Abstract
Background:
The mechanism of action of sweet substance-induced analgesia is thought to involve activation of the endogenous opioid system. The nitric oxide (NO) pathway has a pivotal role in pain modulation of analgesic compounds such as opioids.
Objectives:
We investigated the role of NO and the opioid receptor-mediated system in the analgesic effect of sucrose ingestion in mice.
Materials and Methods:
We evaluated the effect of intraperitoneal administration of 10 mg/kg of NO synthase inhibitor, N-nitro-L-arginine methyl ester (L-NAME) and 20 mg/kg of opioid receptor antagonist, naltrexone on the tail flick response in sucrose ingesting mice.
Results:
Sucrose ingestion for 12 days induced a statistically significant increase in the latency of tail flick response which was unmodified by L-NAME, but partially inhibited by naltrexone administration.
Conclusions:
Sucrose-induced nociception may be explained by facilitating the release of endogenous opioid peptides. Contrary to some previously studied pain models, the NO/cyclic guanosine monophosphate (cGMP) pathway had no role in thermal hyperalgesia in our study. We recommend further studies on the involvement of NO in other animals and pain models.
KEY WORDS: Naltrexone, N-nitro-L-arginine methyl ester, opioid, tail flick test
Introduction
Sweet substance-induced analgesia has been widely acknowledged.[1] Out of all available sweetening substances, sucrose (table sugar) has great potential for routine use. Single doses reduce pain in newborns undergoing commonly performed medical procedures.[1] National/international pain management guidelines accordingly promote widespread use of sucrose.[2] Ingestion of sucrose for a relatively long time (14 days) has analgesic effects, potentiated by increased days of intake.[3] More recent studies have used a 12-day regimen to investigate the analgesic properties of various sweetening agents.[4]
The mechanism of action is thought to be mediated by the activation of endogenous opioid system through taste. There is also a role for nitric oxide (NO) pathway in pain modulation in animals and humans.[5] NO also mediates the peripheral and central antinociceptive effect of analgesic compounds such as opioids.[6] The NO-cyclic guanosine monophosphate (NO/cGMP) signaling pathway has also been shown to play a pivotal role in developing tolerance to opioid analgesia.[7] Nevertheless, the role for NO in nociception is very complex and diverse. Both inhibitory[8] and promotive[5] actions of NO have been reported in nociception and pain.
The present study aims to investigate the analgesic effect of sucrose ingestion in mice and the possible involvement of the opioid receptor-mediated system and NO pathway. We used the tail flick response to thermal pain, which is mainly a spinal reflex especially when the heating slope is faster and there is a decrease in reaction time.
Materials and Methods
Animals
Male Naval Medical Research Institute (NMRI) mice weighing 20-25 g (5-6-weeks-old, Pasteur Institute of Iran) were used. Animals were housed 10 per cage in a room maintained at 22 ± 1°C with an alternating 12 h light-dark cycle starting at 7.00 am. Animals had free access to food and drinking solutions and were group housed during testing procedures. All procedures were approved and carried out in accordance with institutional guidelines for laboratory animal care and use. Each mouse was used only once.
Treatment Groups
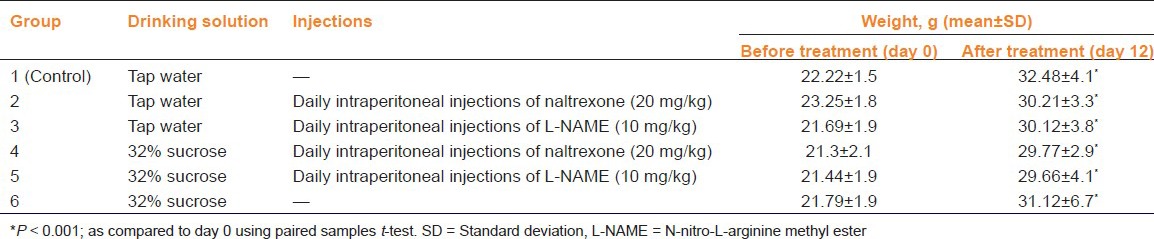
The animals were randomly distributed into six groups of 10 [Table 1]. The daily injections were administered between 8.00 and 10.00 am. Animals were given 12 days to adapt to the dietary conditions. Drinking solutions were measured daily to ensure that enough liquid was supplied for each animal. The animals were weighed before and after the treatment period. The weight was recorded in grams approximately 60 min prior to antinociception recording.
Table 1.
