye for 3 h and completion of the assay was confirmed by microscopic examination of the plate for purple-colored formazan crystals. The crystals formed in each well were dissolved with 100 μl of DMSO and protected from light and absorbance (OD) was immediately read in an ELISA reader at 560 nm. All test sample treatment was conducted in triplicate and the entire experiment was run in duplicate.
The drug-induced cytotoxicity was calculated using the following formula. IC50 was defined as the concentration of drug that produces 50% killing.
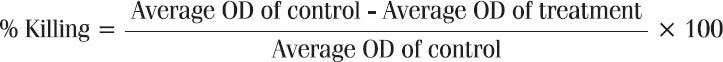
Different extracts were reconstituted in phosphate buffer saline (final concentration<0.1%) in order screen the anticancer activity of the. The extracts were screened at the concentration of 800, 400, 200, 100, 50, 25, 12.5, and 6.25 μg/ml for their in-vitro anticancer activity.
Results
Phytochemical screening
C. pyrenoidosa extract obtained was dark green in color. Phytochemical screening revealed the presence of flavonoids, carbohydrates, phenolics, aminoacids, and alkaloids.
Effect of vegf and C. pyrenoidosa extract on CAM
On the 12th day of incubation, the developed CAM that was treated with VEGF showed profuse growth of blood vessels around the cover slip [Figure 1b]. At microscopic level, a higher vascular density was recognizable below the VEGF containing ovalbumin-coated cover slips as compared to the normal [Figure 1a]. At the boundary between cover slip and CAM mesenchyma, numerous capillaries were detectable around the cover slip. In contrast, treatment with CP extracts decreased the blood vessel density in a dose-dependent manner. Figure 1 indicating the representative sample showing the effect of normal, VEGF, VEGF+thalidomide, VEGF with different concentrations of CP extracts. The thalidomide and CP at all dose levels showed reduction in blood vessel density and were found to be statistically significant as compared to untreated VEGF control [Table 1].
Figure 1.
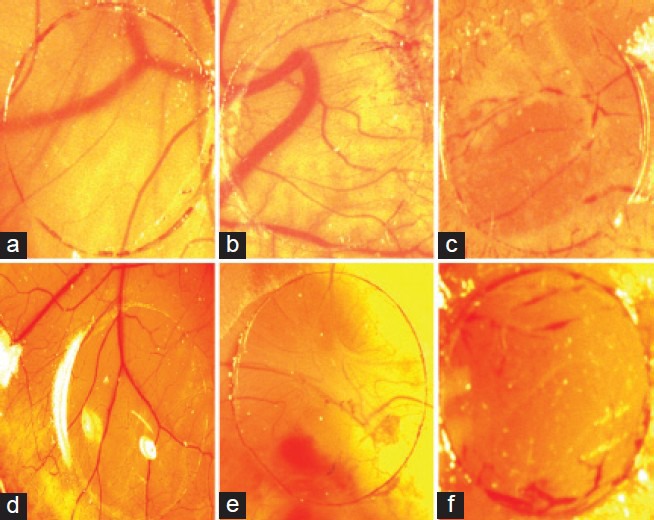
Indicated the chorioallantoic membrane of a 12-day old chick embryo incubated for 4 days with a cover slip treated with or without treatment. (a) Showing normal; (b) showing VEGF (50 ng); (c) showing VEGF (50 ng) + thalidomide (10 μg); (d-f) Indicating VEGF (50 ng) + CP at the concentrations of 25, 50, and 100 μg, respectively
Table 1.
Quantification of antiangiogenic response of the VEGF vs. normal, thalidomide and C. pyrenoidosa extract (25, 50, and 100 μg/egg) in the CAM assay on the 12th-day incubation. Number of experiment is three (n=3)

Effect of C. pyrenoidosa extract on corneal neovascularization
On the initial day the blood vessels in the limbal area were dilated, and there was no neovascularization on the cornea. On the third day, neovascularization was seen towards the central portion of the cornea from the limbal region [Figure 2b]. On the fifth day, the mean area of neovascularization of the control, bevacizumab and C. pyrenoidosa extract-treated groups were found to be 15.37 ± 0.49, 4.95 ± 0.33, 9.07 ± 1.09, respectively [Figure 2c and 2d]. The bevacizumab and CP reduced the formation of total blood vessels which were found to be statistically significant (P < 0.001) [Figure 3].
Figure 2.
