Abstract
Targeted cancer therapy concepts often aim at the induction of adjuvant antitumor immunity or stimulation of tumor cell apoptosis. There is further evidence that combined application of immune stimulating and tumor apoptosis-inducing compounds elicits a synergistic antitumor effect. Here, we describe the development and characterization of bifunctional fusion proteins consisting of a single-chain variable fragment (scFv) domain derived from the CD40-specific monoclonal antibody G28-5 that is fused to the N-terminus of stabilized trimeric soluble variants of the death ligand TNF-related apoptosis-inducing ligand (TRAIL). As shown before by us and others for other cell surface antigen-targeted scFv-TRAIL fusion proteins, scFv:G28-TRAIL displayed an enhanced capacity to induce apoptosis upon CD40 binding. Studies with scFv:G28 fusion proteins of TRAIL mutants that discriminate between the two TRAIL death receptors, TRAILR1 and TRAILR2, further revealed that the CD40 binding-dependent mode of apoptosis induction of scFv:G28-TRAIL is operable with each of the two TRAIL death receptors. Binding of scFv:G28-TRAIL fusion proteins to CD40 not only result in enhanced TRAIL death receptor signaling but also in activation of the targeted CD40 molecule. In accordance with the latter, the scFv:G28-TRAIL fusion proteins triggered strong CD40-mediated maturation of dendritic cells. The CD40-targeted TRAIL fusion proteins described in this study therefore represent a novel type of bifunctional fusion proteins that couple stimulation of antigen presenting cells and apoptosis induction.
Keywords: apoptosis, CD40, dendritic cells, TRAIL
Ligands and receptors of the tumor necrosis factor (TNF) family fulfill central roles in immune regulation and maintenance of tissue homeostasis mainly by control of inflammatory and cell death-inducing pathways. TNF receptors are characterized by a conserved cysteine-rich domain (CRD) that is present in one to six copies in their extracellular domain1 With the exception of a few soluble or GPI-anchored decoy receptors, all TNF receptors are single spanning transmembrane receptors that have the capability to trigger proinflammatory signaling pathways. Structurally, a subgroup of TNF receptors can be defined as the so-called death domain-containing receptors. The intracellular domain of these receptors contains a conserved protein–protein interaction domain, the death domain, and some of the death receptors are able to directly trigger signaling pathways that result in apoptotic and/or necrotic cell death, for example, CD95 and TRAILR1 and TRAILR2, two of the five receptors of the TNF-related apoptosis-inducing ligand (TRAIL).1
Defects in ligands and receptors of the TNF family or their deregulated expression are of pivotal relevance in a variety of diseases and pathologies. Indeed, antibodies and recombinant proteins targeting RANKL or TNF and its receptors are already in use in clinical practice and many more reagents targeting ligands and receptors of the TNF family are currently tested in clinical trials.2 With respect to tumor therapy, a whole range of TNF receptors are considered as useful targets. On the one hand, immune stimulatory TNF receptors, such as the T-cell coactivating molecules 41BB, CD27, OX40 and GITR or the dendritic cell (DC) activator CD40 are of interest for immuno-therapeutic treatments. On the other hand, the above mentioned death receptors attract attention as apoptosis-inducing targets. This especially applies for TRAILR1 and TRAILR2 because there is good evidence that TRAIL predominately acts on tumor cells and the tumor vasculature but largely spares non-transformed cells and tissue.3 It is noteworthy that concomitant activation of immune stimulatory and tumor cell death-inducing TNF receptors can result in a synergistic antitumoral effect as presentation of tumor-derived peptides and thus the effects of immune stimulatory TNF receptors are improved if there is in parallel an increase in dead tumor cells.
Exogenous activation of TNF receptors is typically achieved by the use of two types of reagents, antibodies and recombinantly produced soluble TNF ligand variants. The development and use of both types of reagents require the consideration of a couple of issues. With respect to TNF receptor-specific antibodies, for example, one has to keep in mind that their agonistic activity can be highly dependent on binding to Fcγ receptors (FcγRs). Thus, the activity of such agonistic antibodies is in vivo highly dependent on the availability of FcγRs-expressing cells in the microenvironment of target-expressing cells as well as on the isotype of the antibody.4, 5, 6, 7Another important issue in this context is that in vivo agonistic antibodies not only act by TNF receptor activation but also, and often even more prominent, by the recruitment of immune cells and stimulation of immune effector functions, such as antibody-dependent cellular cytotoxicity (ADCC) and CDC. However, stimulation of TNF receptors with soluble TNF ligands is also not ever straightforwardly possible for the following reason: ligands of the TNF family are normally expressed as membrane-bound proteins, although soluble variants also occur physiologically due to proteolytic processing or alternative splicing. Now, the interaction of TNF receptors with membrane-bound TNF ligands always results in strong receptor activation, whereas TNF receptors respond differently to binding of soluble ligand molecules. Some TNF receptors are strongly activated by the binding of soluble ligands, whereas others bind soluble ligands but then fail to trigger intracellular signaling.8 In the latter case, the lacking or poor response to the binding of a soluble TNF ligand can be overcome by two means, either by ligand oligomerization or by artificial cell surface anchoring of the ligand exploiting an additional TNF receptor-independent interaction with a cell surface exposed molecule.8 Indeed, this is also relevant in the TRAIL–TRAILR system. Especially for TRAILR2, there is broad experimental evidence that binding of soluble TRAIL trimers is insufficient to trigger a significant apoptotic response, whereas secondarily oligomerized TRAIL trimers or trimeric single-chain variable fragment (scFv)–TRAIL fusion protein that have bound to a cell surface antigen are potent inducers of apoptosis.9, 10, 11, 12
Here, we describe the development of trimeric CD40-specific scFv fusion proteins of TRAIL and TRAILR1- and TRAILR2-specific TRAIL mutants that do not only show high CD40-restricted apoptotic activity but also concomitant activation of CD40. Thus, this new type of bifunctional fusion proteins combines a strong immune stimulating effect on DCs with the concomitant induction of tumor cell death via the TRAIL death receptors.
