Abstract
Tumor multidrug resistance (MDR) can result from overexpression of drug transporters and deregulation of cellular signaling transduction. New agents and strategies are required for overcoming MDR. Here, we report that tanshinone-1, a bioactive ingredient in traditional Chinese medicine, directly killed MDR tumor cells and their corresponding parental cells, which was potentiated by inhibition of secondary activation of signaling networks. Tanshinone-1 was slightly more potent at inducing cytotoxicity and apoptosis in MDR cells than in corresponding parental cells. Tanshinone-1-induced MDR cell killing was independent of the function and expression of drug transporters but was partially correlated with the phosphatase-dependent reduction of phospho-705-Stat3, which secondarily activated p38-, AKT-, and ERK-involved signaling networks. Cotreatments with p38, AKT, and ERK inhibitors potentiated the anti-MDR effects of tanshinone-1. Our study presents a model for MDR cell killing using a compound of natural origin. This model could lead to new therapeutic strategies for targeting signaling network(s) in MDR cancers as well as new strategies for multitarget design.
Keywords: tanshinone-1, multidrug resistance, Stat3, p38, AKT, ERK
About 90% of tumor treatment failure results from drug resistance.1 Multidrug resistance (MDR) appears to have a far more important role in treatment failure than individual drug resistance due to its involvement in almost all types of tumors and with many clinical anticancer drugs, including the naturally derived or synthetic drugs used in conventional chemotherapeutics or new molecularly targeted therapies.2 There have been only few successes in overcoming MDR despite the enormous amounts of effort devoted toward investigating the development of MDR and possible curative approaches.3 Therefore, explorations of new agents and strategies are required to address this severe therapeutic hurdle.
One of the most common mechanisms that mediates MDR is the expression of ATP-binding cassette drug transporters including P-glycoprotein (P-gp), MDR-associated protein 1 (MRP1), and breast cancer resistance protein (BCRP) in tumor cells.4 These transporters function to increase efflux and decrease influx of anticancer drugs to reduce cellular drug accumulation.5 It has long been considered that the key to overcoming this type of MDR is to enhance cellular accumulation of the drug. Therefore, MDR reversal agents, such as verapamil, have been used to inhibit the function of drug transporters,4 and other agents have also been investigated for their abilities to suppress drug transporter expression.6, 7, 8, 9 These approaches could lead to severe toxicity, however, as the majority of drug transporters are also physiologically expressed in important organs or tissues where they have roles in detoxification or protection.2
Deregulation of cellular signaling transduction frequently occurred in MDR tumor cells. For example, signal transducer and activator of transcription 3 (Stat3) is frequently overactivated not only in many types of human cancers10 but also in MDR tumors.11 Constitutively activated Stat3 is phosphorylated at its Tyr705 (p-705-Stat3). In addition to promoting cell proliferation and survival, p-705-Stat3 may suppress apoptosis by increasing the expression of various antiapoptotic factors.11 The level of Tyr705 phosphorylation is regulated by upstream Janus kinases (Jaks) and phosphatases, including SH-2-containing phosphatase 2 (Shp2) and protein-tyrosine-phosphatase 1B (PTP1B).12 Reducing p-705-Stat3 either by targeting Stat3 upstream signaling or by activating the phosphatases could result in significant anticancer activity.13, 14, 15, 16 Moreover, methyl spongoate, a natural marine product, mediates an apparent killing effect on liver cancer cells overexpressing various drug transporters by depleting Tyr705 phosphorylation of Stat3.17 Targeting Stat3 with a decoy oligonucleotide has also been recently shown to decrease the expression of its antiapoptotic target genes within cancers in a phase 0 clinical trial.18 Close relationships between Stat3 and other signaling pathways such as p38, AKT, and ERK19, 20, 21also suggest that activation of these signaling pathways may impair the effect of targeting Stat3 in cancer therapy.
Salvia miltiorrhiza (Danshen) is a famous traditional Chinese medicine that has been used to treat various diseases, including cardiovascular diseases, for centuries.22, 23 Several preparations that contain its major bioactive ingredients still have important clinical roles, especially in the treatment of angina pectoris in China.24 Danshen contains two types of major constituents: water-soluble phenolic acids and lipophilic tanshinones.23 Tanshinones, including tanshinone-1 (Supplementary Figure S1) and tanshinone-2A, are abietanediterpenes characterized by an ortho-quinone C-ring.22 Tanshinone-1 accounts for ∼2% of the ethanolic fraction of crude Danshen,25 indicating its high content in this plant as well as its clinical safety. Tanshinones have also been shown to elicit anticancer effects.22, 23, 26, 27, 28, 29 Nevertheless, the mechanisms of action and their molecular target(s) remain unknown.
In the search for MDR-overcoming drugs, we found that tanshinone-1 exerted more potent killing effects on MDR tumor cells than tanshinone-2A with resistance factor (RF) 0.9 versus 3.6 (unpublished data). In this study, we compared the capability of tanshinone-1 for inducing cytotoxicity and apoptosis to its impact on the function and expression of important drug transporters in MDR and corresponding parental tumor cell lines. To investigate its potential mechanism of anticancer action, we further explored whether and how tanshinone-1 changed the phosphorylation levels of Stat3, p38, AKT, and ERK in these cells. Finally, we examined how cotreatments with p38, AKT, and ERK inhibitors affected the anticancer and anti-MDR activities of tanshinone-1. Our results reveal that tanshinone-1 has a potent capability for directly killing MDR tumor cells, independent of drug transporters but partially dependent on reduced Tyr705 phosphorylation of Stat3. Moreover, inhibiting the secondary effects of increased phosphorylation of other signaling molecules, especially p38 and AKT, potentiates its cytotoxicity in both MDR and parental tumor cells.
Results
Tanshinone-1 kills MDR cells in a drug-transporter-independent manner
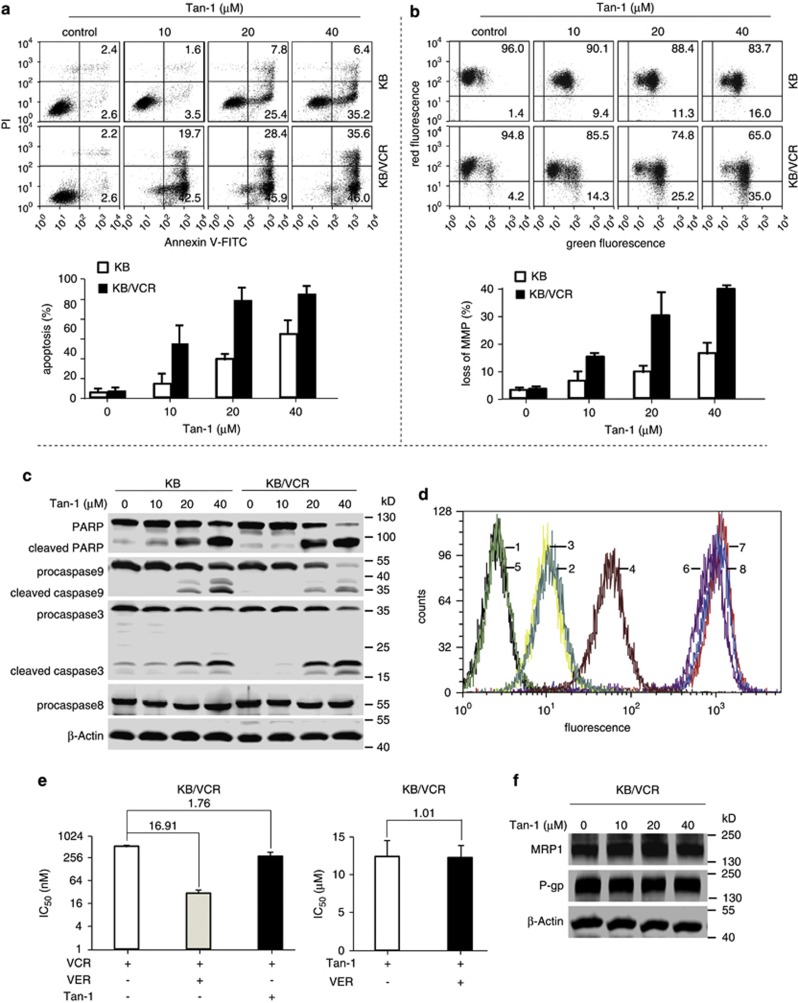
To determine whether tanshinone-1 could kill MDR tumor cells, we used three MDR sublines, K562/A02, KB/VCR, and MCF-7/ADR, that express drug transporters, including P-gp and MRP1.6, 7, 17 Tanshinone-1 elicited a more potent cytotoxicity against MDR cells than the respective parental cells with an average RF of 0.83 (Table 1). In contrast, the average RF of adriamycin and vincristine reached 162.7 (Table 1). However, tanshinone-1 was less toxic to the normal cells (human liver QSG7701 and HL7702 cells and mouse fibroblast NIH3T3 cells) (Table 1 and Table 2). Tanshinone-1 induced more apoptosis (Figure 1a and Supplementary Figure S2a) by triggering increased loss of mitochondria membrane potential (MMP) (Figure 1b) and stronger activation of caspase-3 and caspase-9 (Figure 1c) in KB/VCR cells than in KB cells in a concentration-dependent manner. However, tanshinone-1 did not seem to affect caspase-8 in either MDR or parental cells (Figure 1c). The data indicate that tanshinone-1 activates the intrinsic, rather than extrinsic, apoptosis pathway, which leads to the killing of both MDR and parental cells.
