Abstract
Triptans are 5HT1B/1D receptor agonists commonly prescribed for migraine headache. Although originally designed to constrict dilated intracranial blood vessels, the mechanism and site of action by which triptans abort the migraine pain remain unknown. We showed recently that sensitization of peripheral and central trigeminovascular neurons plays an important role in the pathophysiology of migraine pain. Here we examined whether the drug sumatriptan can prevent and/or suppress peripheral and central sensitization by using single-unit recording in our animal model of intracranial pain. We found that sumatriptan effectively prevented the induction of sensitization (i.e., increased spontaneous firing; increased neuronal sensitivity to intracranial mechanical stimuli) in central trigeminovascular neurons (recorded in the dorsal horn), but not in peripheral trigeminovascular neurons (recorded in the trigeminal ganglion). After sensitization was established in both types of neuron, sumatriptan effectively normalized intracranial mechanical sensitivity of central neurons, but failed to reverse such hypersensitivity in peripheral neurons. In both the peripheral and central neurons, the drug failed to attenuate the increased spontaneous activity established during sensitization. These results suggest that neither peripheral nor central trigeminovascular neurons are directly inhibited by sumatriptan. Rather, triptan action appears to be exerted through presynaptic 5HT1B/1D receptors in the dorsal horn to block synaptic transmission between axon terminals of the peripheral trigeminovascular neurons and cell bodies of their central counterparts. We therefore suggest that the analgesic action of triptan can be attained specifically in the absence, but not in the presence, of central sensitization.
Migraine is a recurring neurological disorder of unilateral headache affecting 18% of women and 6% of men (1, 2). Migraine pain is thought to be driven by activation and sensitization of peripheral neurons in the trigeminal ganglion that innervate the meninges (i.e., meningeal nociceptors) and central trigeminovascular neurons in the upper cervical and medullary dorsal horn. Several lines of evidence suggest that activation of meningeal nociceptors can be initiated locally by release of inflammatory mediators around meningeal blood vessels (3, 4). Upon such activation, meningeal nociceptors become hyperresponsive (sensitized) to the otherwise innocuous fluctuation in intracranial pressure, resulting in the characteristic throbbing of migraine pain (5–7). The sustained firing of sensitized meningeal nociceptors eventually leads to activation and subsequent sensitization of central trigeminovascular neurons (8), which process sensory signals that originate not only from the dura, but also from the periorbital skin, resulting in increased responsiveness not only to mild changes in intracranial pressure but also to innocuous skin stimulation. This central sensitization, which occurs during migraine in many patients (9), is manifested as cutaneous allodynia (i.e., enhanced periorbital skin sensitivity).
The distinction between peripheral and central sensitization is important not only for better understanding migraine pathophysiology, but also for improving on current therapy, as well as designing future therapeutic strategies. Among the most prescribed antimigraine drugs to date is a family of 5HT1B/1D receptor agonists collectively known as triptans (10). Based on a series of clinical and preclinical studies, we have shown that triptans must be administered as early as possible before the establishment of central sensitization to abort migraine, abolish throbbing and prevent the development of cutaneous allodynia (11, 12). If given too late, after the establishment of central sensitization, triptan therapy can still mitigate throbbing, but not migraine pain or cutaneous allodynia. These findings have led us to propose that triptans block the flow of sensory signals to the dorsal horn selectively from the meninges, but not the skin. Because 5HT1B/1D receptors are present on both peripheral and central axonal branches of meningeal nociceptors, but absent from nociceptive neurons in the dorsal horn (13–15), we hypothesized that the neuronal actions of triptans could be exerted either by direct inhibition of the meningeal nociceptors or by blockade of synaptic transmission between the meningeal nociceptors and the central trigeminovascular neurons in the dorsal horn. To differentiate between these possibilities, we examined the effects of systemic triptan administration on the induction and maintenance of sensitization in meningeal nociceptors compared to the central trigeminovascular neurons. The results obtained in this study help identifying the site of action critical for terminating of migraine with triptans.