Results
Binding to CD40 enhances apoptosis induction by scFv:G28-TRAIL
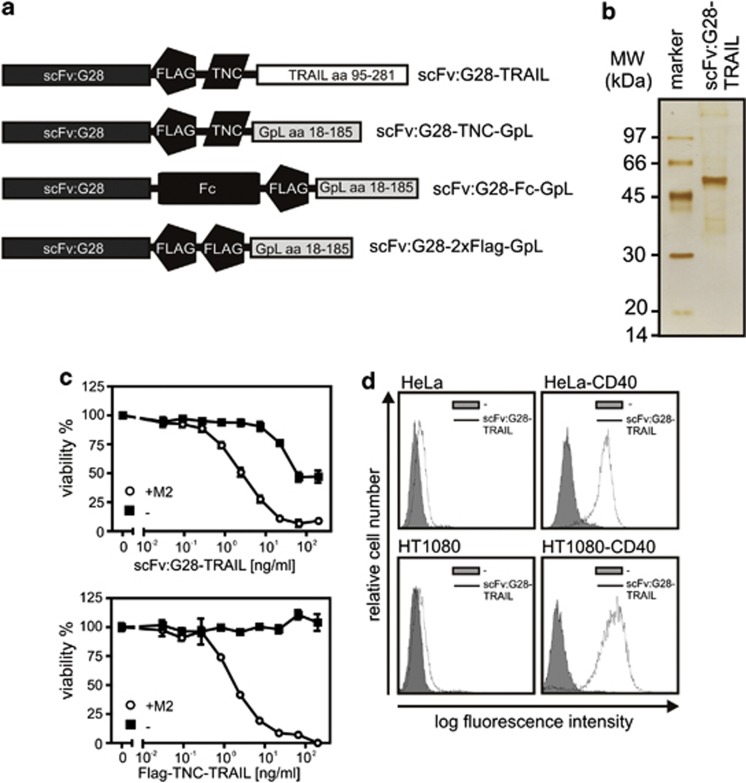
To achieve targeting of soluble trimeric TRAIL to CD40, we constructed a fusion protein, termed in the following as scFv:G28-TRAIL, consisting of a Ig signal peptide followed by a scFv derived from the human CD40-specific mAb G28-5 and aa 95-281 of human TRAIL encompassing its C-terminal TNF homology domain. An internal Flag epitope and the short 3 kDa trimerization domain of tenascin-C (TNC) were placed between the scFv and TRAIL domains (Figure 1a). The Flag epitope facilitates the detection and purification, whereas the TNC domain stabilizes the trimeric assembly of the fusion protein, which is driven by the TRAIL domain (Figure 1a). Supernatants collected from Hek293 cells stably transfected with the scFv:G28-TRAIL-encoding expression plasmid were subjected to affinity chromatography purification on anti-Flag agarose resulting in fairly pure preparations of the scFv-TRAIL fusion protein (Figure 1b). To initially evaluate the inherent functionality of the scFv and TRAIL domain of scFv:G28-TRAIL, the cytotoxic effect on CD40-negative Jurkat cells and binding to CD40 transfectants were analyzed. In contrast to Flag-TNC-TRAIL, the scFv:G28-TRAIL fusion protein showed a significant cytotoxic effect on Jurkat cells between 20 and 200 ng/ml. More important, however, both molecules elicited a comparable strong cytotoxic effect on Jurkat cells upon oligomerization with the antibody M2 recognizing the internal Flag tag of the two molecules (Figure 1c). It is very well established that soluble TRAIL trimers only induce efficient cell death in Jurkat cells upon oligmerization.11, 12 Thus, scFv:G28-TRAIL preparations appear to contain a minor fraction of oligomerized molecules causing the observed apoptosis induction at higher concentrations but otherwise have the same intrinsic apoptotic capacity as Flag-TNC-TRAIL. Analysis of the binding of scFv:G28-TRAIL to HeLa cells and HeLa-CD40 transfectants as well as to HT1080 and HT1080-CD40 transfectants by FACS further demonstrated the functionality of the scFv domain (Figure 1d).
Figure 1.
Domain architecture of the scFv:G28 fusion proteins used in this study and initial analysis of scFv:G28-TRAIL. (a) Schemes of scFv:G28-TRAIL, scFv:G28-2xFlag-GpL, scFv:G28-Fc-GpL and scFv:G28-TNC-GpL. scFv:G28, scFv derived from the human CD40-specific mAb G28-5; Flag epitope; TNC, trimerization domain, aa 110-139 of chicken tenascin-C; TRAIL, aa 95-281 of human TRAIL; Fc, CH3 domain of hIgG1; GpL, luciferase of Gaussia princeps. scFv:G28-Fc-GpL and scFv:G28-2xFlag-GpL were used as competitors for scFv:G28-TRAIL. scFv:G28-TNC-GpL were used to analyze the effect of trimerization of scFv:G28 on CD40 activity. (b) Anti-Flag affinity chromatography purified scFv:G28-TRAIL (100 ng) was subjected to SDS-PAGE and visualized by silver staining. (c) Jurkat cells (60 × 103 per well; 96-well plate) were challenged in the absence and presence of the anti-Flag mAb M2 (1 μg/ml) with the indicated concentrations of scFv:G28-TRAIL or its conventional counterpart Flag-TNC-TRAIL. Next day, cellular viability was determined by using the MTT assay (d) HeLa and HT1080 cells along with the corresponding CD40 transfectants were incubated with scFv:G28-TRAIL (500 ng/ml) and after three washes, bound molecules were detected by FACS using anti-Flag mAb M2-FITC