Treatment group regimens and mean weight of mice at baseline and after 12 days of treatment in animals treated with sucrose (n = 60)
Chemicals
Sucrose (Merck, Germany) was dissolved in water to a 32% concentration and administered orally as the drinking solution in selected groups. L-NAME and naltrexone (Sigma, UK) were dissolved in sterile physiological saline and administered intraperitoneally (i.p.) at a volume of 10 and 20 mg/kg of body weight, respectively. The doses were based upon the previous studies and shown to be pharmacologically active.[4]
Assessment of Antinociception
Tail flick measurement was done using the Ugo Basile Tail Flick Unit (Ugo Basile, Comerio, Italy) based on modification of a method described originally by D’Amour and Smith (1941). The tail flick unit consisted of an infrared heat source (50 W bulb) of adjustable intensity that was set at 40 units. The infrared source focused on the lower one-third of the tail. Tail flick latency (TFL) time (in seconds) required by the mouse to reach the thermal threshold for pain and flick its tail was recorded. A cutoff of 8 s was set to avoid tissue damage. The animals were allowed to acclimatize to the experimental environment for 30 min before the tests and to the apparatus at least 2 min before eliciting a tail flick. The operator was unaware of the specific treatment group to which an animal belonged.
For each group, three baseline TFLs were taken with 5 min intervals on the day before the treatment. Antinociception testing was repeated on the last day of treatment (day 12) and 10 recordings were obtained from each mouse at minutes 0, 5, 15, 25, 35, 45, 55, 65, 75, and 90. All nociceptive tests were performed at similar times in the afternoon. Animals were weighed daily to calculate the proper amount of drug administration. The study protocol was approved by the local ethics committee of Tehran University of Medical Sciences.
Statistical Analysis
All data are shown as mean ± standard error of the mean (SEM) of the value for corresponding parameters. Statistical comparisons between groups in each experiment were done with one-way analysis of variance (ANOVA) followed by post hoc Student-Newman-Keuls test. In cases in which only two groups were to be compared, a t-test was used. A P-value less than 0.05 was considered statistically significant. Statistical analysis was performed using commercially available software (Statistical Package for Social Sciences (SPSS) 16.0, SPSS, Chicago, IL, USA).
Results
Measuring daily intake of fluid in groups receiving 32% sucrose solution showed an average sucrose consumption of 18.53 g per day for each animal. Weighing the animals before and after the treatment period showed no statistically significant difference (P > 0.05) between groups either before or after receiving the treatment [Table 1]. However, all groups gained weight significantly during this period as assessed by paired samples t-test (P < 0.001). Hence, it can be presumed that the treatment regimen had no effect on the animals in terms of weight gaining and nutrition. Weight gain had no impact on pain threshold during the experiment period since there was no significant difference between TFLs before and after the treatment (day 0 versus day 12) in the control group.
Effect of Sucrose Ingestion on Tail Flick Test
The TFLs for groups receiving tap water and sucrose after 12 days of treatment were significantly different with the group ingesting sucrose solution showing higher pain thresholds at all testing times (***P < 0.001). The mean TFL of the sucrose treated group was also significantly higher than that of the tap water control group (1.33 versus 0.98 s, P < 0.001) [Figure 1].
Figure 1.
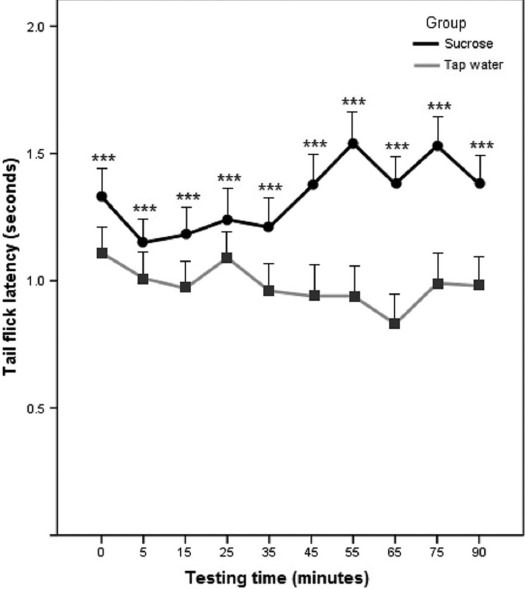
Tail flick latency (TFL) of mice receiving water or sucrose solution (32%) for 12 days. Antinociception was recorded on the 12th day of treatment and expressed in seconds. Ten recordings were obtained for each animal at 0, 5, 15, 25, 35, 45, 55, 65, 75, and 90 min. Each point is the mean ± standard error (SE) of 10 animals. The difference between water and sucrose-treated groups is significant at ***P< 0.001
Effect of the Long-acting Opioid Receptor Antagonist Naltrexone on Sucrose-induced Antinociception
Cotreatment with a long-acting opioid receptor antagonist (naltrexone, 20 mg/kg, i.p.) reversed the effect of sucrose ingestion (32% solution) on the latency of tail flick response (¥P < 0.05) [Figure 2]. TFLs of the group receiving sucrose along with naltrexone were 13% lower compared to those receiving sucrose alone. It was also observed that naltrexone (20 mg/kg) administration was not effective in the tap water treated control group (P > 0.05) [Figure 2].