Table 1. Cytotoxicity of tanshinone-1 in MDR and corresponding parental tumor cells.
| Compound |
IC50a
(μM) |
RFb |
IC50
(μM) |
RF |
IC50
(μM) |
RF | |||
|---|---|---|---|---|---|---|---|---|---|
| KB | KB/VCR | MCF-7 | MCF-7/ADR | K562 | K562/A02 | ||||
| Adriamycin | — | — | — | 0.11±0.06 | 9.7±2.9 | 88.2 | 0.05±0.03 | 7.3±3.3 | 146.0 |
| Vincristine | 0.002±0.0007 | 0.5±0.2 | 254.0 | — | — | — | — | — | — |
| Tanshinone-1 | 8.7±1.2 | 8.3±0.1 | 0.9 | 7.7±1.2 | 5.6±1.0 | 0.7 | 21.2±6.4 | 20.1±5.5 | 0.9 |
The drug concentration required for 50% growth inhibition (IC50) of tumor cells was determined from three separate experiments and expressed as mean±S.D. Each drug concentration was tested in triplicate for 72 h
The resistance factor (RF) was calculated as the ratio of the IC50 value of the MDR cells to that of corresponding parental cells
Table 2. Cytotoxicity (IC50 a, μM) of tanshinone-1 in normal cell lines.
| Cell lines | QSG7701 | HL7702 | NIH3T3 |
|---|---|---|---|
| Tanshinone-1 (μM) | >80 | 40.1±4.3 | 28.6±5.5 |
The drug concentration required for 50% growth inhibition (IC50) of the tested cells was determined from three separate experiments and expressed as mean±S.D. Each drug concentration was tested in triplicate for 72 h
Figure 1.
Tanshinone-1 induced apoptosis independent of drug transporters. (a) Tanshinone-1 (Tan-1) increased Annexin V-positive cells. Cells were treated with Tan-1 for 24 h, then stained with Annexin V/propidium iodide (PI) and analyzed by flow cytometry. Upper panel, representative histograms; lower panel, mean±S.D. (b) Tan-1 disrupted the mitochondria membrane potential. Cells were exposed to Tan-1 for 18 h, stained with 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine iodide, and then detected by flow cytometry. Upper panel, representative histograms; lower panel, mean±S.D. (c) Tan-1 induced the cleavage of procaspase 3/9 and PARP. Cells were treated with Tan-1 for 18 h and analyzed by western blotting. (d) Tan-1 did not affect Rh123 export. Cells were pretreated with Tan-1 or verapamil for 90 min, cultured in medium with or without 1 μg/ml Rh123, and then followed by flow cytometry detection. 1–4: KB/VCR, blank; Rh123; Rh123 and 40 μM Tan-1; Rh123 and 5 μM verapamil; 5–8: KB, blank; Rh123; Rh123 and 40 μM Tan-1; Rh123 and 5 μM verapamil. (e) Verapamil did not significantly enhance the sensitivity of KB/VCR cells to Tan-1. Cells were treated with 5 μM verapamil or 5 μM Tan-1 in combination with vincristine (left panel), or treated with 5 μM verapamil plus Tan-1 for 72 h (right panel). The IC50 was expressed as mean±S.D. (f) Tan-1 did not decrease expression of drug transporter proteins. Cells were treated with Tan-1 for 12 h and analyzed by western blotting. All the data were obtained from at least three independent experiments
The results suggest that the expression of drug transporters in MDR cells does not impair the biological effect of tanshinone-1. To clarify this point, we analyzed the efflux of rhodamine 123 (Rh123, a fluorescent dye known as a substrate of P-gp).30 Rh123 stayed in the parental KB cells but was transported out of KB/VCR cells (Figure 1d). This was prevented by treating with the well-known P-gp blocker verapamil31, 32 instead of tanshinone-1. Similarly, verapamil, but not tanshinone-1, significantly sensitized KB/VCR cells to vincristine (Figure 1e, left) but did not affect the sensitivity of KB/VCR cells to tanshinone-1 (Figure 1e, right). KB/VCR cells were also observed to accumulate slightly more tanshinone-1 than the parental cells (Supplementary Figure S2b), and tanshinone-1 did not change the expression of the mdr-1 and MRP1 genes at either protein (Figure 1f) or mRNA (Supplementary Figure S2c) levels. These data indicate that tanshinone-1-induced killing of MDR cells is independent of drug transporters.
Tanshinone-1 depletes the Tyr705 phosphorylation of cellular Stat3
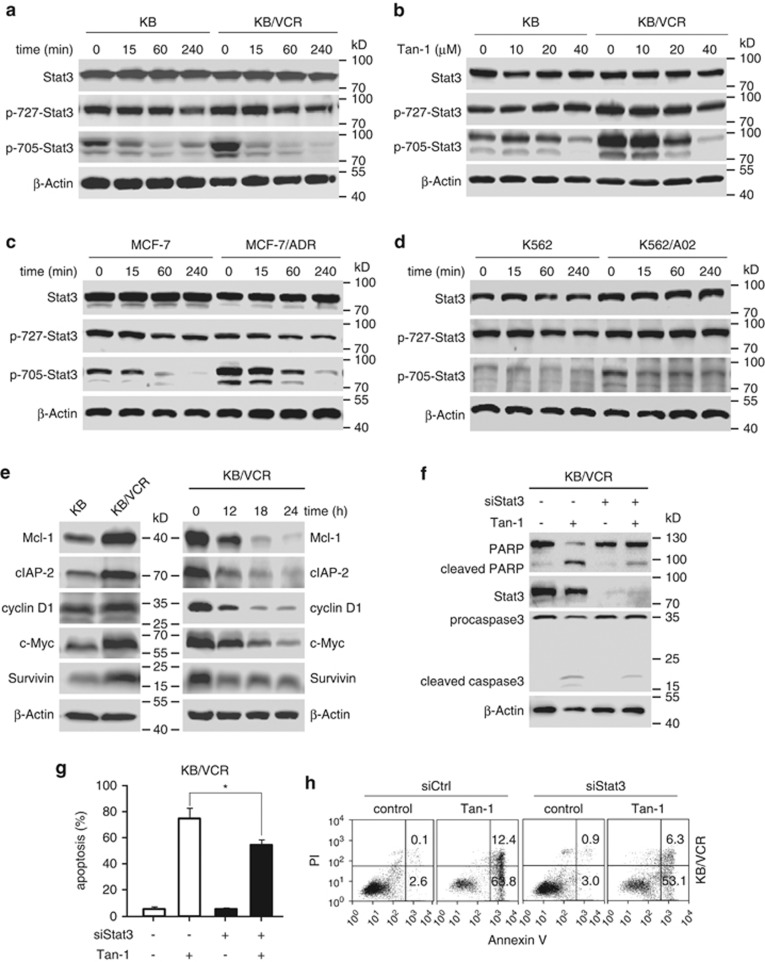
As the tanshinone-1-induced cytotoxicity and apoptosis in MDR cells occurs in a drug-transporter-independent fashion, additional factor(s) most likely contribute to the sensitivity of those cells to this natural product. Stat3 has been reported to be involved in tumor drug resistance.33, 34, 35, 36, 37 Therefore, we examined Stat3 protein expression and phosphorylation levels of Tyr705 and Tyr727; phosphorylation of these residues differentially regulates the transcriptional activity of Stat3.38, 39 There was no apparent difference in the levels of total Stat3 and p-727-Stat3 in all three tested MDR sublines when compared with their respective parental cell lines (Figures 2a–d). However, a significant enhancement of Tyr705 phosphorylation of Stat3 was observed in the MDR cells relative to the parental cells (Figures 2a–d). Importantly, treating both MDR and parental cells with tanshinone-1 resulted in a quick depletion of p-705-Stat3 but only a slight change in the p-727-Stat3 levels and no discernible change in total Stat3 protein levels (Figures 2a–d). Similarly, MDR KB/VCR cells had higher protein expression of Stat3-target genes, including Mcl-1, cIAP-2, cyclin D1, c-Myc and Survivin than KB cells (Figure 2e, left). Tanshinone-1 also significantly reduced these proteins in KB/VCR cells (Figure 2e, right). Additionally, downregulating Stat3 expression partially rescued apoptotic induction and reversed the tanshinone-1-induced cleavage of caspase-3 and PARP in KB/VCR cells (Figures 2f–h). These results suggest that the depletion of p-705-Stat3 caused by the treatment with tanshinone-1 could partially contribute to its killing of MDR cells. Unexpectedly, however, reduction of Stat3 expression in MDR MCF-7/ADR and KB/VCR cells did not enhance the cellular sensitivity to the anticancer drugs vincristine and adriamycin (Supplementary Figures S3a and b). Moreover, constitutive activation of Stat3 in parental KB cells did not significantly change their sensitivity to tanshinone-1 (Supplementary Figures S3c–h). The data indicate that other mechanism(s) may be involved in the response to tanshinone-1 in these cells.