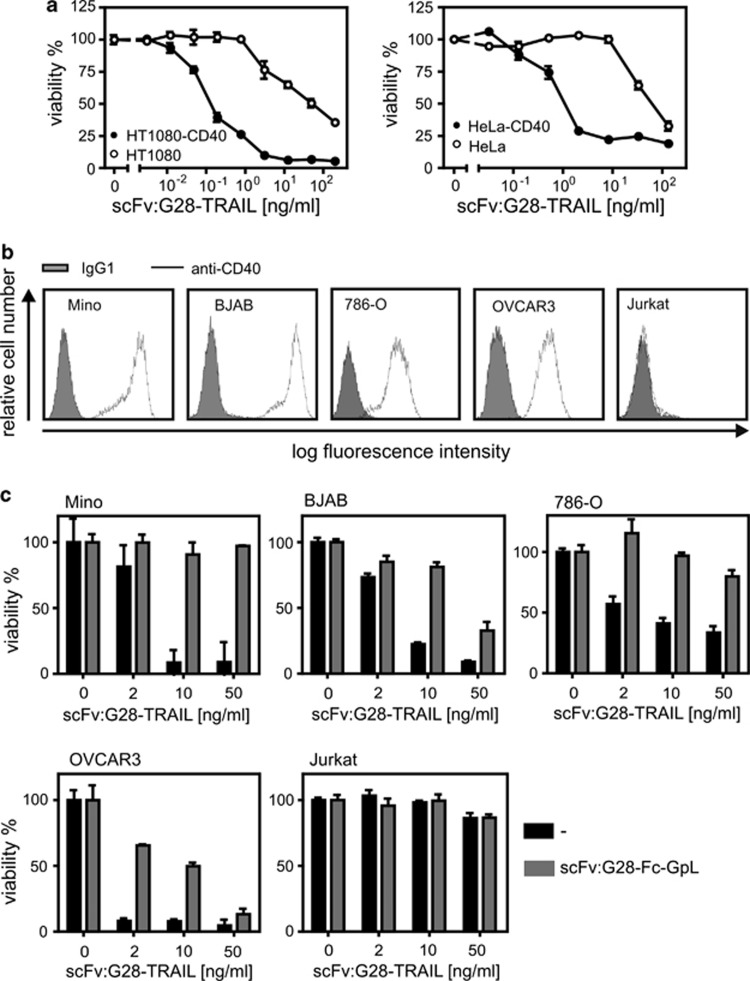
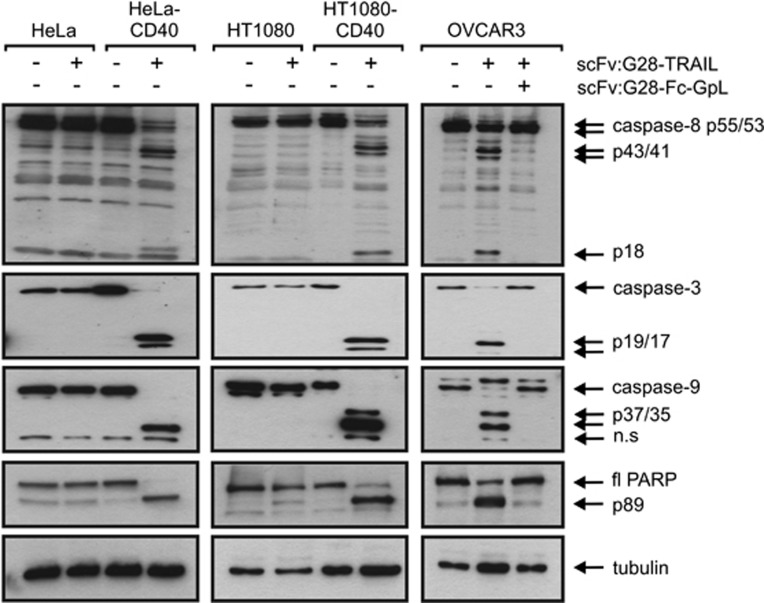
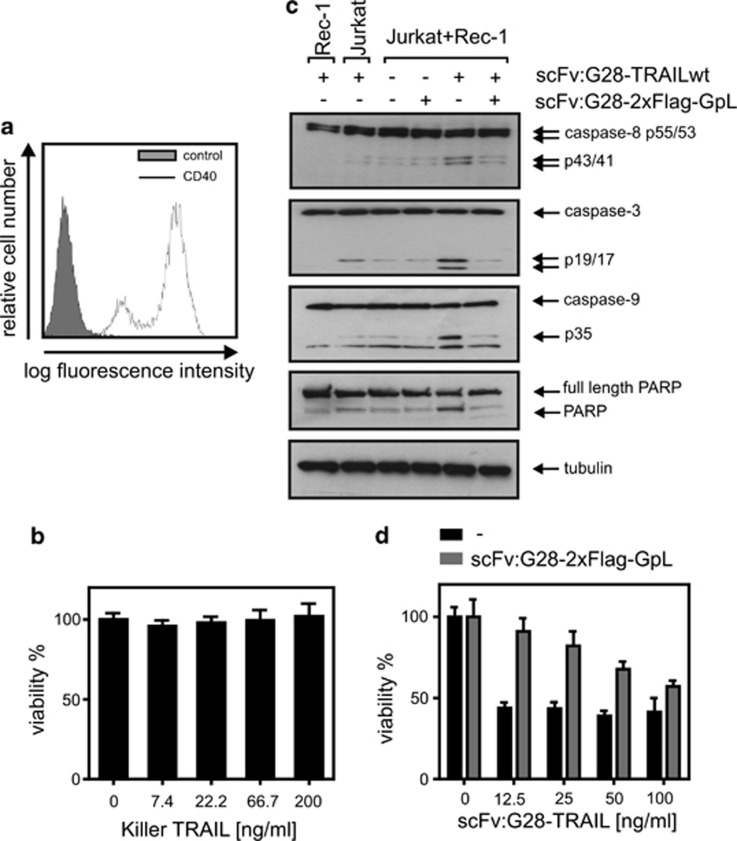
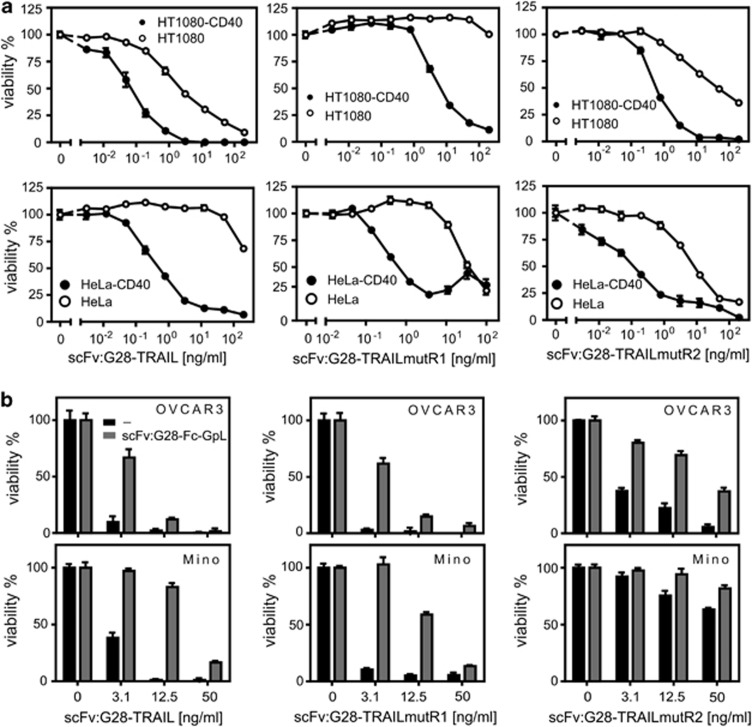
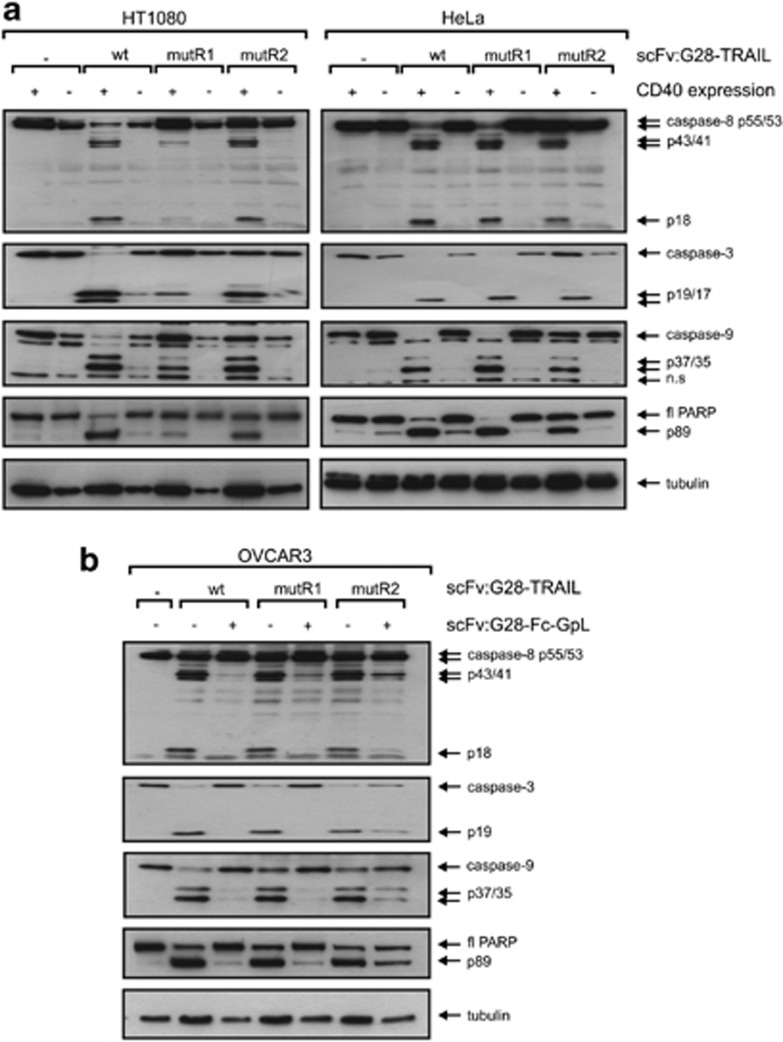
Next, we analyzed whether, and if yes to which extent, CD40 cell surface expression influences the apoptotic activity of scFv:G28-TRAIL. HT1080-CD40 and HeLa-CD40 transfectants along with their corresponding CD40-negative counterparts were challenged with increasing concentrations of scFv:G28-TRAIL, and the following day the viability of the cells were determined by crystal violet staining. In parental HeLa and HT1080 cells, there were only partial killing even at the highest concentration of 200 ng/ml used in these experiments (Figure 2a). In parallel, the CD40-expressing transfectants were almost completely killed at concentrations of around 10 ng/ml and showed a shift of the ED50-values for cell death induction of approximately two orders of magnitude toward lower concentrations (Figure 2a). CD40-dependent enhancement of apoptosis induction by scFv:G28-TRAIL was also evident from experiments, where four different cell lines with endogenous CD40 expression were challenged in the presence and absence of a Fc fusion protein of scFv:G28 that competes with scFv:G28-TRAIL for CD40 binding (Figures 2b and c). Not unexpected, enhanced cell death induction of CD40-expressing cells by scFv:G28-TRAIL was reflected by stronger activation of caspases (Figure 3). This is in accordance with the idea that the enhanced cytotoxic effects of scFv:G28-TRAIL on CD40-expressing cells are due to enhanced stimulation of TRAIL death receptor-mediated apoptosis. To further proof the possibility that CD40-bound scFv:G28-TRAIL also acts on neighboring CD40-negative cells, we exploited that CD40-expressing Rec-1 cells on the one hand are TRAIL-resistant (Figures 4a and b) and that on the other hand Jurkat cells are only killed by oligomerized or cell surface immobilized TRAIL trimers.10, 12, 13 As shown in Figure 1c, without oligomerization scFv:G28-TRAIL induced cell death in Jurkat cells only between 20 and 200 ng/ml and there was no significant cell death at lower concentrations. Indeed, there was almost no caspase activation in individual cultures of Jurkat and Rec-1 cells treated with 5 ng/ml of scFv:G28-TRAIL, whereas in cocultures of these two cell lines there were significant induction of caspase activation in response to treatment with scFv:G28-TRAIL (Figure 4c). In accordance with the idea of paracrine killing of CD40-negative Jurkat cells by Rec-1-bound scFv:G28-TRAIL, cell death induction and caspase activation in cocultures by the latter were diminished in the presence of scFv:G28-2xFlag-GpL as a competitor (Figures 4c and d). Together these experiments argue for the ability of scFv:G28-TRAIL to induce bystander apoptosis in CD40-negative cells.