Materials and Methods
Surgical Preparation. All experiments were approved by the standing committee on animals of Harvard Medical School. Male Sprague–Dawley rats weighing 350–500 g were anesthetized with urethane (1.2 g/kg i.p.) and fitted with an intratracheal metal tube to allow artificial ventilation and a cannula inserted into the femoral vein for later administration of sumatriptan. Rats were placed in a stereotaxic apparatus, with core temperature kept at 37°C by using a heating blanket. End-tidal CO2 was continuously monitored and kept within physiological range (3.5–4.5 pCO2). To apply chemical and mechanical stimulation onto the dura mater, we carefully removed the bone above the dorsal surface of the left hemisphere and transverse sinus and kept the overlying dura moist by using a modified synthetic interstitial fluid (135 mM NaCl/5 mM KCl/1 mM MgCl2/5 mM CaCl2/10 mM glucose/10 mM Hepes, pH 7.2). To record the activity of meningeal nociceptors, we performed a second, more rostral craniotomy and lowered a platinum-coated tungsten microelectrode (impedance 500 KΩ) into the left trigeminal ganglion. To record the activity of central trigeminovascular neurons, we exposed the area between the obex and C2, which was kept moist with mineral oil, and lowered a platinum-coated tungsten microelectrode (impedance 4–6 MΩ) into the medullary dorsal horn. Anatomical localizations of central recording sites were identified postmortem as described (8).
Meningeal Nociceptors Recording. Single electrical stimulations (0.5–5 mA, 0.5-ms pulse duration) were delivered to the dura overlying the ipsilateral transverse sinus at 0.5 Hz. Meningeal nociceptors were then identified by their constant latency responses, and classified as either C-units (conduction velocity ≤1.5 m/s) or A-units (conduction velocity >1.5 m/s). Action potentials were processed by using a real-time waveform discriminator (spike 2, Cambridge Electronic Design) and acquired for both online and offline analyses. Neuronal responses to mechanical stimulation were determined by using a servo, force-controlled mechanical stimulator (Aurora Scientific, Ontario) with a flat-ended cylindrical plastic probe (0.5 mm) that delivers graded mechanical stimuli to the dural surface at the site of the lowest threshold (7). Stimulus trials for testing mechanically sensitive units consisted of four “ramp and hold” stimuli (rise time 100 ms, stimulus width 2 s, interstimulus interval 60 s), which included a subthreshold, a threshold, and two suprathreshold stimuli, delivered in ascending order. Stimuli that evoked 1–2 spikes per s above baseline were considered as threshold. Subthreshold stimuli were applied at 40–50% of the threshold force, and did not evoke any response during baseline testing. The two suprathreshold stimuli were 2- and 4-fold higher than the threshold stimulus. Throughout the experiment, stimulus trials were delivered at constant interval of 10 min, during which spontaneous firing rate was recorded.
Central Trigeminovascular Neurons Recording. Dorsal horn trigeminovascular neurons receiving both Aδ- and C-fiber inputs from the dura were identified by using electrical stimulation as describe above. Each neuron was characterized for its baseline responses to mechanical indentation of the dura (using calibrated von Frey hairs; range, 0.06–3.6 g) and classified as wide-dynamic range or high-threshold according to its responses to skin brushing and pressing (using paint brush and loose arterial clip). Stimuli were repeated at least three times at 30-min intervals and only neurons showing <10% variability in responses were further studied. The selected neurons were then sampled for their baseline firing rate (spikes per s) over a period of 10 min.
Induction of Sensitization. Topical application (for 5 min) of an inflammatory soup (IS) containing a mixture of inflammatory mediators (histamine, serotonin, bradykinin, all at 1 mM, and 0.1 mM prostaglandin E2, pH 5.5) (16–18) was used to induce activation and mechanical sensitization of meningeal nociceptors and central trigeminovascular neurons as described earlier (8, 19). Our earlier studies have shown that a brief 5-min exposure of the dura to IS rapidly induces long-lasting sensitization in central trigeminovascular neurons (8). In a pilot study preceding this one, we found that such chemical stimulation of the dura induced long-lasting sensitization in meningeal nociceptors as well (n = 5). This peripheral sensitization was manifested as an increase in ongoing firing rate (from 0.41 ± 0.16 spikes per s at baseline to 0.87 ± 0.24 at 1 h and 0.92 ± 0.45 at 2 h) and an increase in firing produced in response to threshold mechanical stimulation of the dura (from 2.20 ± 0.37 spikes per s at baseline to 7.58 ± 2.50 at 1 h and 5.52 ± 0.99 at 2 h).
Triptan Treatment. Sumatriptan solution (300 μg/ml) was infused intravenously at a rate of 15 μg/min over 7–10 min to a final dose of 300 μg/kg of body weight. This dose was chosen to maximize potential inhibitory action of the drug on trigeminovascular neurons and minimize the probability of negative results due to lower ineffective dose. We used two paradigms to test the effects of sumatriptan on sensitization of peripheral and central trigeminovascular neurons. In one paradigm, sumatriptan was administered intravenously 1 h after topical application of IS on the dura (i.e., delayed treatment), to examine whether the drug can disrupt the maintenance of a well established sensitization. In the second paradigm, we administered sumatriptan and applied IS concurrently (initiated within 5 min of each other) to examine whether the drug can prevent the induction of sensitization. The induction of peripheral and central sensitization by IS and the effects of sumatriptan were assessed by monitoring (i) changes in neuronal spontaneous activity, and (ii) changes in magnitude of threshold responses to mechanical dural stimulation.