Figure 2.
Tanshinone-1 reduced Tyr705 phosphorylation of cellular Stat3, which contributed to apoptotic induction. (a–d) Tanshinone-1 (Tan-1) led to the dephosphorylation of Stat3 at Tyr705 in a time- and concentration-dependent manner in MDR and corresponding parental cells. The cells were treated with 40 μM tanshinone-1 for the indicated time (a, c and d) or with gradient concentrations of tanshinone-1 for 4 h (b), and then detected by western blotting. (e) Tanshinone-1 reduced the protein expression of the Stat3-targeted genes. KB and KB/VCR cells were treated with or without 20 μM tanshinone-1 for the indicated time and then western blotting for cellular levels of Mcl-1, cIAP-2, cyclin D1, c-Myc, and Survivin proteins was done. Basal levels (left) and reduced levels (right) of the examined proteins. (f) The siRNA for Stat3 (siStat3) decreased Stat3 expression and reversed the cleavage of procaspase 3 and PARP induced by tanshinone-1. KB/VCR cells were transfected with siStat3 for 48 h and then treated with 20 μM tanshinone-1 for another 24 h. The cell lysates were subjected to western blotting. (g and h) siStat3 rescued apoptotic induction by tanshinone-1. KB/VCR cells were transfected with siStat3 for 48 h and then treated with 20 μM tanshinone-1 for another 18 h. The resulting cells were stained with Annexin V/PI and analyzed by flow cytometry. The apoptosis rates from three independent experiments were expressed as mean±S.D.; *P<0.05 (g). Representative histograms are shown in h
Tanshinone-1 activates cellular phosphatases
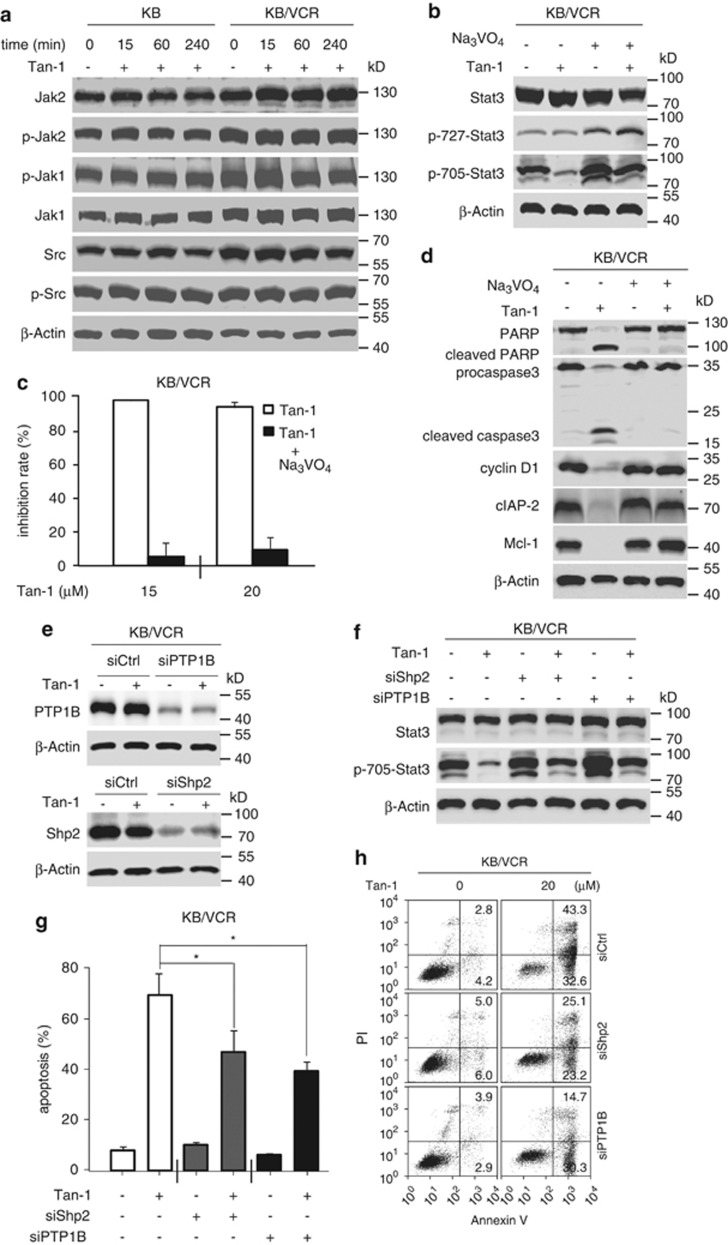
We next examined how tanshinone-1 causes depletion of p-705-Stat3 in these cells. One possibility is that tanshinone-1 reduces upstream kinase(s) of Stat3, such as Jak1, Jak2, and Src,10, 21 at either protein levels or the levels of their phosphorylated (active) counterparts. However, no apparent changes were observed in either their total protein or phosphorylation levels in both KB/VCR and KB cells (Figure 3a). Tanshinone-1 did not inhibit the kinase activity of the Jak1, Jak2 and Src (Supplementary Table S1). Therefore, we further examined phosphatases, as they also regulate the phosphorylation levels of cellular proteins.12 Pretreatments of KB/VCR cells for 30 min with Na3VO4, a pan phosphatase inhibitor, prevented the reduction of p-705-Stat3 (Figure 3b), proliferation inhibition and apoptotic induction mediated by tanshinone-1 (Figures 3c and d), suggesting that phosphatases may participate in the biological effects of tanshinone-1.
Figure 3.