Figure 2.
CD40 binding enhances apoptosis induction by scFv:CD40-TRAIL. (a) HT1080-CD40 and HeLa-CD40 transfectants and the corresponding parental cell lines were seeded in 96-well plates and were stimulated the next day in triplicates with the indicated concentration of scFv:G28-TRAIL. After an additional day, cellular viability was determined by crystal violet staining and normalized the help of untreated cells and a cell sample treated with mixture of toxic compounds. Cells were sensitized with CHX (2.5 μg/ml) to enhance apoptosis. (b) OVCAR3, BJAB, Mino, 786-O and CD40-negative Jurkat cells were analyzed for endogenous CD40 expression by FACS. (c) Triplicates of cells were treated in 96-well plates with the indicated concentrations of scFv:G28-TRAIL in the presence and absence of scFv:G28-Fc-GpL (2 μg/ml) as a competitor. Next day, cellular viability was determined by crystal violet staining or using the MTT assay
Figure 3.
Cells were treated as indicated with mixtures of scFv:G28-TRAIL (10 ng/ml), CHX (2.5 μg/ml) and scFv:G28-Fc-GpL (2 μg/ml) for 5 h and total cell lysates were analyzed by western Blot for the presence of the indicated proteins
Figure 4.
Binding to apoptosis-resistant CD40+ cells enables scFv:G28-TRAIL to induce apoptosis in sensitive CD40− bystander cells. (a) Rec-1 cells were analyzed by FACS for CD40 cell surface expression. (b) Rec-1 cells were challenged with the indicated concentrations of Killer-TRAIL and analyzed the next day for cellular viability using the MTT assay. (c) Rec-1 and Jurkat cells were individually cultivated or cocultivated and were stimulated with scFv:G28-TRAIL (5 ng/ml) in the presence and absence of scFv:G28-2xFlag-GpL (2 μg/ml), a competitor interfering with CD40 binding of scFv:G28-TRAIL. Cells were harvested after 6 h for western blot analysis of caspase processing (d) Rec-1 and Jurkat cells were cultivated together and were stimulated with the indicated concentrations of scFv:G28-TRAIL in the presence and absence of scFv:G28-2xFlag-GpL (2 μg/ml). Cells were then analyzed next day with respect to cellular viability using the MTT assay
CD40 binding-dependent activity of TRAILR1- and TRAILR2-specific scFv:G28-TRAIL variants
In the recent years several mutations have been described for TRAIL that confer at least to some extent specificity for TRAILR1 and TRAILR2 (for review see Wajant et al.8). To evaluate the potential of such mutations for the generation of TRAILR1- or TRAILR2-specific scFv-TRAIL fusion proteins with CD40-restricted activity, we replaced the TRAIL wild-type domain of scFv:G28-TRAIL by the TRAIL mutants G131R/R149I/S159R/N199R/K201H/S215D (mutR1) and Y189Q/R191K/Q193R/H264R/I266L/D267Q (mutR2) that preferentially bind to TRAILR1 (TRAILmutR1) and TRAILR2 (TRAILmutR2)14, 15 and showed good discrimination for TRAILR1 and TRAILR2 in the cellular binding assays and functional assays (manuscript submitted). In the following, we analyzed to which extent CD40 binding impacts the apoptotic activity of scFv:G28-TRAILmutR1 and scFv:G28-TRAILmutR2. Side by side analysis of CD40 transfectants with their CD40-negative counterparts as well as the analysis of cells with endogenous CD40 expression in the absence and presence of the competitor scFv:G28-Fc-GpL revealed significantly enhanced apoptotic activity for both constructs upon CD40 binding (Figures 5a, b and 6a, b).
Figure 5.
CD40 binding enhances apoptosis induction by scFv:G28 fusion proteins of TRAILR1- and TRAILR2-specific TRAIL mutants. (a) HT1080-CD40 and HeLa-CD40 transfectants and the respective parental cell lines were stimulated in 96-well plates in triplicates with the indicated concentrations of scFv:G28-TRAIL, scFv:G28-TRAILmutR1 and scFv:G28-TRAILmutR2 in the presence of CHX (2.5 μg/ml). Next day, cellular viability was determined by crystal violet staining. (b) OVCAR3 and Mino cells were treated in triplicates in 96-well plates with the various scFv:G28-TRAIL fusion proteins with and without pretreatment of the cells with scFv:G28-Fc-GpL (30 min, 2 μg/ml) as a competitor. Cellular viability was determined next day by crystal violet staining or the MTT assay
Figure 6.
CD40 binding enhances caspase activation by scFv:G28 fusion proteins of TRAILR1- and TRAILR2-specific TRAIL mutants. (a) HeLa and HT1080 cells along with the corresponding CD40 transfectants were incubated for 5 h with the various scFv:G28-TRAIL variants (10 ng/ml) as indicated. Total cell lysates were analyzed by western blot for the presence of the indicated proteins. (b) OVCAR3 cells were stimulated with 10 ng/ml of the various scFv:G28-TRAIL variants in the presence and absence of scFv:G28-Fc (30 min pretreatment). Cells were analyzed for caspase processing after 5 h
Induction of DC maturation by scFv:G28-TRAIL
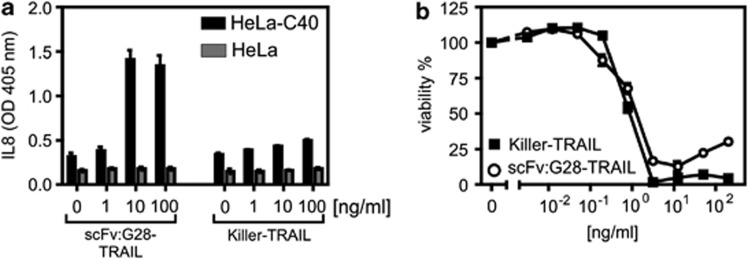
Against the background that the parental G28-5 antibody acts at least in some scenarios as an agonist and furthermore due to reports showing that other antibodies targeting members of the TNF receptor family exert agonistic activity after oligomerization or FcγR binding,4, 6 we wondered whether scFv:G28-TRAIL could act as a stimulator of CD40 signaling. Initially, we investigated Hela cells and HeLa-CD40 transfectants with respect to the activation of signaling pathways leading to IL-8 production. scFv:G28-TRAIL indeed triggered the latter in HeLa-CD40 cells but not in HeLa cells (Figure 7a). In principle, TRAIL and the TRAIL death receptors can also trigger the aforementioned proinflammatory response, but we found in a former study that this depends in HeLa cells on sensitization for TRAILR1/2 signaling by CHX and blockade of apoptosis.16 Indeed, Killer-TRAIL, a commercially available active form of TRAIL exerted only a weak IL-8-inducing effect in HeLa-CD40 cells, although it triggered apoptosis with high efficiency under CHX-sensitized conditions (Figure 7b).