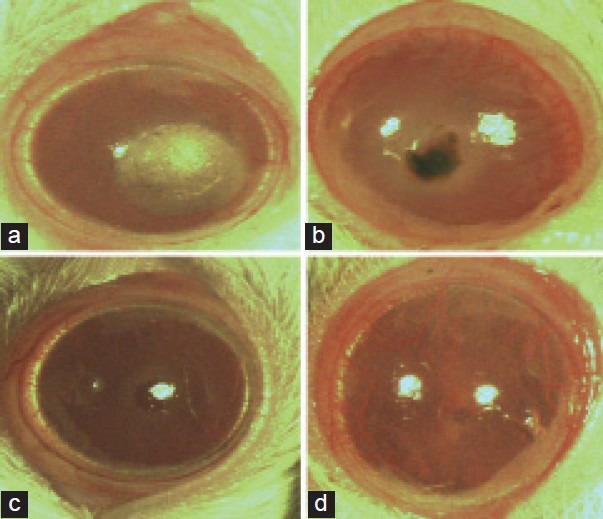
Indicated the results of corneal neovascularization (CN) of rat cornea. (a) Showing the induction of cautery in rat cornea; (b) showing CN after alkali burn in eye without treatment of drug, (c) showing inhibition of CN after alkali burn in eyes treated with C. pyrenoidosa extract on fifth day of treatment; (d) showing CN after alkali burn in eyes treated with Bevacizumab
Figure 3.
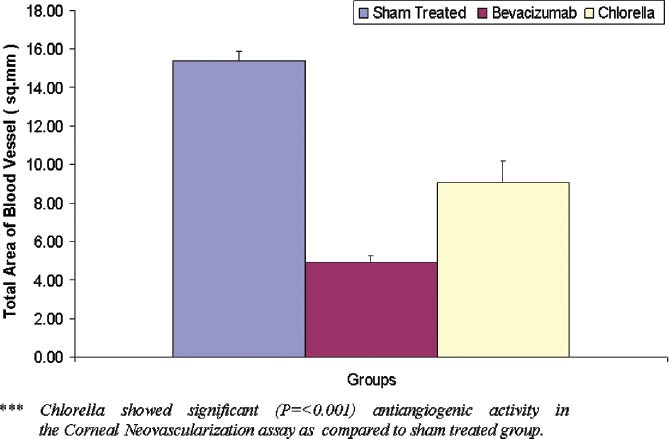
Graph indicating the mean vessel density of Sham (sterile PBS), Bevacizumab (4 mg/ml) and Chlorella pyrenoidosa (1 mg/ml) treated in corneal neovascularization. The values are shown as mean ± SEM
Effect of C. pyrenoidosa extract on antiproliferative study by MTT assay
In preliminary experiments, the extract of Chlorella pyrenoidosa was screened at concentration of 800, 400, 200, 100, 50, 25, 12.5, and 6.25 μg/ml for duration of 24 h. The C. pyrenoidosa extract negatively affected the viability of HeLa cells. About 40% increment in killing was seen when the dose of the extract was increased from 6.25 to 400 μg/ml. IC50 values of the extract was 570 μg/ml, respectively [Figure 4].
Figure 4.