Statistical Analysis. We studied one neuron (either peripheral or central) per animal. Data are presented as means ± SE. Statistical analyses were conducted by using nonparametric statistics (20, 21). Spontaneous activity and neuronal responses to dural stimulation were analyzed over three time points (0, 1, and 2 h after IS or sumatriptan), by using Friedman two-way analysis of variance. Post hoc paired comparisons were performed by using one-tailed Wilcoxon matched-pairs signed-ranks test. Level of significance was set at 0.05.
Results
We tested 15 meningeal nociceptors, of which 7 were C units, (conduction velocity range 0.39–1.40 m/s) and 8 were Aδ-units (conduction velocity range 1.90–15.0 m/s), and 14 wide-dynamic range, central trigeminovascular neurons that exhibited both C- and Aδ-fiber input from the dura.
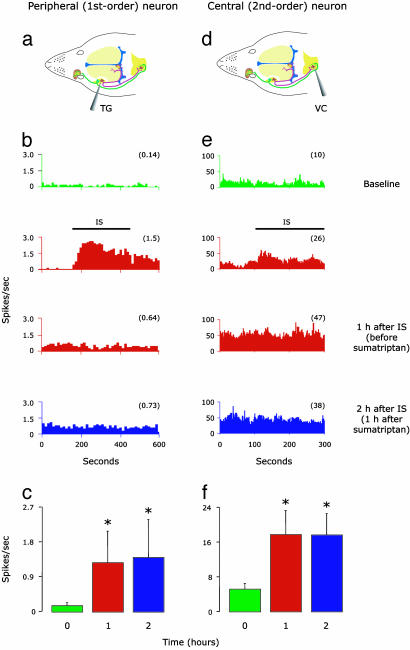
Spontaneous Activity. The induction of peripheral and central sensitization by application of IS on the dura was manifested as a significant increase in the mean (±SE) firing rate (spikes per s) of meningeal nociceptors (from 0.14 ± 0.1 at baseline to 1.26 ± 0.8 1 h later; P < 0.02; Fig. 1c) and central trigeminovascular neurons (from 5.1 ± 1.3 at baseline to 17.7 ± 5.6 1 h later; P < 0.04; Fig. 1f). Individual examples are shown in Fig. 1 b and e. After sensitization was established in peripheral and central neurons, delayed sumatriptan administration failed to inhibit the increased firing rate in either meningeal nociceptors (Fig. 1 b and c) or central trigeminovascular neurons (Fig. 1 e and f).
Fig. 1.
Effects of late sumatriptan administration on IS-induced increase in firing rate of peripheral (Left) and central (Right) trigeminovascular neurons. (a and d) Schematic localization of recording sites for meningeal nociceptors in the trigeminal ganglion (TG) and for central trigeminovascular neurons in the spinal trigeminal nucleus (VC). (b and e) Peristimulus time histograms showing mean firing rate of one meningeal nociceptor and one central trigeminovascular neuron sampled before (baseline/green), during and 1 h after the application of IS (red), and after a delayed (1 h) administration of sumatriptan (blue). Notice that sumatriptan did not block the long-lasting increase in firing rate induced by IS in both peripheral and central neurons. (c and f) Mean (±SE) spontaneous firing rate in peripheral (n = 7) and central (n = 7) trigeminovascular neurons sampled at 1-h intervals before (baseline/green) and after (red) the application of IS, and after a delayed (1 h) administration of sumatriptan (blue). Numbers in parentheses indicate mean firing rate (spikes per s). *, P < 0.05 compared to baseline.
