Abstract
Aims and Objectives:
The aim of this study is to compare the efficacy, safety and cost-effectiveness of topical Whitfield's ointment plus oral fluconazole with topical 1% butenafine in tinea infections of the skin.
Materials and Methods:
Patients were randomly allocated to the two treatment groups and advised to apply either agent topically twice-a-day for 4 weeks on the lesions and fluconazole (150 mg) was administered once a week for 4 weeks in the study group applying Whitfield's ointment. Patients were followed-up at an interval of 10 days for clinical score and global evaluation response was assessed at baseline and during each follow-up.
Results:
Out of 120 patients enrolled in the study 103 completed the study. Patients treated with Whitfield's ointment and oral fluconazole reduced mean sign and symptom score from 8.81 ± 0.82 to 0.18 ± 0.59 while butenafine treated patients reduced it from 8.88 ± 0.53 to 0.31 ± 0.67 at the end of the treatment. Nearly, 98% patients were completely cleared of the lesion on the 3rd follow-up with both treatments.
Conclusion:
Whitfield's ointment with oral fluconazole is as efficacious, safe and cost-effective as compared with 1% butenafine in tinea infections of the skin.
KEY WORDS: Butenafine, fluconazole, tinea infections, Whitfield's ointment
Introduction
Dermatophytoses are fungal infections of keratinized tissue (hair, skin, and nails).[1] Whitfield's ointment is one of the oldest and cheapest antifungal agent containing 3% of salicylic acid and 6% of benzoic acid.[2,3] Fluconazole is a fungistatic that impairs fungal cell wall synthesis by inhibiting the enzyme 14-α lanosterol demethylase.[4] Their combination enhances the efficacy and minimizes the chances of relapse. Butenafine is a benzylamine group of fungicidal drug, which impairs fungal cell wall synthesis by inhibiting the enzyme squalene epoxidase.[4] The present study was under taken to compare the efficacy, safety and cost-effectiveness of topical Whitfield's ointment plus oral fluconazole with topical 1% butenafine in tinea infections of the skin. The primary objective was to assess the cure rate, relapse, safety and cost-effectiveness of both treatments while secondary objective was to assess the Clinicomycological correlation and to study the distribution of different species of tinea.
Materials and Methods
The study was registered with Clinical Trial Registry of India (CTRI/2012/08/002914 and was approved by ethics committee of the teaching hospital. It was a prospective, randomized, open-label, controlled, comparative clinical study, conducted in patients attending the skin out-patient Department of C U Shah Medical College and Hospital, Surendranagar from May 2009 to November 2009. Diagnosis of tinea infections was performed by the Dermatologist. Patients newly diagnosed with tinea infections of skin were included in the study while follow-up cases, pregnant or lactating women, patients having an allergy to imidazoles or allylamines were excluded. All patients were explained about the study and written informed consent was obtained. Patients were randomly allocated in two groups. Group A received topical Whitfield's ointment to be applied twice-a-day for 4 weeks plus oral fluconazole (150 mg once a week for 4 weeks) while Group B received topical 1% butenafine cream twice-a-day for 4 weeks. All patients were administered oral chlorpheniramine maleate (4 mg) twice-a-day for a month to relieve pruritus. Whitfield’ ointment, fluconazole (150 mg) and 1% butenafine were purchased from the pharmacy store of the hospital.
Skin scraping was collected on the slides having 1-2 drops of 20% KOH and observed under ×10 and ×45 of the microscope at each follow-up [Figure 1].[1,5] The scraping were cultured on the plates of Sabouraud's agar supplemented with 1% chloramphenicol in the incubator at 32-35°C for 7-10 days. The species were identified by lactophenol cotton blue preparation. Photographs of both gross cultures and microscopic appearance of lactophenol cotton blue preparation were taken. Patients were followed-up at the interval of 10 days for 4 weeks to assess the relapse. Outcome of the treatment was assessed by the clinical and mycological care.[6,7]
Figure 1.