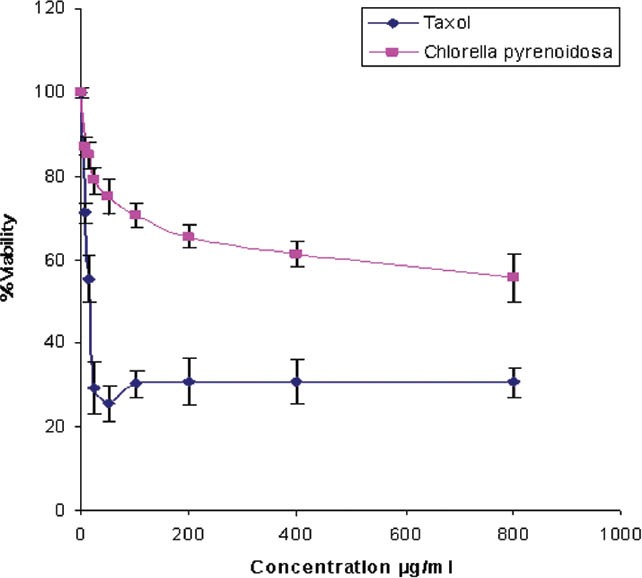
The antiproliferative activity of dichloromethane/methanolic extract of Chlorella pyrenoidosa on HeLa cells. Cells were incubated with different concentrations of the plant extracts (ranged from 6.25 to 800 ìg/ml) for 48 h. Cell viability was determined by the MTT assay (n = 4). Viability curves: Percentage viability = absorbance of test wells/ absorbance of control wells) × 100) plotted against the concentration of extract
Discussion
Angiogenesis inhibitors combined with chemotherapeutic drugs have significant efficacy in the treatment of a variety of cancers.[10] Marine natural products, such as okadaic acid, brevetoxins, lyngbyatoxin A, caulerpenyne, bryostatins, and isocyano terpenes are reported to have significant biological activities.[11] Algae isolates obtained from fresh and marine resources could be one of the richest sources of novel bioactive secondary metabolites expected to have pharmaceutical significance for new drug development. The red alga Sphaerococcus coronopifolius has been reported to have antibacterial activity and the green alga Ulva lactuca was shown to possess an anti-inflammatory compound.[12] A cytotoxin metabolite, stypoldione, which was reported to inhibit microtubule polymerization and there by prevents mitotic spindle formation was isolated from tropical brown alga, Stypopodium zonale.[13,14] Angiogenesis is an important process in the body, both during normal and pathological conditions. The growth and spread of cancer cells especially requires the formation of blood vessels. VEGF seems to be a very promising target for the tumor vasculature normalization hypothesis. VEGF-A has been studied extensively and plays a critical role in both vasculogenesis and angiogenesis.[15,16,17]
VEGF signaling is modulated by angiopoietins that bind to Tie-2 receptors.[18] Soluble Tie-2 receptors curb and regress CN independent of VEGF in murine models of mechanical-alkali injury-induced CN.[19] The avascular cornea owes it transparency to soluble VEGFR-1 (sFlt-1) that acts as a decoy receptor for VEGF-A, a potent stimulator of CN.[20]
Various chemicals such as steroids, methotrexate, heparin, cyclosporine -A and thalidomide, have been studied as inhibitors of corneal neovascularization. However, when inflammation is not the cause of angiogenesis, such as disease associated with deficiency of limbal cells or CN secondary to corneal hypoxia, anti-inflammatory corticosteroids have little or no effect on capillary growth. The side effects of steroids, such as glaucoma and cataract formation, should not be underestimated. Hence, for such reasons bioactive compounds from natural products are used to be a new source for molecule acting through a different pathway. Therefore, in this study, organic extract of CP was used for the evaluation of its possible anti-angiogenic activity in the experimental models of neovascularization of C. pyrenoidosa extract to inhibit corneal neovascularization.
The extract of C. pyrenoidosa showed a promising chemo preventive activity against human liver neoplasia and carcinogenesis induced by heterocyclic amines such as 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline.[21] Dietary C. pyrenoidosa when given to patients with malignant glioma, effects on immunocompetence improves quality of life and survival.[22] Chlorella is also a good commercial source of many carotenoids, such as lutein, zeaxanthin, β-carotene, and astaxanthin.
Blood vessels in the CAM exhibited an organized branching pattern. High-order vessels branch into several lower order vessels, which then again branch into smaller vessels.[23] Implantation of the cover slip coated with vehicle control did not disturb this pattern. In contrast, blood vessels seemed to exhibit branching and changed direction abruptly with the VEGF treated group [Figure 1]. In the treatment group, VEGF with C. pyrenoidosa extract showed significant reduction of growth of blood vessels when compared with the control group, which indicated antiangiogenic effect of C. pyrenoidosa [Figure 1]. In this study, CP at the concentrations of 25, 50, and 100 μg showed a dose-dependent increase in antiangiogenic activity as compared to untreated control. Although, different methods of corneal neovascularizations such as burning, suturing, sodium hydroxide, injection of calf albumin into the cornea are reported in the literature, in order to induce controlled inflammatory neovascularization, silver nitrate cauterization was preferred in this study [Figure 2a]. In this model, C. pyrenoidosa extract significantly reduced the corneal neovascularization as compared to sham treated. However, as compared to the standard bevacizumab (4.95 ± 0.33) treatment, the observed effect in CP was inferior and this could be due to the presence of the active compound in very small quantities.