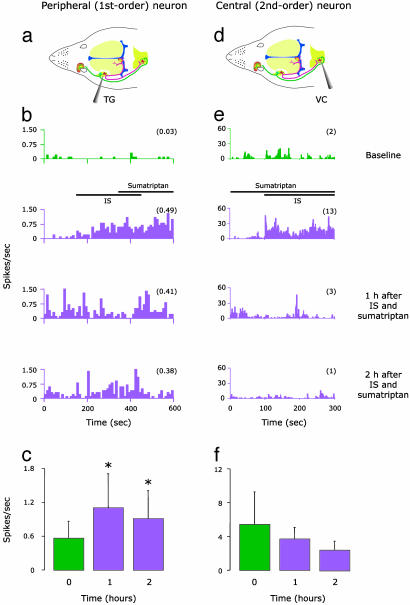
When given simultaneously with IS, however, sumatriptan selectively blocked the induction of central sensitization but did not interfere with the induction of peripheral sensitization (Fig. 2). Thus, mean firing rate did not increase in central trigeminovascular neurons; in fact, it decreased (although not significantly) from 5.4 ± 3.9 at baseline to 3.7 ± 1.4 at 1 h and 2.4 ± 1.2 at 2 h after IS plus sumatriptan (Fig. 2f). In meningeal nociceptors, on the other hand, firing rate increased significantly (P < 0.03) from 0.56 ± 0.3 at baseline to 1.10 ± 0.6 at 1 h and 0.91 ± 0.5 at 2 h after IS plus sumatriptan (Fig. 2c). Individual examples are shown in Fig. 2 b and e.
Fig. 2.
Effects of early sumatriptan administration on IS-induced increase in firing rate of peripheral (Left) and central (Right) trigeminovascular neurons. (a and d) Schematic localization of recording sites for meningeal nociceptors in the trigeminal ganglion and for central trigeminovascular neurons in the spinal trigeminal nucleus. (b and e) Peristimulus time histograms showing mean firing rate of one meningeal nociceptor and one central trigeminovascular neuron sampled before (baseline/green), during, and 1 and 2 h after (purple) simultaneous application of IS and sumatriptan. Notice that sumatriptan effectively blocked the long-lasting increase in firing rate induced by IS in the central but not the peripheral unit. (c and f) Mean (±SE) spontaneous firing rate in peripheral (n = 8) and central (n = 7) trigeminovascular neurons after simultaneous application of IS and sumatriptan. Numbers in parentheses indicate mean firing rate (spikes per s). Color scheme is the same as in b and e. *, P < 0.05 compared to baseline.
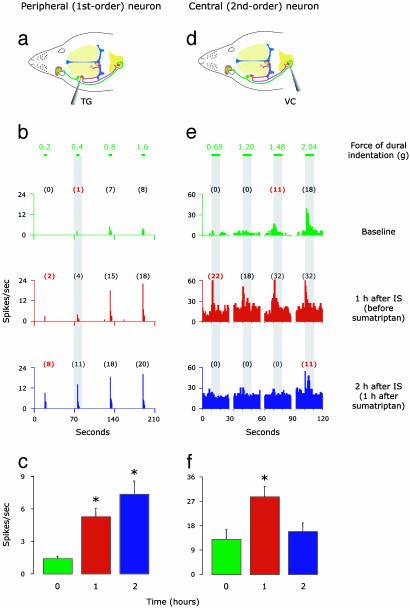
Responses to Mechanical Stimulation of Dura. The induction of peripheral and central sensitization by application of IS onto the dura was also manifested as enhanced responses of meningeal nociceptors and central trigeminovascular neurons to mechanical dural stimulation. After exposing the dural receptive field to IS, mean threshold response magnitude increased by 361 ± 67% above baseline in meningeal nociceptors (P < 0.02, Fig. 3c) and by 135 ± 38% above baseline (P < 0.02) in central trigeminovascular neurons (Fig. 3f). After the establishment of peripheral sensitization, delayed sumatriptan administration failed to reduce the response magnitude; in fact, the responsiveness of meningeal nociceptors continued to increase by additional 40 ± 17% after sumatriptan treatment (P < 0.02, Fig. 3c). Individual examples are shown in Fig. 3 b and e. On the other hand, delayed sumatriptan administration successfully reduced the response magnitude of central trigeminovascular neurons back to baseline values (P < 0.03, Fig. 3f).
Fig. 3.
IS-induced sensitization to mechanical dural stimulation in peripheral (Left) and central (Right) trigeminovascular neurons: effects of late sumatriptan administration. (a and d) Schematic localization of recording sites for meningeal nociceptors in the trigeminal ganglion and for central trigeminovascular neurons in the spinal trigeminal nucleus. (b and e) Response magnitude and response threshold to mechanical dural stimulation sampled before (baseline/green), 1 h after application of IS (red), and 1 h after a delayed administration of sumatriptan (blue). Numbers above lines depict force values (g) applied to dural receptive field. Numbers in parentheses indicate mean spikes per s, and those in red mark the threshold response. Notice that response magnitude increased and response threshold decreased after IS in both peripheral and central neurons, indicating mechanical hypersensitivity. Notice also that delayed sumatriptan reversed this mechanical hypersensitivity in the central but not the peripheral neuron. (c and f) Mean (±SE) response magnitude of peripheral (n = 8) and central (n = 7) trigeminovascular neurons to mechanical stimulation of the dura before (baseline/green) and after (red) the application of IS, and after a delayed (1 h) administration of sumatriptan (blue). These means were calculated for the minimum force that produced a response (i.e., threshold) at baseline. For example, in the individual cases shown in b, baseline threshold was 0.4 g; it produced 1, 4, and 11 spikes per s at the different time points. *, P < 0.05 compared to baseline.