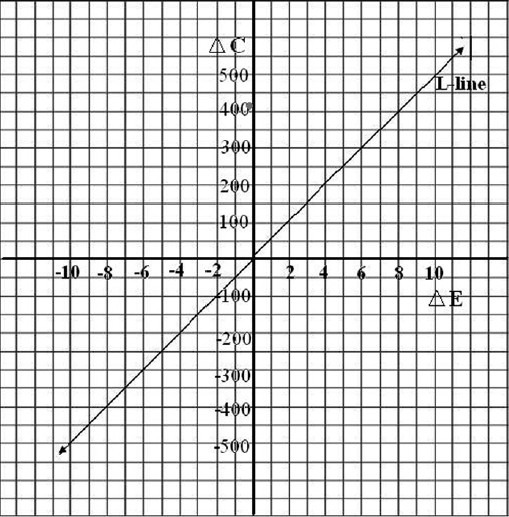
Butenafine is less cost-effective as compared to the combination treatment of Whitfield's ointment and oral fluconazole. ΔC-Incremental cost, ΔE-Incremental effectiveness, L-line – Line passing from 0–, Red Dot-Showing cost-effectiveness
Clinical cure was assessed by scoring of three parameters that is erythema; pruritus and scaling, Each parameter was categorized into - mild - 1, moderate - 2, severe - 3. Global Evaluation Response was assessed at each follow-up.[7]
Mycological cure was assessed by examining skin scraping microscopically and culture. Both KOH and culture negative specimens were considered mycologically cured.
Statistical Analysis
Sample size calculation
Sample size was calculated by nMaster 1.0. As no previous studies have been conducted between combination of Whitfield's ointment and oral fluconazole, a pilot study on 10 patients was carried out to assess the cure rate. As per the result of the pilot study, cure rate with Whitfield's ointment + oral fluconazole was taken as 90% and as per study by Tschen et al.[8] cure rate with butenafine was taken as 88%. Assuming population difference of proportions as 0.085, setting alpha error at 5% and power of study at 80% and using two sided test, 104 patients were needed. Assuming a dropout rate of approximately 15 20%, a total 120 patients were enrolled for the study.
Graph Pad Instat 3.0 was used for the statistical analysis. Normality of the data was checked by Kolmogorov Smirnov test. Mann Whitney test was used to compare the groups with respect to age. Fisher's exact test was used to find the difference in both groups in terms of gender distribution. Baseline comparison of sign and symptom score between the groups was performed by Mann Whitney test. Total score of erythema, pruritus and scaling before and after treatment was compared by Wilcoxon matched pair test. Chi-square test was used to compare global evaluation score between both groups at each follow-up. P < 0.05 was considered to be statistically significant. Cost-effectiveness was calculated on the basis of total expenditure incurred on medicines, cost of conveyance at the end of treatment in 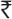 Indian rupee and cure rate in percentage. Incremental cost (ΔC) and incremental effectiveness (ΔE) were calculated.[9]
Indian rupee and cure rate in percentage. Incremental cost (ΔC) and incremental effectiveness (ΔE) were calculated.[9]
Results
Out of 120 patients enrolled in the study, 8 patients from group A and 9 patients from group B were lost to follow-up and 103 patients completed the study.
Demographic Characteristics
Median age in both groups was 35 years. Both groups were also similar in terms of gender distribution (P = 0.1209). Tinea corporis was the most common diagnosis (37.84%) followed by tinea corporis and cruris mixed infection (30%). Most of the patients (96.12%) were suffering from severe tinea infections at the first visit. Trichophyton mentagrophytes (60.94%) was the most common species followed by Trichophyton rubrum (28.12%).
Efficacy
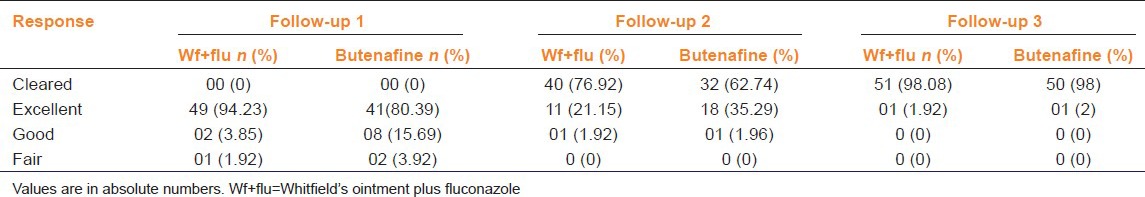
Combination of Whitfield's ointment and oral fluconazole treatment and butenafine significantly reduced mean sign and symptom score (P < 0.0001). Global Evaluation Response revealed that skin lesions were completely cleared with Whitfield's ointment + oral fluconazole and butenafine creamin majority of the patients (98%) [Table 1]. At the end of treatment, 97% of the patients in both groups were mycologically cured and no relapse was observed in both treatment groups at the end of 4 weeks.