New scientific strategies for the evaluation of natural products with biological activity require the implementation of large-scale screening programs. It has been suggested that aqueous and ethanolic extracts from plants used in allopathic medicine are potential sources of biological active agents.[24] Furthermore, the selection of crude plant extracts for screening programs has the potential of being more successful in its initial steps than the screening of pure compound isolated from natural products.[25,26]
In this study, the antiproliferative activity of algal crude extracts was investigated. Dichloromethane/methanolic extract was prepared from dried lyophilized powder mass and the required concentration was made by using sterile Phosphate Buffer Saline (PBS) and tested for their antiproliferative activity on HeLa cells using the MTT assay. MTT assay was used to evaluate the reduction of viability of cell cultures in the presence and absence of the extracts. Taxol was used as a positive control. Cells were incubated with different concentrations of the algal extracts (ranged from 6.25 to 800 μg/ml) for 48 h.
With the available literature of various algal strains, it has been noticed that the many of the strains had not been screened yet for its fine chemicals that are potentially responsible for various biological activities. Therefore, in this study, the investigation of antiproliferative potential of algal crude extract of C. pyrenoidosa was performed and concluded that the extracts of C. pyrenoidosa exhibited antiproliferative activity of 570 μg/ml. The MTT assays data are presented in [Figure 4].
To be a good drug candidate, the IC50 value of such agent should be sufficiently low to avoid any possible unspecific effects. The American National Cancer Institute assigns a significant cytotoxicity effect of promising anticancer product for future bioguided studies if it exerts an IC50 value ≤ 30 μg/ml.[27] In this preliminary study, the focus of our interest was on crude extracts; the antiproliferative activity could be due to the presence in the dichloromethane/methanolic extract of active products that could probably have highly antigrowth effects. The results obtained in this preliminary study indicate that the extract of the algae C. pyrenoidosa was shown to induce significant and dose-dependent inhibitory activities against human cervical cancer cell lines HeLa.
This study provides an important basis for further investigation into the isolation, characterization, and mechanism of antiangiogenic and antiproliferative compounds from the screened algal extracts. Further research is required to carry more biological activities, including the in-vivo studies and the order of inhibition. Thus, C. pyrenoidosa extract could be as a source for new lead structures in drug design to combat cancer.
To conclude, this study showed the possible antiangiogenic and anti-proliferative activity of the organic extract of fresh water green alga C. pyrenoidosa in vovo and in vivo models of neovascularization and by MTT assay. However, further studies are required to evaluate their individual activity and mechanism of action.
Footnotes
Source of Support: Nil
Conflict of Interest: No
References
- 1.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–6. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 2.Daniel TO, Abrahamson D. Endothelial signal integration in vascular assembly. Annu Rev Physiol. 2000;62:649–71. doi: 10.1146/annurev.physiol.62.1.649. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267:10931–4. [PubMed] [Google Scholar]
- 4.Ciardiello F, Caputo R, Bianco R, Damiano V, Pomatico G, De Placido S, et al. Antitumor effect and potentiation of cytotoxic drugs activity in human cancer cells by ZD-1839 (Iressa), an epidermal growth factor receptor-selective tyrosine kinase inhibitor. Clin Cancer Res. 2000;6:2053–63. [PubMed] [Google Scholar]
- 5.Simmons TL, Andrianasolo E, McPhail K, Flatt P, Gerwick WH. Marine natural products as anticancer drugs. Mol Cancer Ther. 2005;4:333–42. [PubMed] [Google Scholar]
- 6.Ferrara N. In: Antiangiogenic Agents in Cancer Therapy. Teicher BA, editor. Totowa, NJ: Humana Press; 1999. pp. 119–41. [Google Scholar]