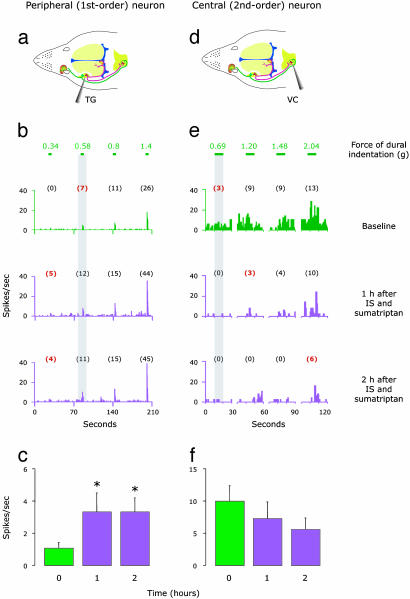
When given simultaneously with IS, sumatriptan selectively blocked the induction of mechanical hypersensitivity to dural stimulation in central trigeminovascular neurons (Fig. 4f) but not in meningeal nociceptors (Fig. 4c). Thus, mean response magnitude of central trigeminovascular neurons to dural stimulation did not increase above baseline level; in fact, it decreased (although not significantly) by ≈44 ± 14.6% (Fig. 4f). In meningeal nociceptors, on the other hand, mean response magnitude to dural stimulation increased by 200 ± 49% above baseline (P < 0.002, Fig. 4c). Individual examples are shown in Fig. 4 b and e.
Fig. 4.
IS-induced sensitization to mechanical dural stimulation in peripheral (Left) and central (Right) trigeminovascular neurons: effects of early sumatriptan administration. (a and d) Schematic localization of recording sites for meningeal nociceptors in the trigeminal ganglion and for central trigeminovascular neurons in the spinal trigeminal nucleus. (b and e) Response magnitude and response threshold to mechanical dural stimulation sampled at 1-h intervals before (baseline/green) and after (purple) simultaneous application of IS and sumatriptan. Numbers above lines depict force values (g) applied to dural receptive field. Numbers in parentheses indicate mean spikes per s, and those in red mark the threshold response. Threshold response values are marked in red. Notice that in the peripheral neuron response magnitude increased and response threshold decreased, indicating hypersensitivity, whereas, in the central neuron, response magnitude decreased and response threshold increased, indicating hyposensitivity. (c and f) Mean (±SE) response magnitude of peripheral (n = 8) and central (n = 7) trigeminovascular neurons to mechanical stimulation of the dura before (baseline/green) and after (purple) simultaneous application of IS and sumatriptan. Mean response magnitude was calculated as above (Fig. 3). *, P < 0.05 compared to baseline.
Discussion
In the present study, we show that the 5HT1B/1D agonist, sumatriptan, can effectively prevent the induction of sensitization in central trigeminovascular neurons but not in meningeal nociceptors (Fig. 5). These results rule out the peripheral terminals of meningeal nociceptors in the dura, or their cell bodies in the trigeminal ganglion, as the sites through which sumatriptan aborts migraine headache. The finding that sumatriptan did not intercept the maintenance of central sensitization (as reflected by the enhanced spontaneous activity) is consistent with the absence of 5HT1B/1D receptors from central trigeminovascular neurons in the dorsal horn (13–15). These findings rule out a postsynaptic site of action through which sumatriptan terminates the headache. A final piece in the puzzle is the finding that sumatriptan effectively abated intracranial hypersensitivity in central trigeminovascular neurons but not in meningeal nociceptors. Viewed together, these differential effects provide compelling evidence that sumatriptan prevents the development of central sensitization and terminates migraine headache by blocking synaptic transmission between meningeal nociceptors and central trigeminovascular neurons through presynaptic 5HT1B/1D receptors on central terminals of meningeal nociceptors in the dorsal horn. This central site of action is consistent with the evidence that sumatriptan can rapidly cross the blood–brain barrier into the central nervous system after systemic administration (22). A presynaptic site of action explains why triptans cannot suppress central sensitization hours after its establishment (11, 12) when the activity in the central neuron has become independent of incoming input from the dura. Considering that calcitonin gene-related peptide (CGRP), substance P, and glutamate contribute to the transmission of nociceptive information in the dorsal horn (23–26), our proposal of presynaptic inhibition by sumatriptan is consistent with the presence of 5HT1B/1D receptors on peripheral nociceptors that express CGRP, substance P, and glutamate (27–29), and the evidence that sumatriptan represses secretion of these neurotransmitters from central branches of such nociceptors through modulation of intracellular calcium (30–32).