Tanshinone-1 (Tan-1) activated cellular phosphatases. (a) Tan-1 did not inhibit upstream kinases of Stat3. Cells were treated with 40 μM Tan-1 and analyzed by western blotting. (b) Na3VO4 prevented the decrease of phospho-705-Stat3. Cells were pretreated with 20 μM Na3VO4 for 30 min, followed by treatment with 40 μM Tan-1 for 4 h, and were then analyzed by western blotting. (c) Na3VO4 completely prevented the Tan-1-mediated proliferation inhibition. Cells were pretreated with 20 μM Na3VO4 for 30 min, followed by the treatment with Tan-1 for 72 h, and were then analyzed by SRB assays and expressed as mean±S.D. (d) Na3VO4 decreased cleavage of procaspase 3 and PARP and the reduction of cyclin D1, Mcl-1, and cIAP-2. Cells were pretreated with 20 μM Na3VO4 for 30 min, exposed to 20 μM Tan-1 for 24 h, and then analyzed by western blotting. (e and f) Knockdown of Shp2 or PTP1B reversed the reduction of phospho-705-Stat3. Cells were transfected with siRNA against Shp2 and PTP1B for 48 h (e). The cells were then treated with 20 μM Tan-1 for 4 h (f) and analyzed by western blotting. (g and h) Knockdown of Shp2 and PTP1B reduced the number of Annexin V/PI-positive cells. KB/VCR cells were transfected with siRNA against Shp2 and PTP1B for 48 h, followed by treatment with 20 μM Tan-1 for 18 h, stained with Annexin V/PI, and then analyzed by flow cytometry. The apoptosis rates from three independent experiments were expressed as mean±S.D.; *P<0.05 (g). Representative histograms are shown in h
There are at least three phosphatases, Shp1, Shp2, and PTP1B, which have been reported to regulate the phosphorylation levels of cellular Stat3 at its Tyr705.14, 40, 41 Compared with Shp2 and PTP1B, Shp1 was expressed at very low levels in the MDR KB/VCR and K562/A02 sublines and their respective parental cell lines, whereas higher expression was found in the MCF-7 cell lines (Supplementary Figure S4). The differential expression of Shp1 indicates only a slight possibility that Shp1 mediates the effect of tanshinone-1. Therefore, we focused on Shp2 and PTP1B. Treatments with tanshinone-1 did not significantly change their protein levels in KB/VCR cells (Figure 3e). However, reduction of either Shp2 or PTP1B with specific siRNA against Shp2 (siShp2) or PTP1B (siPTP1B) reversed the tanshinone-1-mediated depletion of p-705-Stat3 (Figures 3e and f). Additionally, both siShp2 and siPTP1B mitigated the apoptotic induction by tanshinone-1 in KB/VCR cells (Figures 3g and h). In a cell-free system, however, tanshinone-1 did not cause significant alterations in the enzymatic activities of Shp2, PTP1B, and four other phosphatases (Supplementary Table S2). The data indicate that tanshinone-1 likely activates cellular Shp2 and PTP1B indirectly, resulting in dephosphorylation of p-705-Stat3.
Tanshinone-1 enhances phosphorylation levels of AKT, ERK, and p38
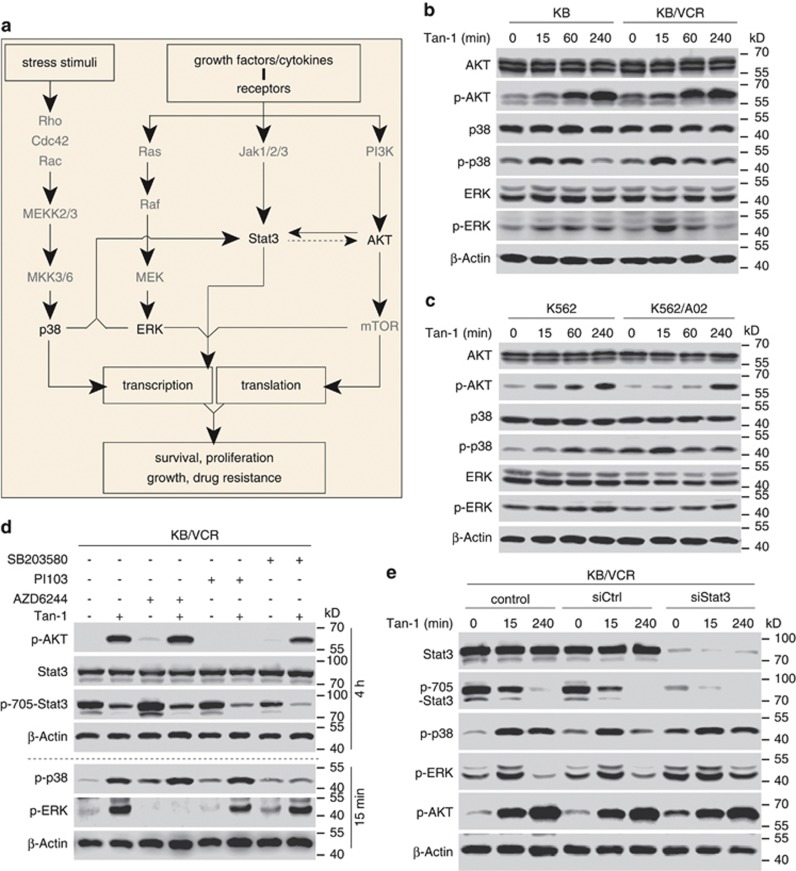
The Stat3 signaling pathway has been shown to interact with the PI3K-AKT-mTOR, MAPK, and p38 pathways20, 42, 43, 44, 45, 46 (Figure 4a). Therefore, we examined the effects of tanshinone-1 on the key signaling molecules, AKT, ERK, and p38, of these pathways. Treatments with tanshinone-1 led to increased phosphorylation of AKT, ERK, and p38 in distinct patterns but did not change their total protein (Figure 4b). In both MDR KB/VCR and parental KB cells exposed to tanshinone-1, AKT phosphorylation increased progressively; in contrast, both ERK and p38 phosphorylation rose relatively rapidly at first, before returning to basal levels (Figure 4b). A similar phenomenon was observed in MDR K562/A02 and parental K562 cells (Figure 4c). However, tanshinone-1 did not change the enzymatic activities of the upstream tyrosine kinases of these pathways, including EGFR, FGFR1, PDGFR-β, and c-Src (Supplementary Table S1). Pretreatments with the respective phosphorylation inhibitors of AKT (PI103), ERK (AZD6244), and p38 (SB203580) eliminated the increased phosphorylation but did not reverse the reduction of p-705-Stat3 in cells exposed to tanshinone-1 (Figure 4d and Supplementary Figure S5). Moreover, specific siRNA against stat3 slightly increased phosphorylation of AKT, ERK, and p38 in MDR KB/VCR cells and decreased changes in ERK and p38 phosphorylation mediated by tanshinone-1 (Figure 4e). The results suggest that the increased phosphorylation of AKT, ERK, and p38 may be due to the decreased Tyr705 phosphorylation of Stat3. The secondary activation of the pathways containing AKT, ERK and p38 could alleviate, to a certain degree, the deleterious effects of tanshinone-1 on both MDR and parental cells, thereby acting as a compensatory mechanism.
Figure 4.