Table 1.
Global evaluation response of patient treated with Wf+flu and topical butenafine at different time interval
Safety
Both drug treatments were well-tolerated. However, two patients complained of burning and one patient complained of redness with Whitfield's ointment. Gastritis was reported in one patient with fluconazole. No adverse event was reported with 1% butenafine.
Cost-Effectiveness
Cost per patient for complete treatment for Whitfield's ointment plus fluconazole was 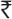 293.49, while that for butenafine was
293.49, while that for butenafine was 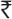 707.60. ΔC was
707.60. ΔC was 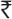 414.11 and ΔE was 0.08 (ΔE). The line passing from 0 is called L-line. The zone below the line is acceptable zone. In the present study, the difference lies above L-line shown by the red dot. This represents that butenafine was less cost-effective as compared to Whitfield's ointment + oral fluconazole.
414.11 and ΔE was 0.08 (ΔE). The line passing from 0 is called L-line. The zone below the line is acceptable zone. In the present study, the difference lies above L-line shown by the red dot. This represents that butenafine was less cost-effective as compared to Whitfield's ointment + oral fluconazole.
Discussion
This study combined Whitfield's ointment and oral fluconazole to minimize the chances of relapse as both are fungistatic and most of the patients in the study were suffering from severe tinea infections justifying their combination while butenafine being fungicidal reduces the chance of relapse when used singly.[10] The present study showed that 80.77% of the patients were completely cleared of the lesion clinically and mycologically at the end of second follow-up (20 days) and 98.08% were completely cleared at third follow-up (30 days) with topical Whitfield's ointment plus oral fluconazole. In a study by Crevits et al., 82% (37/45) of patients by oral fluconazole had shown complete clearance of the lesion, 90% and 88% respectively for tinea corporis and cruris respectively.[11] Whitfield's ointment produced 67% clinical cure at the end of 3 weeks treatment.[12]
This study demonstrated that 62.74% of the patients were completely cleared with the lesion clinically and mycollogically at the end of second follow-up (20 days) and 98% were completely cleared at third follow-up (30 days) with topical 1% butenafine cream. Present study found 97% of the patients mycologically cured at the end of treatment in both groups. In the study by Tschen et al. in interdigital tinea pedis, 88% mycological cure was achieved at the end of for weeks with the treatment of butenafine.[8] The present study found no statistical significant difference between the two groups in clearing of the lesion and in the development of excellent clinical response (P > 0.05). In the butenafine group, the score declined from a mean of 7.36 at baseline to 1.5 ± f 2.3 at 8 weeks.[13] Burning, pruritus, striae and erythema with atrophy has been reported with topical buetinafine[13] while no adverse drug reactions have been observed with fluconazole.[14] Our results were synonymous with other existing studies, Moreover, no relapse was observed in either groups during the 4 week surveillance period. Residual protection for 4 weeks with butenafine has been reported.[8] Clayton and Connor et al. have reported that transient burning and irritation with Whitfield ointment with relapse at the end of 4 weeks.[15] Treatment with Whitfield's ointment plus oral fluconazole was 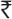 414.11 cheaper than that with 1% butenafine. Thus, it can be inferred that Whitfield's ointment plus oral fluconazole is as efficacious as topical 1% butenafine in tinea infections of the skin. Both treatments are safe and provide residual protection for 4 weeks after the completion of therapy. However, Whitfield's ointment plus oral fluconazole is more cost-effective as compared with topical 1% butenafine in tinea infections of the skin.
414.11 cheaper than that with 1% butenafine. Thus, it can be inferred that Whitfield's ointment plus oral fluconazole is as efficacious as topical 1% butenafine in tinea infections of the skin. Both treatments are safe and provide residual protection for 4 weeks after the completion of therapy. However, Whitfield's ointment plus oral fluconazole is more cost-effective as compared with topical 1% butenafine in tinea infections of the skin.
Acknowledgment
We are thankful to the Dean of the institute for allowing us to carry out the study in the hospital and are also grateful to all participated in the study.