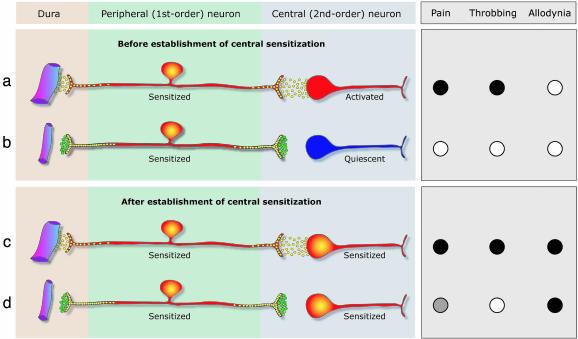
Fig. 5.
Proposed mechanism of action for 5HT1B/1D agonists during migraine. (a) Minutes after the onset of migraine, activated meningeal nociceptors enter a new physiological state, denoted peripheral sensitization, and release a host of neuropeptides such as CGRP, substance P, and neurokinin A (yellow circles), that promote local vasodilatation and plasma extravasation (i.e., neurogenic inflammation) through their peripheral branch in the meninges, and activation of central trigeminovascular neurons through their central branch in the dorsal horn. After peripheral sensitization sets in, rhythmic pulsation of the meninges generate bursts of action potentials that activate the central trigeminovascular neuron (shown in red) and the pain (•) begins to throb (•). (b) Systemically administered triptan molecules (green circles) bind to presynaptic 5HT1B/1Dreceptors on terminals of both the peripheral and central branches of the meningeal nociceptor, resulting in blockade of neuropeptide release. At this stage, blockade of neuropeptide release from the peripheral terminal has no effect on the hyperexcitability of the meningeal nociceptor. However, blockade of neuropeptide release from the central terminal of meningeal nociceptor renders the central trigeminovascular neuron inactive (shown in blue), resulting in termination of pain (○) and throbbing (○). (c) After the establishment of central sensitization, the pain continues to throb (•) and the skin becomes allodynic (•). (d) At this stage, blockade of neuropeptide release from the central terminals of the meningeal nociceptor cannot reverse the hyperexcitability of the central trigeminovascular neuron because its activity no longer depends on input from the meningeal nociceptor. In the face of the autonomous activity of the central trigeminovascular neuron, this blockade of synaptic transmission provides only partial pain relief ( ), terminates the throbbing (○), but does not resolve the allodynia (•).
), terminates the throbbing (○), but does not resolve the allodynia (•).
Our animal model of intracranial pain is based on the evidence that brief activation of trigeminovascular neurons induces neuropeptide-mediated neurogenic inflammation (33), which includes dilatation of blood vessels and plasma protein extravasation (34). It is generally accepted that neurogenic inflammation is critical for the maintenance of ongoing activity and mechanical hypersensitivity of meningeal nociceptors during migraine (35, 36). Considering the convincing evidence that systemic sumatriptan (100–300 μg/kg) produces both constriction of dilated meningeal blood vessels (37–39) and inhibition of neurogenic inf lammation (39, 40), we were surprised that sumatriptan did not inhibit the IS-induced sensitization of meningeal nociceptors. Thus, contrary to the prevailing view, these findings suggest that, once peripheral sensitization has been established, the ongoing activity and mechanical hypersensitivity of meningeal nociceptor are no longer dependent on neurogenic inflammation or vasodilatation. Along this line, we propose that the vasoconstricting effects of sumatriptan and its inhibition of plasma extravasation do not play a significant role in the termination of migraine pain.