Figure 7.
scFv:G28-TRAIL stimulate CD40 signaling. (a) HeLa cells and HeLa-CD40 transfectants were challenged in triplicates with scFv:G28-TRAIL and Killer-TRAIL and after 8 h supernatants were analyzed by ELISA for the presence of IL-8. (b) HeLa-CD40 transfectants were sensitized for apoptosis induction with CHX (2.5 μg/ml) and otherwise treated as in a. Cell viability was determined next day by crystal violet staining
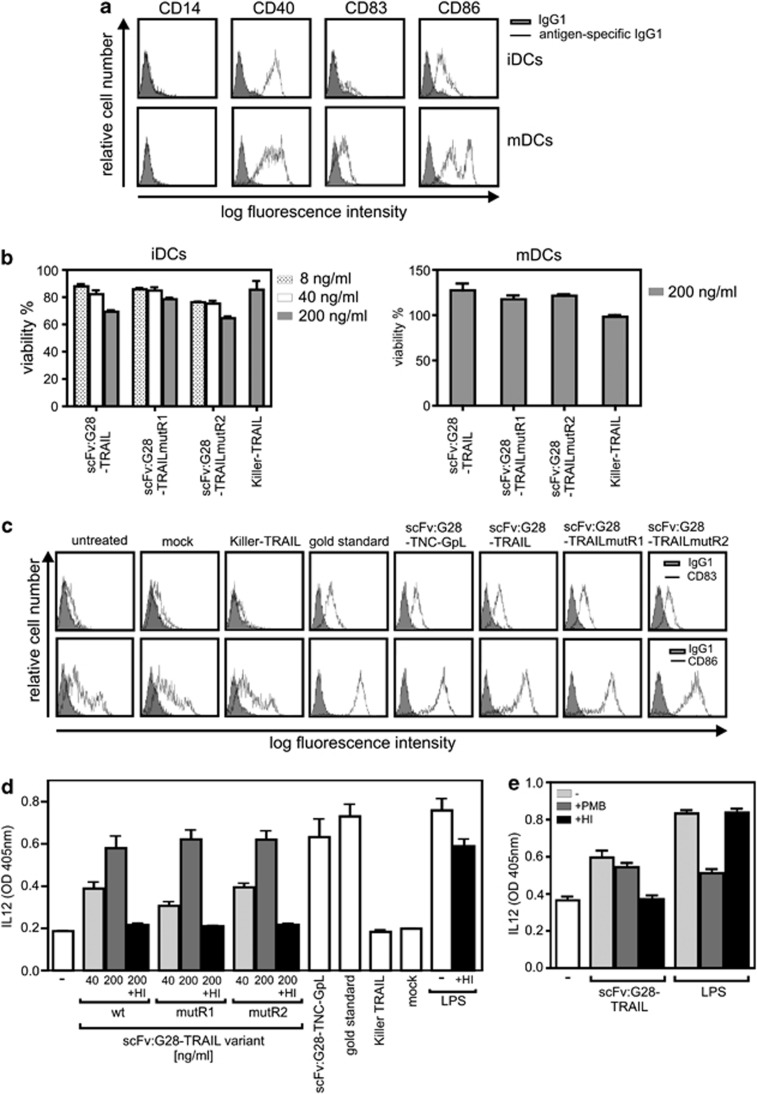
The major aim of many therapeutic concepts that base on CD40 targeting is the stimulation of DC maturation to enhance antitumor immunity.6 We therefore analyzed the effect of scFv:G28-TRAIL on immature and mature monocyte-derived DCs. Despite the strong expression of CD40 on DCs, scFv:G28-TRAIL variants were not or only poorly toxic against immature and mature DCs (Figures 8a and b). Noteworthy, however, scFv:G28-TRAIL was similarly effective with respect to the stimulation of DC maturation as a mixture of TNF, IL-6, IL1-β and prostaglandin E2 (PGE2), the gold standard for in vitro DC maturation. All scFv:G28-TRAIL variants as well as a tenascin-C trimerization domain-containing variant of scFv:G28 domain (scFv:G28-TNC-GpL) triggered the upregulation of CD83 and CD86 and also induced IL12 release (Figures 8c and d). In contrast, Killer-TRAIL did not show any effects indicating DC maturation (Figures 8c and d). Although heat-inactivation had no major effect on lipopolysaccharide (LPS)-induced IL12 release, it completely destroyed the IL12-inducing activity of the various scFv:G28-TRAIL samples (Figures 8c and d). Vice versa, polymyxin B treatment inhibited LPS-induced IL12 release, whereas it left scFv:G28-TRAIL-induced IL12 production largely unaffected (Figure 8e). The reciprocal effect of heat-inactivation and polymyxin B treatment on LPS- and scFv:G28-TRAIL-induced IL12 production indicates that the latter was primarily triggered by scFv:G28-TRAIL and not by endotoxin contaminations. In sum, our data suggest that scFv:G28-TRAIL variants act as bifunctional reagents that not only trigger cell death in tumor cells but also stimulate DC maturation.
Figure 8.