Figure 2.
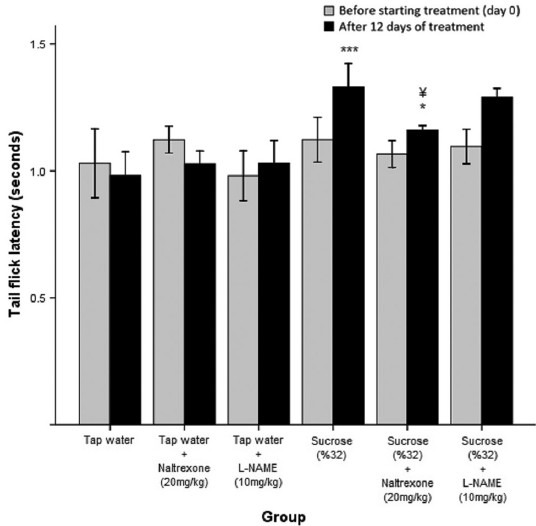
Tail flick latency (TFL) of mice receiving water or sucrose solution (32%) for 12 days along with daily intraperitoneal (i.p.) injections of L-NAME (10 mg/kg) or naltrexone (20 mg/kg). Baseline and final antinociception recordings are shown. The sucrose treated-group shows higher TFLs compared to the groups receiving tap water alone or along with L-NAME or naltrexone (***P< 0.001). The group receiving sucrose and naltrexone shows higher TFLs than the group receiving water (*P< 0.05) and significantly lower values than the group receiving sucrose (¥P< 0.05)
Effect of the Nonselective NOS Inhibitor on Antinociceptive Effect of Sucrose
Daily administration of L-NAME (10 mg/kg, i.p.) with sucrose (32% solution) had no significant effect on the sucrose-induced antinociception (P > 0.05). L-NAME (10 mg/kg) administration was not effective in the tap water treated control group (P > 0.05) [Figure 2].
Discussion
Previous studies have shown that oral administration of sucrose or glucose solutions provides safe and potentially effective analgesia for pain associated with routine invasive procedures in the newborn and possibly adults.[9] In the present study, 12 day ingestion of 32% sucrose solution demonstrated an analgesic effect in mice. This analgesic effect was partially inhibited by long-acting opioid receptor antagonist naltrexone (20 mg/kg), but was unmodified by the NO synthase (NOS) inhibitor L-NAME (10 mg/kg).
Although the analgesic activity of sucrose is well-recognized, its mechanism remains to be elucidated. The possible role of opioid receptors has been investigated in various studies on different animal models. Blass, et al., reported that intraoral administration of sucrose nearly doubled the pain threshold in young rats in a heat withdrawal test, and this effect was reversed by the opioid receptor antagonist, naloxone.[10] Naloxone also reduced the tail flick analgesic index which had been increased in adult rats feeding on a sucrose solution.[3] Additionally, using a formalin test in mice, it has been found that naloxone is able to block the synergistic effects of sweeteners on morphine-induced antinociception, suggesting the involvement of endogenous opioid systems.[11] On the other hand, in a clinical study aimed at clarifying the mechanism underlying the pain reducing effect of orally administered glucose, Gradin and Schollin showed that administration of an opioid antagonist did not decrease the analgesic effect of orally administered glucose given before blood sampling in newborns, hence questioning the mechanisms underlying this effect.[12]
In the present study, 20 mg/kg of naltrexone significantly modified the analgesic effect of sucrose. This finding supports the hypothesis that sucrose may facilitate release of endogenous opioid peptides from neurons that may ultimately be responsible for the observed antinociceptive effect. Our results are also in line with those of Reboucas, et al.[13] As was the case in their protocol, we used the tail flick test and administered the drugs intraperitoneally. However, we used mice instead of rats in our study. In accordance with previous findings, we observed no analgesic or hyperalgesic effects for the administration of an opioid antagonist alone.[13,14]
NO is an important mediator of nociception in acute[15] and chronic[5] pain states at both central[16] and peripheral[17] levels. Experimental and clinical data have demonstrated that NO is also capable of inducing analgesia.[8] The L-arginine-NO pathway has been shown to play a critical role in the glutamate and N-methyl-D-aspartate (NMDA)-mediated nociceptive responses. Studies have also shown that i.p., intracerebroventricular (i.c.v.), intrathecal (i.t.), and local administration of the NOS inhibitor, N-omega-nitro-L-arginine (L-NOARG), inhibit glutamate-induced nociception. Investigating the role of aspartame on formalin test results in mice supported the hypothesis that activation of NMDA receptors could modulate pain-related behavior via a NO/cGMP-glutamate release cascade.[4] L-NOARG decreased the allodynia caused by sciatic nerve ligation in a dose dependent manner and it was shown that the thermal hyperalgesia after sciatic nerve ligation is mediated by NO.[18] Ferreira, et al. also reported that i.p. administered NOS inhibitors L-NOARG and L-NAME had no effect by themselves and thus argued that the NO/cGMP pathway has no role in the induction of thermal hyperalgesia.[19]. In our study, L-NAME did not affect TFLs in mice whether administered alone or along with sucrose. This is in accordance with the results of Ferreira, et al. Therefore, it may be concluded that in contrast to the nociception induced by glutamate and allodynia caused by sciatic nerve ligation, the NO/cGMP pathway has no role in the induction of thermal hyperalgesia demonstrated by the tail flick test. The discrepancies may be justified by the use of different experimental pain models representing different mechanisms of nociception.