In our recent clinical study (12), we showed that the momentary worsening of migraine pain and throbbing produced by episodic intracranial hypertension in response to bending over (41, 42) was successfully reversed by sumatriptan even in allodynic attacks in which the drug ultimately failed to abort migraine pain. Consistent with these findings, we now show that sumatriptan can effectively reverse the intracranial hypersensitivity of central trigeminovascular neurons even though it cannot suppress ongoing central sensitization. Based on the differential effects of sumatriptan on throbbing vs. pain in allodynic patients, and on mechanical hypersensitivity vs. ongoing activity in sensitized central trigeminovascular neurons in animals, we propose that the neural substrate of throbbing differs from that of the pain. In the absence of central sensitization, both throbbing and pain depend on incoming nociceptive input from the meninges (Fig. 5 a and b). However, after the establishment of central sensitization, throbbing continues to depend on the peripheral input to the central trigeminovascular neuron, whereas the pain becomes largely dependent on intrinsic activity of the central neuron itself (Fig. 5 c and d).
Our proposed neuronal mechanism of action of 5HT1B/1D agonists during migraine is illustrated in Fig. 5. In the early stages of migraine, central trigeminovascular neurons become activated in response to incoming impulses from meningeal nociceptors (Fig. 5a). At this stage, presynaptic inhibition by triptans should be sufficient to render the central neurons quiescent, resulting in a pain-free state (Fig. 5b). In migraine attacks associated with cutaneous allodynia, the central neurons eventually become sensitized and develop their own autonomous activity (Fig. 5c). At this stage, the same presynaptic inhibition by triptans becomes progressively inadequate to render central trigeminovascular neurons quiescent, resulting in only partial pain relief (Fig. 5d). In migraine attacks that remain allodynia-free, the central trigeminovascular neurons do not reach a state of sensitization and continue to be driven by incoming impulses from meningeal nociceptors. Therefore, the central presynaptic inhibition exerted by triptans should be sufficient to render the patient pain-free at any time during the attack.
Acknowledgments
We thank Drs. Clifford Saper and Andrew Strassman for critical comments on the manuscript. This work was supported by a grant from GlaxoSmithKline and by National Institutes of Health Grants DE13347 (National Institutes of Dental and Craniofacial Research) and NS35611 (National Institutes of Neurological Disorder and Stroke) (to R.B.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: IS, inflammatory soup.
References
- 1.Stewart, W. F., Lipton, R. B., Celentano, D. D. & Reed, M. L. (1992) J. Am. Med. Assoc. 267, 64-69. [PubMed] [Google Scholar]
- 2.Olesen, J. & Cutrer, F. M. (2000) in The Headaches, eds. Olesen, J., Tfelt-Hansen, P. & Welch, K. (Raven, New York), pp. 345-357.
- 3.Moskowitz, M. A. & Macfarlane, R. (1993) Cerebrovasc. Brain Metab. Rev. 5, 159-177. [PubMed] [Google Scholar]
- 4.Bolay, H., Reuter, U., Dunn, A. K., Huang, Z., Boas, D. A. & Moskowitz, M. A. (2002) Nat. Med. 8, 136-142. [DOI] [PubMed] [Google Scholar]
- 5.Strassman, A. M., Raymond, S. A. & Burstein, R. (1996) Nature 384, 560-564. [DOI] [PubMed] [Google Scholar]
- 6.Bove, G. M. & Moskowitz, M. A. (1997) J. Neurophysiol. 77, 299-308. [DOI] [PubMed] [Google Scholar]
- 7.Levy, D. & Strassman, A. M. (2002) J. Neurophysiol. 88, 3021-3031. [DOI] [PubMed] [Google Scholar]
- 8.Burstein, R., Yamamura, H., Malick, A. & Strassman, A. M. (1998) J. Neurophysiol. 79, 964-982. [DOI] [PubMed] [Google Scholar]
- 9.Burstein, R., Yarnitsky, D., Goor-Aryeh, I., Ransil, B. J. & Bajwa, Z. H. (2000) Ann. Neurol. 47, 614-624. [PubMed] [Google Scholar]
- 10.Saxena, P. R. & Tfelt-Hansen, P. (2000) in The Headaches, eds. Olesen, J., Tfelt-Hansen, P. & Welch, K. (Lippincot Williams & Wilkins, Philadelphia), Vol. 2, pp. 411-438. [Google Scholar]
- 11.Burstein, R. & Jakubowski, M. (2004) Ann. Neurol. 55, 27-36. [DOI] [PubMed] [Google Scholar]
- 12.Burstein, R., Jakubowski, M. & Collins, B. (2004) Ann. Neurol. 55, 19-26. [DOI] [PubMed] [Google Scholar]
- 13.Longmore, J., Shaw, D., Smith, D., Hopkins, R., McAllister, G., Pickard, J. D., Sirinathsinghji, D. J., Butler, A. J. & Hill, R. G. (1997) Cephalalgia 17, 833-842. [DOI] [PubMed] [Google Scholar]
- 14.Riad, M., Tong, X. K., el Mestikawy, S., Hamon, M., Hamel, E. & Descarries, L. (1998) Neuroscience 86, 1031-1035. [DOI] [PubMed] [Google Scholar]
- 15.Potrebic, S., Ahn, A. H., Skinner, K., Fields, H. L. & Basbaum, A. I. (2003) J. Neurosci. 23, 10988-10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steen, K. H., Steen, A. E., Kreysel, H. W. & Reeh, P. W. (1996) Pain 66, 163-170. [DOI] [PubMed] [Google Scholar]
- 17.Steen, K. H., Steen, A. E. & Reeh, P. W. (1995) J. Neurosci. 15, 3982-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steen, K. H., Reeh, P. W., Anton, F. & Handwerker, H. O. (1992) J. Neurosci. 12, 86-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy, D. & Strassman, A. M. (2002) J. Physiol. 538, 483-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siegel, S. (1956) Nonparametric Statistics for the Behavioral Sciences (McGraw–Hill Kogakusha, Tokyo).