scFv:G28-TRAIL fusion proteins stimulate DC maturation. (a) Immature DCs (iDCs) and DCs (mDCs) that have been matured with the gold standard (mixture of 20 ng/ml TNF, 10 ng/ml IL1β, 20 ng/ml IL-6 and 1 μg/ml PGE2) were analyzed by FACS with respect to the cell surface expression of the indicated proteins. (b) iDCs and mDCs were challenged with scFv:G28-TRAIL variants, Killer-TRAIL or a cytotoxic cocktail (100 ng/ml Fc-CD95L, 5 μg/ml CHX and 0.5% sodium azide). Next day, cellular viability was determined using the MTT assay and normalized according to cells treated with the cytotoxic cocktail. (c) iDCs were treated for 48 h with 200 ng/ml of the various scFv:G28-TRAIL variants, with 200 ng/ml scFv:G28-TNC-GpL, a tenascin-C trimerization domain-containing fusion protein of scFv:G28 and the Gaussia princeps luciferase or with Killer-TRAIL (200 ng/ml). A supernatant of mock transfected HEK293 cells were included as a negative control for scFv:G28-TNC-GpL. To evaluate DC maturation, cells were analyzed by FACS with respect to the cell surface expression of CD83 and CD86 and compared with untreated groups and DCs were incubated with the ‘gold standard' mixture. (d) iDCs were treated in triplicates with the indicated concentrations of the various scFv:G28-TRAIL variants as well as with Killer-TRAIL (200 ng/ml), the gold standard mixture, LPS (100 ng/ml), scFv:G28-TNC-GpL and a control supernatant of mock transfected HEK293 cells. Where indicated, samples were heat-inactivated (HI) at 70 °C for 30 min to check for heat-stable endotoxin contaminations. DC supernatants were assayed after 24 h for the production of IL12 by ELISA. (e) iDCs were treated in triplicates with 200 ng/ml scFv:G28-TRAIL or LPS (20 ng/ml). Where indicated, samples were HI at 70 °C for 30 min or treated with 50 μg/ml polymyxin B (PMB). After 24 h, DC supernatants were again assayed for IL12 production by ELISA
Discussion
Early on after the discovery of TRAIL and the TRAIL death receptors, it became apparent that TRAIL induces apoptosis in a variety of tumor cell lines but sparse the huge majority of non-transformed cells. In view of this finding, it cannot be a real surprise that considerable efforts have been made by several groups and companies to develop and evaluate TRAIL/TRAIL death receptor-based cancer therapeutics. Indeed, a variety of clinical phase I and II trials using recombinant TRAIL or TRAIL death receptor-targeting antibodies are ongoing or have been recently completed.17, 18 These trials confirmed the anticipated good safety profile of TRAIL death receptor-targeting reagents as single therapy and in combination with several other drugs but also revealed comparably moderate antitumor activities.17, 18 The latter might be related to two major issues. First, there is compelling evidence from in vitro studies and preclinical models that there exist many mechanisms conferring TRAIL resistance to tumor cells which, however, can be overcome by combination with appropriate drugs that preferentially resensitize cancer cells. However, the TRAIL death receptor-targeting drug regimens have been applied so far to poorly selected groups of patients, as appropriate biomarkers are missing.17, 18 Second, the currently used TRAIL death receptor-targeting reagents presumably do not fully exploit the apoptotic signaling capabilities of TRAILR1/TRAILR2. In a subset of members of the TNF receptor superfamily, including TRAILR2, the binding of soluble ligand trimers is less efficient in receptor activation than interaction with the corresponding membrane-bound form of the ligand.8 Now, in these cases maximal receptor stimulation by soluble ligand trimers can be restored by secondary ligand oligomerization or artificial cell surface immobilization. Noteworthy, the recombinant TRAIL used in clinical trials corresponds to the less active trimeric soluble form of the molecule. The molecular basis of the different activities of TNF receptor complexes with soluble and membrane-bound (or oligomerized) ligand trimers is still insufficiently understood, but there is evidence that secondary assembly of initially formed ligand–receptor complexes into supramolecular cluster has a decisive role. The need of supramolecular clustering might also explain the finding that oligomerized and FcγR-bound TRAILR2-specific antibodies display enhanced signaling capabilities.7, 19, 20 Thus, TRAIL death receptor-targeting antibodies might elicit in vivo suboptimal receptor stimulation as long as they are not oligomerized by binding to FcγR-expressing cells.
We and others have shown earlier for the TRAIL death receptors and some other members of the TNF receptor family that their per se poor response to binding of soluble ligand trimers can be overcome by anchoring the latter to cell surface antigens. Here, we exploited this principle to develop CD40-targeted scFv-TRAIL fusion proteins that mimic the superior activity of oligomerized soluble TRAIL and membrane TRAIL upon binding to CD40. Noteworthy, by the use of a potentially agonistic CD40-specific scFv domain for CD40 targeting, this approach not only resulted in TRAIL fusion proteins with enhanced apoptosis-inducing activity (Figures 2, 3, 4) but also tightly connected this to CD40 binding and CD40 activation (Figures 7 and 8). The linkage of improved TRAIL activity with binding and activation of CD40 has two considerable advantages compared with non-targeted TRAIL variants. First, CD40 is highly expressed on the huge majority of B-cell malignancies and on many solid tumors. It is also expressed on many immune cells and some other non-transformed cell types but typically at more moderate levels. Thus, CD40 is not a bona fide tumor marker but shows considerable tumor association. Enhanced TRAIL death receptor activation by CD40 binding, as achieved with the constructs described in this work, has therefore the potential to limit the offtarget effects of TRAIL. This property may become particularly relevant in view of the fact that in most TRAIL-based therapeutic scenarios, combination with other drugs that potentially sensitizes normal cells for TRAIL-induced apoptosis has been envisaged.17, 18 Moreover, we found that the cell surface antigen binding-mediated increase in activity can also be achieved with TRAIL mutants that preferentially stimulate TRAILR1 or TRAILR2 (Figures 5 and 6). Thus, the use of such mutants in combination with CD40 targeting principally allows further reduction of potential TRAIL-related side effects in cases where the latter and the apoptotic effect in the targeted tumor make differential use of TRAILR1 and TRAILR2. A second advantage of CD40 targeting with scFv-TRAIL fusions proteins is the accompanied activation of CD40. Indeed, due to its stimulatory effects on antigen presenting cells and cytotoxic myeloid cells, agonistic CD40 antibodies are currently investigated in clinical trials for cancer therapy.6 In this respect, we observed that scFv:G28-TRAIL strongly induces maturation of monocyte-derived immature human DCs without causing cell death in these cells (Figure 8). Thus, CD40-targeting scFv-TRAIL fusion proteins of the type described in our study have the potential to trigger concomitantly two independent, potentially synergistically acting mechanisms namely apoptosis induction in tumor cells and activation of DCs.
Materials and Methods
Cloning, production and purification of recombinant proteins
The expression constructs encoding the various scFv:G28-TRAIL fusion proteins, the monomeric scFv:G28-2xFlag-GpL and the dimeric scFv:G28-Fc-GpL were obtained by combining appropriate DNA fragments encoding scFv G28-5 (accession number AJ853736) derived from the human CD40-specific mAb G28-5,21 TRAIL aa 95-281 (accession number U37518), chicken TNC aa 110-139, human IgG1 aa 222-447, luciferase of Gaussia princeps (GpL) and a Flag epitope by standard cloning techniques into pCR3 behind an Ig leader. The different TRAIL fusion proteins were produced and purified essentially as described elsewhere for similarly structured scFv-TNF ligand fusion proteins.22 In brief, HEK293 cells were electroporated (50 × 106 cells/ml, 4-mm cuvette, 250 V, 1800 μF, maximum resistance) with 40 μg of the corresponding expression vectors in 1 ml of culture medium containing 10% FCS using an Easyject Plus electroporator (PeqLab Biotechnologie, Erlangen, Germany). Electroporated cells were recovered in RPMI 1640 with 10% FCS, 1% Penicillin (PAA Laboratories, Cölbe, Germany) overnight and were then selected by supplementing the medium with 0.5 mg/ml Geneticin disulfate (G418-Sulfate, Carl Roth company, Karlsruhe, Germany) for 4 weeks. For production of the TRAIL fusion proteins, the polyclonal transfectants were cultivated for 7 days in RPMI 1640 with only 2% FCS. Supernatants were then collected and clarified by centrifugation. scFv:G28-TRAIL fusion protein was purified by affinity chromatography on anti-Flag M2 agarose beads (Sigma-Aldrich, St. Louis, MO, USA) and elution with TBS containing 100 μg/ml Flag peptide (Sigma-Aldrich). The fractions containing the recombinant protein were finally dialyzed against PBS and stored at −20°C for further analysis.