In addition, it has been observed that depending on the site of activation, the L-arginine/NO/cGMP pathway could induce opposite effects.[20] Our study suggests that the analgesic effect of intraoral sucrose which was apparently exerted through the opioid receptor-mediated system was not modified by an NOS inhibitor. These findings suggest existence of different subsets of nociceptive primary sensory neurons in which NO plays different roles.
The mechanisms involved in the role of NO in modulating analgesic activities are not well understood and the discrepancies found in the existing studies might be related to differences in animal species, nociceptive stimuli, site of NO donor administration, and/or doses of the inhibitors of NO or NOS substrate used in the studies.[21] We used the intraoral method for delivering sucrose due to the taste mechanism that has been proposed in analgesia induced by sweet substances. We did not assess other methods of administrating sucrose such as oral gavage and injection. We also limited the dosage of opioid antagonist and NOS inhibitor to the standard effective doses used widely in the literature. The effect of varying doses of inhibitors was not addressed and needs further investigation. Measurement of NO production in tested groups may also be helpful in this context.
Conclusions
This study shows that sucrose ingestion for a relatively long period of time is associated with analgesic effect in mice which is partially mediated by the opioidergic system. We observed a reduction in the effect of sucrose on tail flick efficacy by 20 mg/kg naltrexone. In spite of prior evidence of NO mediating the antinociceptive effect of opioids,[6] our results indicate that the NO/cGMP pathway has no role in the thermal nociception induced by the tail flick test in mice. We recommend further studies to evaluate the involvement of NO in nociception in other pain models. The precise mechanism by which sweetening agents interact with the opioid receptor-mediated system also needs further assessment.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil
References
- 1.Stevens B, Yamada J, Ohlsson A. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database Syst Rev. 2010;1:CD001069. doi: 10.1002/14651858.CD001069.pub3. [DOI] [PubMed] [Google Scholar]
- 2.Lefrak L, Burch K, Caravantes R, Knoerlein K, DeNolf N, Duncan J, et al. Sucrose analgesia: Identifying potentially better practices. Pediatrics. 2006;118(Suppl 2):S197–202. doi: 10.1542/peds.2006-0913R. [DOI] [PubMed] [Google Scholar]
- 3.Segato FN, Castro-Souza C, Segato EN, Morato S, Coimbra NC. Sucrose ingestion causes opioid analgesia. Braz J Med Biol Res. 1997;30:981–4. doi: 10.1590/s0100-879x1997000800011. [DOI] [PubMed] [Google Scholar]
- 4.Abdollahi M, Nikfar S, Abdoli N. Potentiation by nitric oxide synthase inhibitor and calcium channel blocker of aspartame-induced antinociception in the mouse formalin test. Fundam Clin Pharmacol. 2001;15:117–23. doi: 10.1046/j.1472-8206.2001.00013.x. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Boettger MK, Reif A, Schmitt A, Uceyler N, Sommer C. Nitric oxide synthase modulates CFA-induced thermal hyperalgesia through cytokine regulation in mice. Mol Pain. 2010;6:13. doi: 10.1186/1744-8069-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hervera A, Leanez S, Negrete R, Pol O. The peripheral administration of a nitric oxide donor potentiates the local antinociceptive effects of a DOR agonist during chronic inflammatory pain in mice. Naunyn Schmiedebergs Arch Pharmacol. 2009;380:345–52. doi: 10.1007/s00210-009-0436-6. [DOI] [PubMed] [Google Scholar]