- 21.Zar, J. H. (1998) Biostatistical Analysis (Prentice-Hall, Englewood Cliffs, NJ).
- 22.Johnson, D. E., Rollema, H., Schmidt, A. W. & McHarg, A. D. (2001) Eur. J. Pharmacol. 425, 203-210. [DOI] [PubMed] [Google Scholar]
- 23.Neugebauer, V., Rumenapp, P. & Schaible, H. G. (1996) Neuroscience 71, 1095-1109. [DOI] [PubMed] [Google Scholar]
- 24.Neugebauer, V., Rumenapp, P. & Schaible, H. G. (1996) Eur. J. Neurosci. 8, 249-260. [DOI] [PubMed] [Google Scholar]
- 25.Yu, L. C., Zheng, E. M. & Lundeberg, T. (1999) Regul. Pept. 83, 21-24. [DOI] [PubMed] [Google Scholar]
- 26.Cheunsuang, O., Maxwell, D. & Morris, R. (2002) Neuroscience 111, 423-434. [DOI] [PubMed] [Google Scholar]
- 27.Hou, M., Kanje, M., Longmore, J., Tajti, J., Uddman, R. & Edvinsson, L. (2001) Brain Res. 909, 112-120. [DOI] [PubMed] [Google Scholar]
- 28.Ma, Q. P., Hill, R. & Sirinathsinghji, D. (2001) Eur. J. Neurosci. 13, 2099-2104. [DOI] [PubMed] [Google Scholar]
- 29.Ma, Q. P. (2001) NeuroReport 12, 1589-1591. [DOI] [PubMed] [Google Scholar]
- 30.Messlinger, K., Ebersberger, A. & Schaible, H. G. (1998) Br. J. Pharmacol. 125, 1726-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Durham, P. L. & Russo, A. F. (2003) J. Neurosci. 23, 807-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Travagli, R. A. & Williams, J. T. (1996) J. Physiol. 491, 177-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Markowitz, S., Saito, K. & Moskowitz, M. A. (1987) J. Neurosci. 7, 4129-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holzer, P. (1988) Neuroscience 24, 739-768. [DOI] [PubMed] [Google Scholar]
- 35.Moskowitz, M. A. (1993) Neurology 43, S16-20. [PubMed] [Google Scholar]
- 36.Sanchez del Rio, M., Reuter, U. & Moskowitz, M. A. (2000) Funct. Neurol. 15, 157-162. [PubMed] [Google Scholar]
- 37.Friberg, L., Olesen, J., Iversen, H. K. & Sperling, B. (1991) Lancet 338, 13-17. [DOI] [PubMed] [Google Scholar]
- 38.Humphrey, P. P. & Feniuk, W. (1991) Trends Pharmacol. Sci. 12, 444-446. [DOI] [PubMed] [Google Scholar]
- 39.Shepherd, S. L., Williamson, D. J., Beer, M. S., Hill, R. G. & Hargreaves, R. J. (1997) Neuropharmacology 36, 525-533. [DOI] [PubMed] [Google Scholar]
- 40.Buzzi, M. G. & Moskowitz, M. A. (1990) Br. J. Pharmacol. 99, 202-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blau, J. N. & Dexter, S. L. (1981) Cephalalgia 1, 143-147. [DOI] [PubMed] [Google Scholar]
- 42.Daley, M. L., Pasupathy, H., Griffith, M., Robertson, J. T. & Leffler, C. W. (1995) IEEE Trans. Biomed. Eng. 42, 420-424. [DOI] [PubMed] [Google Scholar]