Footnotes
Source of Support: Nil
Conflict of Interest: No
References
- 1.Sobera JO, Elewski BE. Fungal diseases. In: Bolognia JL, Jorizzo JL, Rapini RP, editors. Dermatology. 2nd ed. USA: Mosby Elsevier; 2008. pp. 1135–63. [Google Scholar]
- 2.Brunton LS, Lazo JS, Parker KL. Antifungal drugs. In: Brunton LS, Lazo JS, Parker KL, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. USA: McGraw-Hill Publication; 2008. pp. 1225–42. [Google Scholar]
- 3.Gooskens V, Pönnighaus JM, Clayton Y, Mkandawire P, Sterne JA. Treatment of superficial mycoses in the tropics: Whitfield's ointment versus clotrimazole. Int J Dermatol. 1994;33:738–42. doi: 10.1111/j.1365-4362.1994.tb01524.x. [DOI] [PubMed] [Google Scholar]
- 4.Tripahi KD. Antifungal drugs. In: Tripathi KD, editor. Essentials of Medical Pharmacology. 6th ed. New Delhi: Jaypee Brothers; 2008. pp. 757–66. [Google Scholar]
- 5.Verma S, Heffernan MP. Superficial fungal infection: Dermatophytosis, onychomycosis, tinea nigra, piedra. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Dermatology in General Medicine. 6th ed. Vol. 2. USA: McGraw Hill Inc; 2003. pp. 1989–2001. [Google Scholar]
- 6.McNeely W, Spencer CM. Butenafine. Drugs. 1998;55:405–12. doi: 10.2165/00003495-199855030-00006. [DOI] [PubMed] [Google Scholar]
- 7.Jerajani HR, Amladi ST, Bongale R, Adepu V, Tendolkar UM, Sentamilselvi G, et al. Evaluation of clinical efficacy and safety of once daily topical administration of 1% oxiconazole cream and lotion in dermatophytosis: An open label, non-comparative multicentre study. Indian J Dermatol Venereol Leprol. 2000;66:188–92. [PubMed] [Google Scholar]
- 8.Tschen E, Elewski B, Gorsulowsky DC, Pariser DM. Treatment of interdigital tinea pedis with a 4-week once-daily regimen of butenafine hydrochloride 1% cream. J Am Acad Dermatol. 1997;36:S9–14. doi: 10.1016/s0190-9622(97)70316-5. [DOI] [PubMed] [Google Scholar]
- 9.Heitjan DF, Moskowitz AJ, Whang W. Bayesian estimation of cost-effectiveness ratios from clinical trials. Health Econ. 1999;8:191–201. doi: 10.1002/(sici)1099-1050(199905)8:3<191::aid-hec409>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 10.Lesher JL, Jr, Babel DE, Stewart DM, Jones TM, Kaminester L, Goldman M, et al. Butenafine 1% cream in the treatment of tinea cruris: A multicenter, vehicle-controlled, double-blind trial. J Am Acad Dermatol. 1997;36:S20–4. doi: 10.1016/s0190-9622(97)70318-9. [DOI] [PubMed] [Google Scholar]
- 11.Crevits B, Picoto A, Staberg B, Urbanowski S, Silny W. Comparison of efficacy and safety of oral fluconazole and topical clotrimazole in the treatment of tinea corporis, tinea cruris, tinea pedis and cutaneous candidiasis. Curr Ther Res Clin Exp. 1998;59:503–10. [Google Scholar]
- 12.Wright S, Robertson VJ. An institutional survey of tinea capitis in Harare, Zimbabwe and a trial of miconazole cream versus Whitfield's ointment in its treatment. Clin Exp Dermatol. 1986;11:371–7. doi: 10.1111/j.1365-2230.1986.tb00477.x. [DOI] [PubMed] [Google Scholar]
- 13.Ramam M, Prasad HR, Manchanda Y, Khaitan BK, Banerjee U, Mukhopadhyaya A, et al. Randomised controlled trial of topical butenafine in tinea cruris and tinea corporis. Indian J Dermatol Venereol Leprol. 2003;69:154–8. [PubMed] [Google Scholar]
- 14.Assaf RR, Elewski BE. Intermittent fluconazole dosing in patients with onychomycosis: Results of a pilot study. J Am Acad Dermatol. 1996;35:216–9. doi: 10.1016/s0190-9622(96)90327-8. [DOI] [PubMed] [Google Scholar]
- 15.Clayton YM, Connor BL. Comparison of clotrimazole cream, Whitfield's ointment and Nystatin ointment for the topical treatment of ringworm infections, pityriasis versicolor, erythrasma and candidiasis. Br J Dermatol. 1973;89:297–303. doi: 10.1111/j.1365-2133.1973.tb02978.x. [DOI] [PubMed] [Google Scholar]