- 7.Uma R, Sivasubramanian V, Devaraj SN. Preliminary phycochemical analysis and in vitro antibacterial screening of green micro algae, Desmococcus Olivaceous, Chlorococcum humicola and Chlorella vulgaris. J Algal Biomass Utln. 2011;2:74–81. [Google Scholar]
- 8.Harbome JB. New York: Chapman and Hall; 1983. Phytochemical Method; A guide to modern techniques of plants analysis. [Google Scholar]
- 9.Ribatti D, Gualandris A, Bastaki M, Vacca A, Ilularo M, Roncali L, et al. New model for the study of angiogenesis and anti-angiogenesis in the chick embryo chorioallantoic membrane: The gelatin sponge/chorio allantoic membrane assay. J Vasc Res. 1997;34:455–63. doi: 10.1159/000159256. [DOI] [PubMed] [Google Scholar]
- 10.Jain RK, Willett CG, Boucher Y, Tomaso ED, Duda DG, Munn LL, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–7. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paul VJ, Arthur KE, Ritson-Williams R, Ross C, Sharp K. Chemical defenses: From compounds to communities. Biol Bull. 2007;213:226–51. doi: 10.2307/25066642. [DOI] [PubMed] [Google Scholar]
- 12.Faulkner DJ. Marine natural products. Nat Prod Rep. 2002;19:1–48. doi: 10.1039/b009029h. [DOI] [PubMed] [Google Scholar]
- 13.Gerwick WH, Fenical W. Ichthyotoxic and cytotoxic metabolites of the tropical brown alga Stypopodium zonale. J Org Chem. 1981;46:22–7. [Google Scholar]
- 14.Jacobs RS, Culver P, Langdon R, O’Brien T, White S. Some pharmacological observations on marine natural products. Tetrahedron. 1985;41:981–4. [Google Scholar]
- 15.Hiratsuka S, Kataoka Y, Nakao K, Nakamura K, Morikawa S, Tanaka S, et al. Vascular endothelial growth factor A (VEGF-A) is involved in guidance of VEGF receptor-positive cells to the anterior portion of early embryos. Mol Cell Biol. 2005;25:355–63. doi: 10.1128/MCB.25.1.355-363.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakurai Y, Ohgimoto K, Kataoka Y, Yoshida N, Shibuya M. Essential role of Flk1 (VEGF receptor 2) tyrosine residue 1173 in vasculogenesis in mice. Proc Natl Acad SciUSA. 2005;102:1076–81. doi: 10.1073/pnas.0404984102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi H, Shibuya M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin Sci. 2005;109:227–41. doi: 10.1042/CS20040370. [DOI] [PubMed] [Google Scholar]
- 18.Campochiaro PA. Ocular neovascularisation and excessive vascular permeability. Expert Opin Biol Ther. 2004;4:1395–402. doi: 10.1517/14712598.4.9.1395. [DOI] [PubMed] [Google Scholar]
- 19.Singh N, Macnamara E, Rashid S, Ambati J, Kontos CD, Higgins E, et al. Systemic soluble Tie2 expression inhibits and regresses corneal neovascularization. Biochem Biophys Res Commun. 2005b;332:194–9. doi: 10.1016/j.bbrc.2005.04.108. [DOI] [PubMed] [Google Scholar]
- 20.Ambati BK, Nozaki M, Singh N, Takeda A, Jani PD, Suthar T, et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993–7. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takekoshi H, Mizoguchi T, Komasa Y, Chubachi H, Inoue Y, Imanishi H, et al. Suppression of glutathione S-transferase placental form-positive foci development in rat hepatocarcinogenesis by Chlorella pyrenoidosa. Oncol Rep. 2005;14:409–14. [PubMed] [Google Scholar]
- 22.Merchant RE, Rice CD, Young HF. Dietary Chlorella pyrenoidosa for patients with malignant glioma: effects on immunocompetence, quality of life and survival. Phytother Res. 1990;4:220–31. [Google Scholar]
- 23.DeFouw DO, Rizzo VJ, Steinfeld R, Feinberg RN. Mapping of the microcirculation in the chick chorioallantoic membrane during normal angiogenesis. Microvasc Res. 1989;38:136–47. doi: 10.1016/0026-2862(89)90022-8. [DOI] [PubMed] [Google Scholar]
- 24.Chung TH, Kim JC, Kim MK. Investigation of Korean plant extracts for potential phytotherapeutic agents against B-virus Hepatitis. Phytother Res. 1995;9:429–34. [Google Scholar]
- 25.Cordell GA. Changing strategies in natural products chemistry. Phytochemistry. 1995;40:1585–612. [Google Scholar]
- 26.Kusumoto IT, Nakabayashi T, Kida H, Miyashiro H, Hattori M, Namba T, et al. Screening of various plant extracts used in ayurvedic medicine for inhibitory effects on human immunodeficiency virus type 1(HIV-1) protease. Phytother Res. 1995;9:180–4. [Google Scholar]
- 27.Suffness M, Pezzuto JM. Assays related to cancer drug discovery. In: Hostettmann K, editor. Methods in Plant Biochemistry: Assays for Bioactivity. Vol. 6. London: Academic Press; 1999. pp. 71–133. [Google Scholar]