Tanshinone-1 activated p38, AKT, and ERK signaling. (a) Signaling pathways consisting of Stat3, p38, AKT, and ERK, may crosstalk and influence their respective biological effects. (b and c) Phosphorylation was analyzed by western blotting. Tanshinone-1 (40 μM, Tan-1) enhanced phosphorylation of p38, AKT, and ERK in MDR KB/VCR and parental KB cells (b) and in MDR K562/A02 and parental K562 cells (c). (d) The impact of p38, AKT, and ERK inhibitors on Tan-1-mediated enhancement of cellular p38, AKT, and ERK phosphorylation. KB/VCR cells were pretreated with PI103 (10 μM, AKT) or AZD6244 (5 μM, ERK) for 30 min or SB203580 (50 μM, p38) for 10 min. The cells were then exposed to 20 μM of Tan-1 for 15 min (p-p38 and p-ERK) or 4 h (p-AKT and p-705-Stat3) followed by western blotting analyses. (e) The impact of siStat3-induced Stat3 reduction on the Tan-1-mediated phosphorylation of p38, AKT, and ERK. KB/VCR cells were pretreated with siCtrl or siStat3 for 48 h. The cells were then exposed to 40 μM of Tan-1 for the indicated time and were analyzed by western blotting
Phosphorylation inhibitors of AKT, ERK, and p38 potentiate cell killing by tanshinone-1
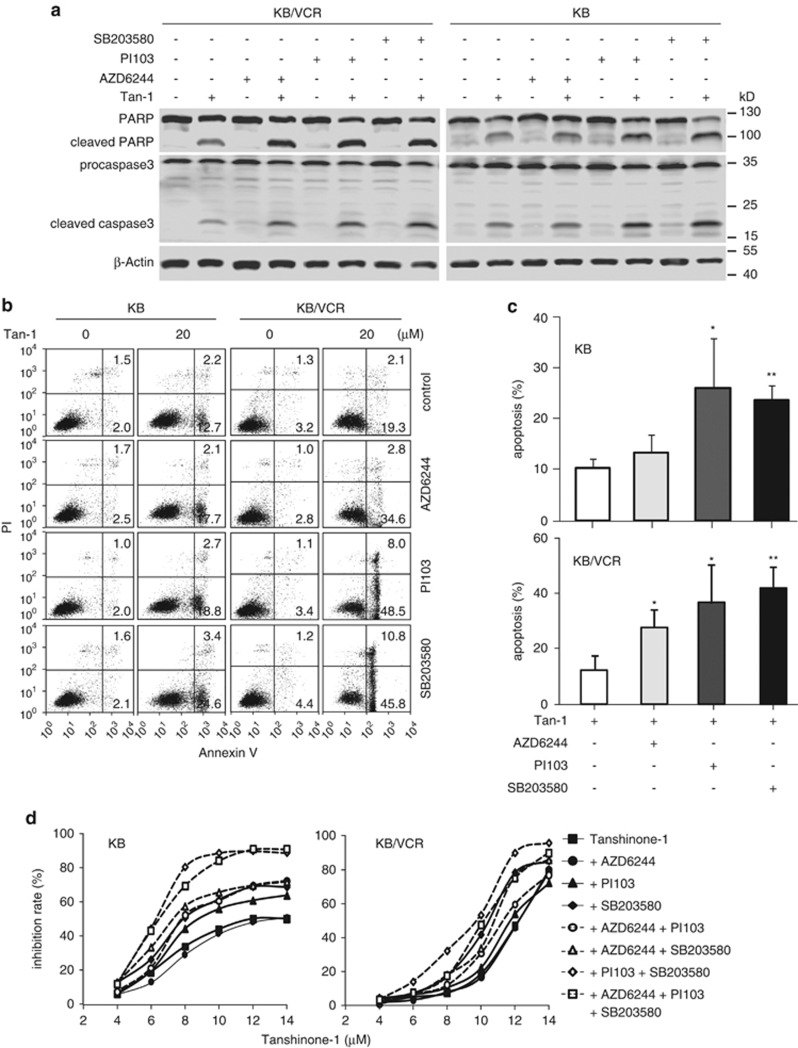
We further examined whether suppressing the tanshinone-1-induced increase in AKT, ERK, and p38 phosphorylation affected cell killing. Cotreatments with phosphorylation inhibitors PI103, SB203580, or AZD6244 significantly enhanced the apoptotic induction by tanshinone-1 in both MDR KB/VCR and parental KB cells. The ERK phosphorylation inhibitor AZD6244 showed a relatively weak potency compared with PI103 and SB203580 (Figures 5a–c). Each inhibitor also sensitized MDR and parental cells to tanshinone-1 to different degrees (Figure 5d). The p38 inhibitor SB203580 resulted in the most potent sensitization to tanshinone-1; the inhibitor PI103 had slightly weaker potentiation; and the inhibitor AZD6244 had the weakest or no potentiation. Notably, the combination of SB203580 with PI103 displayed nearly equivalent (in the parental KB cells) or even slightly more potent (in MDR KB/VCR cells) sensitization than a combination of all three inhibitors (Figure 5d). These data suggest that, although tanshinone-1 may increase the phosphorylation levels of AKT, ERK, and p38, the PI3K-AKT and p38 signaling pathways likely have more important roles in potentiating the cellular sensitivity to tanshinone-1 than the ERK-involving MAPK pathways. Moreover, a combination of phosphorylation inhibitors of AKT and p38 could be the primary choice for the future investigations of experimental therapeutics related to tanshinone-1.
Figure 5.
Cotreatments of p38, AKT and ERK inhibitors potentiated the tanshinone-1 (Tan-1)-induced apoptosis and cytotoxicity. (a–c) MDR KB/VCR and parental KB cells were pretreated with PI103 (10 μM, AKT) or AZD6244 (5 μM, ERK) for 30 min or SB203580 (50 μM, p38) for 10 min. The cells were then exposed to 20 μM of Tan-1 for 14 h. The cleavage of procaspase3 and PARP were detected by western blotting (a) and apoptotic induction was examined by flow cytometry (b). The apoptosis percentage from three independent experiments was expressed as mean±S.D.; *P<0.05; **P<0.01 (c). (d) Cells were pretreated with different combinations of PI103 (10 μM, 30 min), AZD6244 (5 μM, 30 min), or SB203580 (50 μM, 10 min), followed by exposure to gradient concentrations of Tan-1 for 14 h. The growth inhibition rates were analyzed by SRB assays. The data from three independent experiments were expressed as mean (S.D. was not shown for clearness and readability)
Discussion
As a traditional Chinese medicine, Salvia miltiorrhiza has been shown to have reliable therapeutic effectiveness and excellent safety based on its long-term use in clinics.22 In this study, tanshinone-1, one of its major ingredients, was shown to possess the ability to kill MDR tumor cells as shown by six prominent characteristics: 1) tanshinone-1 can directly kill human MDR tumor cells derived from different tissues and established with different anticancer drugs, indicating its potentially wide spectrum of action. 2) Tanshinone-1 is more potent at inducing cytotoxicity and apoptosis in MDR tumor cells than in the corresponding parental cells; one possible explanation of this may be more cellular accumulation of the drug in the former than in the latter. 3) Tanshinone-1 kills MDR cells independently of drug transporters; it is not affected by their drug-transporting function and also does not alter their expression. 4) Unlike classical approaches that target drug transporters, tanshinone-1 kills MDR tumor cells by other mechanism(s) of action. For example, tanshinone-1 reduces the Tyr705 phosphorylation levels of Stat3 and subsequently decreases the expression of various antiapoptotic/prosurvival factors. 5) The tanshinone-1-induced reduction of p-705-Stat3 is likely associated with activating phosphatases of Stat3 (Shp2 and PTP1B but is independent of Stat3 upstream signaling molecules such as Jaks, Src, or receptors. And finally 6) tanshinone-1-induced transcriptional inactivation of Stat3 may result in compensatory activation of other related signaling networks, as evidenced by the increased phosphorylation of p38, AKT, and ERK; additionally, a combination of the phosphorylation inhibitors for p38, AKT, and ERK potentiates tanshinone-1-induced cytotoxicity (Supplementary Figure S6a).
These features make tanshinone-1 stand out as a potential candidate of natural origin that can kill MDR tumor cells. The majority of small-molecule anticancer drugs derived from natural products, including taxol and, recently reported, trabectedin, are substrates of drug transporters such as P-gp, MRP1, and/or BRCP, and their therapeutic effectiveness could be severely impaired by MDR mechanisms.47, 48 One advantage of tanshinone-1, compared with those clinical drugs, is that its anticancer effect is independent of the MDR mechanism. Therefore, the compound is toxic to any cancer cells, even those with the expression of the MDR phenotype. Another advantage of tanshinone-1, compared with MDR reversal agents such as verapamil,4 is that it does not interfere with either the expression or function of drug transporters. Therefore, tanshinone-1 kills MDR tumor cells directly while sparing normal tissues that also express drug transporters.
Tanshinone-1-induced cell killing in the MDR and parental cells might partially be dependent on its reduction of p-705-Stat3, an active form of Stat3. Stat3 functions as a transcription factor that promotes survival and prevents apoptosis by regulating the expression of prosurvival/antiapoptotic genes. In MDR KB/VCR cells, Stat3 siRNA led to the decrease in tanshinone-1-induced apoptosis, indicating that its effect is partially dependent on Stat3 signaling. Compared with parental tumor cells, MDR cells have enhanced levels of p-705-Stat3 and its downstream gene products, including Mcl-1, cIAP-2, cyclin D1, c-Myc, and Survivin, suggesting that the Stat3 signaling might have more important roles in MDR cells. Tanshinone-1 elicits significant reduction of these proteins in MDR tumor cells, and this reduction might be another possible cause for the stronger potency of tanshinone-1 for inducing cytotoxicity and apoptosis in MDR tumor cells. However, Stat3 siRNA did not affect the sensitivity of MDR cells to vincristine or adriamycin and constitutively activated Stat3 did not significantly change the sensitivity of parental KB cells to tanshinone-1 either, suggesting that Stat3 is not the common mediator of cellular responses to tanshinone-1 in parental and MDR cells. Therefore, other unknown mechanism(s) may be involved, which deserves further investigating.