Cell death assay
The adherent cell lines HeLa, HeLa-CD40, HT1080, HT1080-CD40, 786-O and OVCAR3 (20 × 103 cells per well) were seeded in 96-well tissue culture plates. The next day, cells were treated in triplicates with the TRAIL fusion proteins in the presence of CHX (2.5 μg/ml). In experiments, where scFv:G28-Fc-GpL (2 μg/ml) was used as a competitor of scFv:G28-TRAIL fusion proteins for CD40 binding, this compound was added to the cells 30 min prior the fusion proteins. Cell viability was determined after an additional day by crystal violet staining. The suspension cell lines Jurkat, Mino, BJAB and Rec-1 were seeded in 96-well tissue culture plates (60 × 103 cells per well) and were treated the same day in triplicates in the absence of CHX in the same manner as described before for adherent cells. Oligomerization of TRAIL fusion proteins with the FLAG-specific mAb M2 (1 μg/ml) was performed on a separate 96-well plate for 30 min before the complexes were added to the cells. Cell viability was determined the next day by MTT staining. In coculture assay, Jurkat (60 × 103 cells per well) and Rec-1 (6 × 103 cells per well) were seeded together 96-well tissue culture plates. scFv:G28-2xFlag-GpL (2 μg/ml) was added to the cells 30 min prior the fusion proteins as a competitor, and the cells were treated as described previously for suspension cells.
FACS analysis
To evaluate the binding of TRAIL fusion proteins to HeLa and HT1080 cells and their corresponding CD40 transfectants, cells were washed with PBS and incubated on ice for 30 min with scFv:G28-TRAIL (500 ng/ml). Cells were washed again three times with PBS and incubated on ice for 30 min with anti-Flag mAb M2-FITC (Sigma-Aldrich). After three washes with ice-cold PBS, samples were analyzed using a FACSCalibur (BD Biosciences, Heidelberg, Germany). To determine cell surface expression of CD40, cells were incubated on ice with a PE-labeled CD40-specific Ab (clone HB14; Miltenyi Biotec, Bergisch Gladbach, Germany) or an appropriate PE-labeled isotype control (mouse IgG1, clone 11711, R&D Systems, Minneapolis, MN, USA). After three washes with ice-cold PBS, cell-associated immunofluorescence was again determined by FACS analysis. DCs were similarly analyzed with respect to cell surface expression of CD14, CD40, CD83 and CD86 using PE-labeled primary antibodies (CD14: clone M5E2, BD Biosciences; CD40: clone HB14, Miltenyi Biotec; CD83: clone HB15e, R&D Systems; and CD86: clone 37301, R&D Systems) and a corresponding isotype control antibody (mouse IgG1, clone 11711, R&D Systems).
Silver staining
The affinity-purified scFv:G28-TRAIL was separated by SDS-PAGE, and gel was subsequently stained by the help of the PageSilverTM Silver Staining kit (Fermentas GmbH, St. Leon Rot, Germany). In brief, after incubation in fixer 1 (50% ethanol, 10% acetic acid) for 60 min and two times in fixer 2 (30% ethanol) for 20 min, gels were washed twice with deionized water. Then gels were incubated for 1 min with a sensitizing solution and washed again twice with deionized water. After 20 min of staining with staining solution and two washes, protein bands were visualized by adding developing solution. When the desired signal intensity was achieved, the developer solution was discarded, and the reaction was terminated by adding stop solution.
Western blotting
For preparation of total cell lysates, cells were harvested in ice-cold phosphate-buffered saline (PBS) and centrifuged (3 min, 2300 r.p.m., 4 °C), and the pellet was directly lysed in 4 × Laemmli sample buffer (8% SDS, 10% β-mercaptoethanol, 40% glycerol, 0.2 M Tris, pH 8.0) supplemented with phosphatase inhibitor mixture II (Sigma) and protease inhibitor (Roche Diagnostics, Mannheim, Germany). Protein samples were boiled for 5 min at 96 °C after 20 s of sonification. Lysates were centrifuged twice (20 min, 14 000 r.p.m.). Samples were separated by SDS-PAGE and transferred to nitrocellulose membranes. Next, nonspecific binding sites were blocked by incubation with Tris-buffered saline containing 0.1% Tween-20 and 5% dry milk. Western blot analyses were performed with primary antibodies specific for caspase-3 (no. 9662S; Cell Signaling Technology, Danvers, MA, USA), caspase-9 (no. 9502S; Cell Signaling Technology), PARP (clone 7D3-6; BD), caspase-8 (clone C15; Enzo Life Sciences, Lörrach, Germany) and tubulin (clone DM1A; Lab Vision/NeoMarkers, Fremont, CA, USA). Secondary HRP-conjugated antibodies were obtained from Dako (Glostrup, Denmark; anti-mouse IgG) and Cell Signaling (anti-rabbit IgG), and antigen-antibody complexes were visualized using the ECL Western blotting detection system (Amersham Biosciences, Freiburg, Germany) according to the protocol delivered by the manufacturer.
IL-8 ELISA analysis
Cells were seeded (2 × 104 cells/well) in 96-well tissue cultures plates in 100 μl of RPMI 1640 medium with 10% FCS. The next day, medium was exchanged to minimize the background of constitutive IL-8 production, and cells were stimulated for 8 h in triplicates with the indicated concentrations of scFv:G28-TRAIL and Killer-TRAIL (Enzo Life Sciences). Supernatants were analyzed for production of IL-8 using the BD OptEIA Human IL-8 ELISA Set (BD Biosciences) according to the manufacturer's instructions.
Isolation, cultivation, and stimulation of monocyte-derived DCs
PBMCs were isolated from the blood from healthy donors by density gradient centrifugation with lymphocyte separation medium (PAA Laboratories). Magnetic bead separation with anti-CD14-coated beads (Miltenyi Biotec) were used to separate monocytes from the PBMCs. Purified monocytes were immediately seeded in 10 cm plates with RPMI 1640, 10% FCS and 1% penicillin. To induce differentiation to immature DCs, 50 ng/ml GM-CSF and 30 ng/ml IL-4 (ImmunoTools, Friesoythe, Germany) were added every second day. After 7 days, immature DCs were evaluated by FACS analysis for cell surface expression of CD14, CD40, CD83 and CD86 (see above). Immature DCs were seeded (4 × 104 cells/well) in 96-well tissue cultures plates in 100 μl of RPMI 1640 medium with 10% FCS. Cells were then treated overnight with the various TRAIL fusion proteins, Killer-TRAIL, scFv:G28-TNC-GpL and gold standard, a mixture composed of TNF (purified protein, produced using HEK293 cells), IL1β (R&D Systems), IL-6 (Miltenyi Biotec) and prostaglandin E2 (PGE2, Biomol, Hamburg, Germany), to evaluate the DC-stimulating activities of these reagents. To test the various scFv-TRAIL fusion proteins for contaminations with endotoxin, the corresponding samples were heat-inactivated for 30 min at 70 °C. DCs treated in parallel with LPS (Sigma-Aldrich) served as a heat-resistant control. As an additional test for endotoxin contaminations, the effect of the LPS-inhibitory antibiotic polymyxin B (InvivoGen, San Diego, CA, USA) on IL12 production was evaluated. On the next day, supernatants were analyzed for production of IL12 using R&D Systems Human IL12 ELISA DuoSet according to the manufacturer's instructions. In addition, cell viability was determined using the MTT assay. Maturation of DCs was further analyzed by FACS analysis of the DC markers CD83 and CD86 48 h post stimulation. To evaluate TRAIL sensitivity of mature DCs, the latter were generated by 24 h treatment with TNF (1 μg/ml) and then challenged with the 200 ng/ml of various TRAIL fusion proteins and Killer-TRAIL. Finally, cell viability was again analyzed by MTT staining.