- 7.Ozdemir E, Bagcivan I, Durmus N, Altun A, Gursoy S. The nitric oxide-cGMP signaling pathway plays a significant role in tolerance to the analgesic effect of morphine. Can J Physiol Pharmacol. 2011;89:89–95. doi: 10.1139/y10-109. [DOI] [PubMed] [Google Scholar]
- 8.Chung E, Burke B, Bieber AJ, Doss JC, Ohgami Y, Quock RM. Dynorphin-mediated antinociceptive effects of L-arginine and SIN-1 (an NO donor) in mice. Brain Res Bull. 2006;70:245–50. doi: 10.1016/j.brainresbull.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Kakeda T. Potential of sucrose-induced analgesia to relieve pain in male adults: A preliminary study. Jpn J Nurs Sci. 2010;7:169–73. doi: 10.1111/j.1742-7924.2010.00150.x. [DOI] [PubMed] [Google Scholar]
- 10.Blass E, Fitzgerald E, Kehoe P. Interactions between sucrose, pain and isolation distress. Pharmacol Biochem Behav. 1987;26(3):483–9. doi: 10.1016/0091-3057(87)90153-5. [DOI] [PubMed] [Google Scholar]
- 11.Abdollahi M, Nikfar S, Habibi L. Saccharin effects on morphine-induced antinociception in the mouse formalin test. Pharmacol Res. 2000;42:255–9. doi: 10.1006/phrs.2000.0682. [DOI] [PubMed] [Google Scholar]
- 12.Gradin M, Schollin J. The role of endogenous opioids in mediating pain reduction by orally administered glucose among newborns. Pediatrics. 2005;115:1004–7. doi: 10.1542/peds.2004-1189. [DOI] [PubMed] [Google Scholar]
- 13.Reboucas EC, Segato EN, Kishi R, Freitas RL, Savoldi M, Morato S, et al. Effect of the blockade of mu1-opioid and 5HT2A-serotonergic/alpha1-noradrenergic receptors on sweet-substance-induced analgesia. Psychopharmacology (Berl) 2005;179:349–55. doi: 10.1007/s00213-004-2045-x. [DOI] [PubMed] [Google Scholar]
- 14.Rezende RM, Franca DS, Menezes GB, dos Reis WG, Bakhle YS, Francischi JN. Different mechanisms underlie the analgesic actions of paracetamol and dipyrone in a rat model of inflammatory pain. Br J Pharmacol. 2008;153:760–8. doi: 10.1038/sj.bjp.0707630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toriyabe M, Omote K, Kawamata T, Namiki A. Contribution of interaction between nitric oxide and cyclooxygenases to the production of prostaglandins in carrageenan-induced inflammation. Anesthesiology. 2004;101:983–90. doi: 10.1097/00000542-200410000-00025. [DOI] [PubMed] [Google Scholar]
- 16.Freire MA, Guimaraes JS, Leal WG, Pereira A. Pain modulation by nitric oxide in the spinal cord. Front Neurosci. 2009;3:175–81. doi: 10.3389/neuro.01.024.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyamoto T, Dubin AE, Petrus MJ, Patapoutian A. TRPV1 and TRPA1 mediate peripheral nitric oxide-induced nociception in mice. PLoS One. 2009;4:e7596. doi: 10.1371/journal.pone.0007596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sousa AM, Prado WA. The dual effect of a nitric oxide donor in nociception. Brain Res. 2001;897:9–19. doi: 10.1016/s0006-8993(01)01995-3. [DOI] [PubMed] [Google Scholar]
- 19.Ferreira J, Santos AR, Calixto JB. The role of systemic, spinal and supraspinal L-arginine-nitric oxide-cGMP pathway in thermal hyperalgesia caused by intrathecal injection of glutamate in mice. Neuropharmacology. 1999;38:835–42. doi: 10.1016/s0028-3908(99)00006-4. [DOI] [PubMed] [Google Scholar]
- 20.Vivancos GG, Parada CA, Ferreira SH. Opposite nociceptive effects of the arginine/NO/cGMP pathway stimulation in dermal and subcutaneous tissues. Br J Pharmacol. 2003;138:1351–7. doi: 10.1038/sj.bjp.0705181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cury Y, Picolo G, Gutierrez VP, Ferreira SH. Pain and analgesia: The dual effect of nitric oxide in the nociceptive system. Nitric oxide. 2011;25:243–54. doi: 10.1016/j.niox.2011.06.004. [DOI] [PubMed] [Google Scholar]