On the other hand, tanshinone-1-triggered reduction of p-705-Stat3 may lead to secondary activation of related signaling networks. In the cells, Jak-Stat, p38MAPK, PI3K-AKT-mTOR, and Ras-Raf-MEK-ERK pathways are interdependent, interconnected signaling pathways that are responsible for regulating, integrating, and balancing various signals from exogenous or endogenous stimulators, including growth factors, cytokines, and stress stimuli (Figure 4a). These interaction networks precisely control cell survival, proliferation, growth, apoptosis, and the cell's stress response to ensure that biological systems function normally. However, the robustness and redundancy of such networks could impair the therapeutic sensitivity of, for example, cancers. Under these conditions, network pharmacology may provide solutions, particularly for drug discovery.49, 50 Our data show that tanshinone-1 activates p38, AKT, and ERK signaling networks as a secondary effect of inhibiting p-705-Stat3 signaling. Phosphorylation inhibitors of these proteins could enhance cytotoxicity and apoptotic induction by tanshinone-1, though to differing degrees. Our results provide important data with regards to combining tanshinone-1 (and its derivatives) with these inhibitors, especially p38 inhibitors and AKT inhibitors. They also lay the foundations for future multitarget drug design targeting p-705-Stat3-involved signaling networks (Supplementary Figure S6a).
These findings on tanshinone-1 may lead to new strategies for overcoming MDR. Typically, the agents used to sensitize cells to anticancer drugs have targeted drug transporters by either interfering with transporter functions or reducing transporter expression. These agents themselves generally lack anticancer activities and may reduce the physiological functions of normal tissues such as the small intestine, liver, and blood-brain barrier4 which would likely lead to severe toxicity. In contrast, agents such as tanshinone-1 can directly kill MDR tumor cells or inhibit their growth and proliferation but are not substrates of MDR transporters and do not interfere with either function or expression of the transporters, resulting in much lower risks for toxicity (Supplementary Figure S6b). Here, we define these agents as anti-MDR drugs. Another potential advantage of anti-MDR drugs is that they are likely to have enhanced oral bioavailability and increased permeability into the brain and other sites where penetration is prevented by drug transporters.4 Therefore, developing anti-MDR drugs could be a feasible strategy for treating MDR cancers in the future.
This study characterizes several important features of tanshinone-1, presents a model compound for MDR cancer therapy, and advances a strategy for overcoming MDR through the use of anti-MDR drugs. Several important issues remain to be resolved, however. Tanshinone-1 has very low solubility, and additionally, its overall anticancer activity is relatively weak. Therefore, chemical modifications are required to allow tanshinone-1 to enter drug development. For this purpose, it is necessary to further investigate and validate the precise molecular target(s) of tanshinone-1 to establish exact structure–effect relationships that can guide chemists in their manipulations to obtain drug candidates with ideal druggable properties. Additionally, more detailed mechanistic studies are required to provide a more in-depth analysis of the impact of tanshinone-1 on cellular signaling networks. These studies may also provide critical data for potential multitarget drug designs.
Materials and Methods
Cell culture
The KB, K562, NIH3T3, and MCF-7 parental cell lines were purchased from the American Type Culture Collection (Rockville, MD, USA). The QSG7701 and HL7702 cell lines were purchased from The Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China).The MDR KB/VCR and MCF-7/ADR sublines were obtained from the Zhongshan University of Medical Sciences (Guangzhou, China) and the MDR K562/A02 subline was from the Institute of Hematology, Chinese Academy of Medical Sciences (Tianjin, China). All the cells were cultured and used as described previously.6, 7, 51
Cytotoxicity assays
Cytotoxic effects on the cells were assessed by sulforhodamine B (SRB) assays for solid tumor cells or by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assays for suspension cells as reported previously.6, 17, 52 The concentration required for 50% inhibition (IC50) of the tested cells was calculated using the Logit method. The RF for each examined drug was calculated as the ratio of the IC50 value in a MDR subline to that in its parental cell line.
Materials
Tanshinone-1 and Tanshinone-2A was purchased from Shanghai Tauto Biotech Co., Ltd (China). Vincristine (VCR), verapamil (VER), SRB, and MTT were purchased from Sigma (St. Louis, MO). Adriamycin (ADR) was obtained from Dalian Meilun Biology Technology Co., Ltd (Dalian, China). Antibodies against phospho-Stat3 (Tyr705), phospho-Stat3 (Ser727), Stat3, Src, phospho-Src (Ser416), caspase-3, caspase-9, phospho-AKT, AKT, phospho-ERK, ERK, phospho-p38, p38, Mcl-1, and cyclin D1 were obtained from Cell Signaling Technology (Beverly, MA, USA). Antibodies against MRP1, cIAP-2, Survivin, Jak1, phospho-Jak1, Jak2, and phospho-Jak2 were obtained from Abcam (Cambridge, MA, USA). The antibody against β-Actin was purchased from Abgent (San Diego, CA, USA). Antibodies against P-gp, Caspase-8 and PARP were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The antibody against Shp2, Shp1, PTP1B was purchased from Epitomics (Burlingame, CA, USA). The antibody against c-Myc was from R&D Systems (Minneapolis, MN, USA). Rhodamine 123 (Rh123) and 5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine iodide (JC-1) were from Beyotime Institute of Biotechnology (Haimen, China). The Annexin V-FITC apoptosis detection kit was purchased from KeyGen Biotech (Naijing, China).
Western blotting analyses
Cell lysates were analyzed using standard western blotting to examine the levels of the indicated proteins.7, 17, 52
Reverse transcript polymerase chain reaction (RT-PCR)
Cells were treated with tanshinone-1 at the indicated concentrations for 12 h. The total cell RNA was extracted with the TRIzol (Invitrogen, Carlsbad, CA, USA) reagent and reverse transcribed into cDNA. The cDNA was then subjected to PCR. The primers were as follows: 5′-GGATGCAGAAGGAGATCACTG-3′ (forward), 5′-CGATCCACACGGAGTACTTG-3′ (reverse) for β-actin;53 5′-GTATTCAACTATCCCACCC-3′ (forward), 5′-GCTTTATTTCTTTGCCATC-3′ (reverse) for mdr-1; 5′-CTGACAAGCTAGACCATGAATGT-3′ (forward), 5′-TTACACCAAGCCGGCGTCTTT-3′ (reverse) for MRP1.
Flow cytometry
Cell apoptosis, Rh123 efflux, and the loss of mitochondria membrane potential (MMP) were detected with a FACS Calibur cytometer (BD Biosciences, San Jose, CA, USA). Cells were treated with tanshinone-1 and collected for apoptosis detection as instructed by the Annexin V-FITC apoptosis detection kit. For the Rh123 efflux assay, cells were treated with 5 μM verapamil or 40 μM tanshinone-1 for 1.5 h before adding Rh123 (1 μg/ml) for 30-min treatments. All the cells were collected and suspended in PBS for 1.5 h. To examine the loss of MMP, cells were treated with tanshinone-1 at the indicated concentrations for 18 h and collected for JC-1 staining (1 μM). Samples were analyzed by flow cytometry, and 10 000 events were counted each time.
Gene knockdown using small interfering RNA (siRNA) technology
Cells were seeded in 12-well plates at 1.5 × 106 cells/ml. The specific siRNA, mixed with Lipofectamine RNAi MAX, was added into the culture medium without antibiotics. Cells were incubated for 48 h before treatment with the indicated drugs. The sequences of the siRNA target genes were: 5′-GAGUUGAAUUAUCAGCUUATT-3′ for stat3 (1); 5′-GUUUGGAAAUAAUGGUGAATT-3′ for stat3 (2);17 5′-GAATCCTATGGTGGAAACA-3′ for Shp2 (1);54 5′-AAGAATATGGCGTCATGCGTG-3′ for Shp2 (2);55 5′-UAGGUACAGAGACGUCAGU-3′ for PTP1B (1); 5′-UGACCAUAGUCGGAUUAAA-3′ for PTP1B (2), and 5′-AAAUCAACGGAAGAAGGGUCU-3′ for PTP1B (3).56
Drug accumulation assays
KB and KB/VCR cells (1 × 107 cells) were seeded in 10-cm dishes, and were treated with tanshinone-1 for the indicated time. The cells were then scraped and washed twice with PBS. The samples were prepared as previously described.57 The 10 μl of final sample was injected into the high performance liquid chromatography with a ZORBAX SB-C18 Column (4.6 × 150 mm, 5 μm, Agilent, Santa Clara, CA, USA) using 77:23(v/v) methanol/water as the mobile phase at a flow rate of 1.0 ml/min. Concentrations of tanshinone-1 in each sample eluted from the column were detected at 254 nm.