Acknowledgments
This work was supported by Deutsche Forschungsgemeinschaft (grant WA 1025/24-1), Deutsche José Carreras Leukämie-Stiftung (project R/06/17) and Deutsche Krebshilfe (project 109922). Mohamed El-Mesery is a German Egyptian Research Long Term Scholarship (GERLS) holder funded by DAAD.
Glossary
- ADCC
antibody-dependent cellular cytotoxicity
- DC
dendritic cell
- FcγR
Fcγ receptor
- scFv
single-chain variable fragment
- TRAIL
TNF-related apoptosis-inducing ligand
The authors declare no conflict of interest.
Footnotes
Edited by M Agostini
References
- Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Gupta SC, Kim JH. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood. 2012;119:651–665. doi: 10.1182/blood-2011-04-325225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsom-Davis T, Prieske S, Walczak H. Is TRAIL the holy grail of cancer therapy. Apoptosis. 2009;14:607–623. doi: 10.1007/s10495-009-0321-2. [DOI] [PubMed] [Google Scholar]
- Dhein J, Daniel PT, Trauth BC, Oehm A, Moller P, Krammer PH. Induction of apoptosis by monoclonal antibody anti-APO-1 class switch variants is dependent on cross-linking of APO-1 cell surface antigens. J Immunol. 1992;149:3166–3173. [PubMed] [Google Scholar]
- Li F, Ravetch JV. Inhibitory Fcgamma receptor engagement drives adjuvant and anti-tumor activities of agonistic CD40 antibodies. Science. 2011;333:1030–1034. doi: 10.1126/science.1206954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonderheide RH, Glennie MJ. Agonistic CD40 antibodies and cancer therapy. Clin Cancer Res. 2013;19:1035–1043. doi: 10.1158/1078-0432.CCR-12-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson NS, Yang B, Yang A, Loeser S, Marsters S, Lawrence D, et al. An Fcgamma receptor-dependent mechanism drives antibody-mediated target-receptor signaling in cancer cells. Cancer Cell. 2011;19:101–113. doi: 10.1016/j.ccr.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Wajant H, Gerspach J, Pfizenmaier K. Engineering death receptor ligands for cancer therapy. Cancer Lett. 2013;332:163–174. doi: 10.1016/j.canlet.2010.12.019. [DOI] [PubMed] [Google Scholar]
- Bremer E, Kuijlen J, Samplonius D, Walczak H, de Leij L, Helfrich W. Target cell-restricted and -enhanced apoptosis induction by a scFv:sTRAIL fusion protein with specificity for the pancarcinoma-associated antigen EGP2. Int J Cancer. 2004;109:281–290. doi: 10.1002/ijc.11702. [DOI] [PubMed] [Google Scholar]
- Bremer E, Samplonius DF, van Genne L, Dijkstra MH, Kroesen BJ, de Leij LF, et al. Simultaneous inhibition of epidermal growth factor receptor (EGFR) signaling and enhanced activation of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) receptor-mediated apoptosis induction by an scFv:sTRAIL fusion protein with specificity for human EGFR. J Biol Chem. 2005;280:10025–10033. doi: 10.1074/jbc.M413673200. [DOI] [PubMed] [Google Scholar]
- Kelley RF, Totpal K, Lindstrom SH, Mathieu M, Billeci K, Deforge L, et al. Receptor-selective mutants of apoptosis-inducing ligand 2/tumor necrosis factor-related apoptosis-inducing ligand reveal a greater contribution of death receptor (DR) 5 than DR4 to apoptosis signaling. J Biol Chem. 2005;280:2205–2212. doi: 10.1074/jbc.M410660200. [DOI] [PubMed] [Google Scholar]
- Wajant H, Moosmayer D, Wuest T, Bartke T, Gerlach E, Schonherr U, et al. Differential activation of TRAIL-R1 and -2 by soluble and membrane TRAIL allows selective surface antigen-directed activation of TRAIL-R2 by a soluble TRAIL derivative. Oncogene. 2001;20:4101–4106. doi: 10.1038/sj.onc.1204558. [DOI] [PubMed] [Google Scholar]
- Bremer E, Samplonius DF, Peipp M, van Genne L, Kroesen BJ, Fey GH, et al. Target cell-restricted apoptosis induction of acute leukemic T cells by a recombinant tumor necrosis factor-related apoptosis-inducing ligand fusion protein with specificity for human CD7. Cancer Res. 2005;65:3380–3388. doi: 10.1158/0008-5472.CAN-04-2756. [DOI] [PubMed] [Google Scholar]
- MacFarlane M, Kohlhaas SL, Sutcliffe MJ, Dyer MJ, Cohen GM. TRAIL receptor-selective mutants signal to apoptosis via TRAIL-R1 in primary lymphoid malignancies. Cancer Res. 2005;65:11265–11270. doi: 10.1158/0008-5472.CAN-05-2801. [DOI] [PubMed] [Google Scholar]
- Reis CR, van der Sloot AM, Natoni A, Szegezdi E, Setroikromo R, Meijer M, et al. Rapid and efficient cancer cell killing mediated by high-affinity death receptor homotrimerizing TRAIL variants. Cell Death Dis. 2010;1:e83. doi: 10.1038/cddis.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajant H, Haas E, Schwenzer R, Muhlenbeck F, Kreuz S, Schubert G, et al. Inhibition of death receptor-mediated gene induction by a cycloheximide-sensitive factor occurs at the level of or upstream of Fas-associated death domain protein (FADD) J Biol Chem. 2000;275:24357–24366. doi: 10.1074/jbc.M000811200. [DOI] [PubMed] [Google Scholar]
- Dimberg LY, Anderson CK, Camidge R, Behbakht K, Thorburn A, Ford HL. On the TRAIL to successful cancer therapy? Predicting and counteracting resistance against TRAIL-based therapeutics. Oncogene. 2013;32:1341–1350. doi: 10.1038/onc.2012.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellwig CT, Rehm M. TRAIL signaling and synergy mechanisms used in TRAIL-based combination therapies. Mol Cancer Ther. 2012;11:3–13. doi: 10.1158/1535-7163.MCT-11-0434. [DOI] [PubMed] [Google Scholar]
- Adams C, Totpal K, Lawrence D, Marsters S, Pitti R, Yee S, et al. Structural and functional analysis of the interaction between the agonistic monoclonal antibody Apomab and the proapoptotic receptor DR5. Cell Death Differ. 2008;15:751–761. doi: 10.1038/sj.cdd.4402306. [DOI] [PubMed] [Google Scholar]
- Natoni A, MacFarlane M, Inoue S, Walewska R, Majid A, Knee D, et al. TRAIL signals to apoptosis in chronic lymphocytic leukaemia cells primarily through TRAIL-R1 whereas cross-linked agonistic TRAIL-R2 antibodies facilitate signalling via TRAIL-R2. Br J Haematol. 2007;139:568–577. doi: 10.1111/j.1365-2141.2007.06852.x. [DOI] [PubMed] [Google Scholar]
- Clark EA, Yip TC, Ledbetter JA, Yukawa H, Kikutani H, Kishimoto T, et al. CDw40 and BLCa-specific monoclonal antibodies detect two distinct molecules which transmit progression signals to human B lymphocytes. Eur J Immunol. 1988;18:451–457. doi: 10.1002/eji.1830180320. [DOI] [PubMed] [Google Scholar]
- Fick A, Wyzgol A, Wajant H. Production, purification, and characterization of scFv TNF ligand fusion proteins. Methods Mol Biol. 2012;907:597–609. doi: 10.1007/978-1-61779-974-7_33. [DOI] [PubMed] [Google Scholar]