In vitro enzymatic activity assays
The activity of phosphatases was detected by BPS Bioscience Inc. (San Diego, CA, USA). All assays were conducted at room temperature for 15 min. The enzymatic reactions for phosphatases were conducted in duplicate in a transparent 96-well plate. Each well contained 200 μl of reaction buffer (20 mM HEPES, pH 7.4, 100 mM NaCl, 1 mM EDTA, 3 mM DTT), 1 mM pNPP, a phosphatase enzyme, and the test compound. The reaction was initiated by adding a phosphatase, and the reaction progress was monitored continuously for 15 min at an absorbance of 405 nm using a Tecan Infinite M1000 plate reader. The luminescence data were analyzed using the computer software, GraphPad Prism. In the absence of compounds, the slope (VT) of the data set was defined as 100% activity. The percent activity in the presence of each compound was calculated according to the following equation: % activity=(VC/VT) × 100, where VC is the slope of the data set in the presence of the compound.
The tyrosine kinase activity assay was detected by enzyme-linked immunosorbent assays as reported previously.51 The inhibitory activity of tanshinone-1 against Jak1 was tested by Reaction Biology Corporation (Malvern, PA, USA); the impact of tanshinone-1 on Jak2 and Jak3 was examined by BPS Bioscience (San Diego, CA, USA).
Expression of constitutively activated Stat3
It is reported that residue substitutions of the mouse Stat3 at A661 and A663 with cysteine result in constitutive activation of Stat3.58, 59 We amplified the human wild-type stat3 expression plasmid from the HG10034-M-H clone (Sino Biological Inc., Beijing, China), and constructed an expression plasmid containing constitutively activated human Stat3 (pCMV-H3C) by site-directed mutagenesis as reported previously;58, 59 the mouse constitutively activated Stat3 plasmids were gifts from Drs Hua Yu and Jie-Hui Deng (Beckman Research Institute, USA) and Dr. James Darnell (The Rockefeller University, USA). Cells were seeded in 12-well plates at 1.5 × 106 cells per ml and incubated overnight. And then the indicated plasmids were transfected into the KB cells for indicated time before the treatment with tanshinone-1.
Statistical analysis
Data were presented as mean±S.D., and statistical significance was assessed using a Student's t-test. Differences were considered significant at P<0.05.
Acknowledgments
We sincerely thank Drs Hua Yu, Jie-Hui Deng (Beckman Research Institute, USA) and Dr James Darnell (The Rockefeller University, USA) for their kind gift of the plasmid of the constitutively activated Stat3. This work was supported by grants from the National Natural Science Foundation of China (NSFC) (No. 81025020, No.81202547 and No. 81021062), the National Basic Research Program of China (No. 2012CB932502), the National Science & Technology Major Project of China (No. 2012ZX09301-001-002), the ‘Interdisciplinary Cooperation Team' Program for Science and Technology Innovation of the Chinese Academy of Sciences, the Science and Technology Commission of Shanghai Municipality (STCSM) (No. 13XD1404200), and the State Key Laboratory of Drug Research.
Glossary
- ADR
adriamycin
- BCRP
breast cancer resistance protein
- cIAP-2
cellular inhibitor of apoptosis 2
- EC-Stat3
ectopic Stat3
- EGFR
epidermal growth factor receptor
- EN-Stat3
endogenous Stat3
- ERK
extracellular signal regulated kinase
- FGFR1
fibroblast growth factor receptor 1
- H3C
constitutively activated human Stat3
- IC50
concentration required for 50% inhibition
- Jak
Janus kinase
- MAPK
mitogen-activated protein kinases
- M3C
mouse constitutively activated Stat3
- Mcl-1
myeloid cell leukemia 1
- MDR
multidrug resistance
- MEK
mitogen-activated protein kinase kinase
- MMP
mitochondria membrane potential
- MRP1
MDR-associated protein 1
- mTOR
mammalian target of rapamycin
- p38
p38 MAP kinase
- PARP
poly(ADP-ribose) polymerase
- PDGFR-β
platelet-derived growth factor receptor β
- P-gp
P-glycoprotein
- PI3K
phosphatidylinositide 3-kinases
- PTP1B
protein-tyrosine-phosphatase 1B
- RF
resistance factor
- Shp1
SH-2-containing phosphatase 1
- Shp2
SH-2-containing phosphatase 2
- Stat3
signal transducer and activator of transcription 3
- VCR
vincristine
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by C Munoz-Pinedo
Supplementary Material
References
- Pluchino KM, Hall MD, Goldsborough AS, Callaghan R, Gottesman MM. Collateral sensitivity as a strategy against cancer multidrug resistance. Drug Resist Updat. 2012;15:98–105. doi: 10.1016/j.drup.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozik A, Hegedus C, Erdei Z, Hegedus T, Ozvegy-Laczka C, Szakacs G, et al. Tyrosine kinase inhibitors as modulators of ATP binding cassette multidrug transporters: substrates, chemosensitizers or inducers of acquired multidrug resistance. Expert Opin Drug Metab Toxicol. 2011;7:623–642. doi: 10.1517/17425255.2011.562892. [DOI] [PubMed] [Google Scholar]
- Gillet JP, Gottesman MM. Overcoming multidrug resistance in cancer: 35 years after the discovery of ABCB1. Drug Resist Updat. 2012;15:2–4. doi: 10.1016/j.drup.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Amiri-Kordestani L, Basseville A, Kurdziel K, Fojo AT, Bates SE. Targeting MDR in breast and lung cancer: discriminating its potential importance from the failure of drug resistance reversal studies. Drug Resist Updat. 2012;15:50–61. doi: 10.1016/j.drup.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- Miao ZH, Tang T, Zhang YX, Zhang JS, Ding J. Cytotoxicity apoptosis induction and downregulation of MDR-1 expression by the anti-topoisomerase II agent, salvicine, in multidrug-resistant tumor cells. Int J Cancer. 2003;106:108–115. doi: 10.1002/ijc.11174. [DOI] [PubMed] [Google Scholar]
- Miao ZH, Ding J. Transcription factor c-Jun activation represses mdr-1 gene expression. Cancer Res. 2003;63:4527–4532. [PubMed] [Google Scholar]
- Choi BH, Kim CG, Lim Y, Shin SY, Lee YH. Curcumin down-regulates the multidrug-resistance mdr1b gene by inhibiting the PI3K/Akt/NF kappa B pathway. Cancer Lett. 2008;259:111–118. doi: 10.1016/j.canlet.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Yu B, Li MH, Wang W, Wang YQ, Jiang Y, Yang SP, et al. Pseudolaric acid B-driven phosphorylation of c-Jun impairs its role in stabilizing HIF-1alpha: a novel function-converter model. J Mol Med (Berl) 2012;90:971–981. doi: 10.1007/s00109-012-0865-4. [DOI] [PubMed] [Google Scholar]
- Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- Guo Y, Xu F, Lu T, Duan Z, Zhang Z. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat Rev. 2012;38:904–910. doi: 10.1016/j.ctrv.2012.04.007. [DOI] [PubMed] [Google Scholar]
- Levy DE, Darnell JE. STATs: Transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- Yue P, Turkson J. Targeting STAT3 in cancer: how successful are we. Expert Opin Investig Drugs. 2009;18:45–56. doi: 10.1517/13543780802565791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blechacz BR, Smoot RL, Bronk SF, Werneburg NW, Sirica AE, Gores GJ. Sorafenib inhibits signal transducer and activator of transcription-3 signaling in cholangiocarcinoma cells by activating the phosphatase shatterproof 2. Hepatology. 2009;50:1861–1870. doi: 10.1002/hep.23214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi A, Asano T, Kuroda K, Sato A, Asakuma J, Ito K, et al. STAT3 inhibitor WP1066 as a novel therapeutic agent for renal cell carcinoma. Br J Cancer. 2010;102:1592–1599. doi: 10.1038/sj.bjc.6605691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimoe GI, Liu A, Lin L, Wei CC, Schwartz EB, Bhasin D, et al. Small molecules, LLL12 and FLLL32, inhibit STAT3 and exhibit potent growth suppressive activity in osteosarcoma cells and tumor growth in mice. Invest New Drugs. 2012;30:916–926. doi: 10.1007/s10637-011-9645-1. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Miao ZH, Xu L, Yu B, Gong JX, Tong LJ, et al. Drug transporter-independent liver cancer cell killing by a marine steroid methyl spongoate via apoptosis induction. J Biol Chem. 2011;286:26461–26469. doi: 10.1074/jbc.M111.232728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen M, Thomas SM, Kim S, Yeh JI, Ferris RL, Johnson JT, et al. First-in-human trial of a STAT3 decoy oligonucleotide in head and neck tumors: implications for cancer therapy. Cancer Discov. 2012;2:694–705. doi: 10.1158/2159-8290.CD-12-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintas-Cardama A, Kantarjian H, Cortes J, Verstovsek S. Janus kinase inhibitors for the treatment of myeloproliferative neoplasias and beyond. Nat Rev Drug Discov. 2011;10:127–140. doi: 10.1038/nrd3264. [DOI] [PubMed] [Google Scholar]
- Lu Y, Zhou J, Xu C, Lin H, Xiao J, Wang Z, et al. JAK/STAT and PI3K/AKT pathways form a mutual transactivation loop and afford resistance to oxidative stress-induced apoptosis in cardiomyocytes. Cell Physiol Biochem. 2008;21:305–314. doi: 10.1159/000129389. [DOI] [PubMed] [Google Scholar]
- Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–2488. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- Dong Y, Morris-Natschke SL, Lee KH. Biosynthesis, total syntheses, and antitumor activity of tanshinones and their analogs as potential therapeutic agents. Nat Prod Rep. 2011;28:529–542. doi: 10.1039/c0np00035c. [DOI] [PubMed] [Google Scholar]
- Wang X, Morris-Natschke SL, Lee KH. New developments in the chemistry and biology of the bioactive constituents of Tanshen. Med Res Rev. 2007;27:133–148. doi: 10.1002/med.20077. [DOI] [PubMed] [Google Scholar]
- Wu LC, Lin X, Sun H. Tanshinone IIA protects rabbits against LPS-induced disseminated intravascular coagulation (DIC) Acta Pharmacol Sin. 2012;33:1254–1259. doi: 10.1038/aps.2012.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WY, Zhou X, Or PM, Kwan YW, Yeung JH. Tanshinone I increases CYP1A2 protein expression and enzyme activity in primary rat hepatocytes. Phytomedicine. 2012;19:169–176. doi: 10.1016/j.phymed.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Nizamutdinova IT, Lee GW, Son KH, Jeon SJ, Kang SS, Kim YS, et al. Tanshinone I effectively induces apoptosis in estrogen receptor-positive (MCF-7) and estrogen receptor-negative (MDA-MB-231) breast cancer cells. Int J Oncol. 2008;33:485–491. [PubMed] [Google Scholar]
- Nizamutdinova IT, Lee GW, Lee JS, Cho MK, Son KH, Jeon SJ, et al. Tanshinone I suppresses growth and invasion of human breast cancer cells, MDA-MB-231, through regulation of adhesion molecules. Carcinogenesis. 2008;29:1885–1892. doi: 10.1093/carcin/bgn151. [DOI] [PubMed] [Google Scholar]
- Li Y, Gong Y, Li L, Abdolmaleky HM, Zhou JR. Bioactive tanshinone I inhibits the growth of lung cancer in part via downregulation of Aurora A function. Mol Carcinog. 2012;52:535–543. doi: 10.1002/mc.21888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CY, Sher HF, Chen HW, Liu CC, Chen CH, Lin CS, et al. Anticancer effects of tanshinone I in human non-small cell lung cancer. Mol Cancer Ther. 2008;7:3527–3538. doi: 10.1158/1535-7163.MCT-07-2288. [DOI] [PubMed] [Google Scholar]
- Hegewisch-Becker S, Hanania EG, Fu S, Korbling M, Deisseroth AB, Andreeff M. Transduction of MDR1 into human and mouse haemopoietic progenitor cells: use of rhodamine (Rh123) to determine transduction frequency and in vivo selection. Br J Haematol. 1995;90:876–883. doi: 10.1111/j.1365-2141.1995.tb05209.x. [DOI] [PubMed] [Google Scholar]
- Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, et al. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9:215–236. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj NS, Washington MK, Merchant NB. Combined blockade of Src kinase and epidermal growth factor receptor with gemcitabine overcomes STAT3-mediated resistance of inhibition of pancreatic tumor growth. Clin Cancer Res. 2011;17:483–493. doi: 10.1158/1078-0432.CCR-10-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Xiao W, Wang L, Tian Z, Zhang J. Deactivation of signal transducer and activator of transcription 3 reverses chemotherapeutics resistance of leukemia cells via down-regulating P-gp. PLoS One. 2011;6:e20965. doi: 10.1371/journal.pone.0020965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvendiran K, Ahmed S, Dayton A, Kuppusamy ML, Rivera BK, Kalai T, et al. HO-3867, a curcumin analog, sensitizes cisplatin-resistant ovarian carcinoma, leading to therapeutic synergy through STAT3 inhibition. Cancer Biol Ther. 2011;12:837–845. doi: 10.4161/cbt.12.9.17713. [DOI] [PubMed] [Google Scholar]
- Chen KF, Tai WT, Liu TH, Huang HP, Lin YC, Shiau CW, et al. Sorafenib overcomes TRAIL resistance of hepatocellular carcinoma cells through the inhibition of STAT3. Clin Cancer Res. 2010;16:5189–5199. doi: 10.1158/1078-0432.CCR-09-3389. [DOI] [PubMed] [Google Scholar]
- Alas S, Bonavida B. Inhibition of constitutive STAT3 activity sensitizes resistant non-Hodgkin's lymphoma and multiple myeloma to chemotherapeutic drug-mediated apoptosis. Clin Cancer Res. 2003;9:316–326. [PubMed] [Google Scholar]
- Selvendiran K, Koga H, Ueno T, Yoshida T, Maeyama M, Torimura T, et al. Luteolin promotes degradation in signal transducer and activator of transcription 3 in human hepatoma cells: an implication for the antitumor potential of flavonoids. Cancer Res. 2006;66:4826–4834. doi: 10.1158/0008-5472.CAN-05-4062. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A, Xu B, Frank DA, Feldman GM, Cathcart MK. Monocyte 15-lipoxygenase expression is regulated by a novel cytosolic signaling complex with protein kinase C delta and tyrosine-phosphorylated Stat3. J Immunol. 2006;177:3771–3781. doi: 10.4049/jimmunol.177.6.3771. [DOI] [PubMed] [Google Scholar]
- Akasaki Y, Liu G, Matundan HH, Ng H, Yuan X, Zeng Z, et al. A peroxisome proliferator-activated receptor-gamma agonist, troglitazone, facilitates caspase-8 and -9 activities by increasing the enzymatic activity of protein-tyrosine phosphatase-1B on human glioma cells. J Biol Chem. 2006;281:6165–6174. doi: 10.1074/jbc.M505266200. [DOI] [PubMed] [Google Scholar]
- Rajendran P, Li F, Shanmugam MK, Vali S, Abbasi T, Kapoor S, et al. Honokiol inhibits signal transducer and activator of transcription-3 signaling, proliferation, and survival of hepatocellular carcinoma cells via the protein tyrosine phosphatase SHP-1. J Cell Physiol. 2012;227:2184–2195. doi: 10.1002/jcp.22954. [DOI] [PubMed] [Google Scholar]
- Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J. 2010;429:403–417. doi: 10.1042/BJ20100323. [DOI] [PubMed] [Google Scholar]
- Chen J. Multiple signal pathways in obesity-associated cancer. Obes Rev. 2011;12:1063–1070. doi: 10.1111/j.1467-789X.2011.00917.x. [DOI] [PubMed] [Google Scholar]
- Kristensen DM, Kalisz M, Nielsen JH. Cytokine signalling in embryonic stem cells. APMIS. 2005;113:756–772. doi: 10.1111/j.1600-0463.2005.apm_391.x. [DOI] [PubMed] [Google Scholar]
- Dhingra S, Bagchi AK, Ludke AL, Sharma AK, Singal PK. Akt regulates IL-10 mediated suppression of TNFalpha-induced cardiomyocyte apoptosis by upregulating Stat3 phosphorylation. PLoS One. 2011;6:e25009. doi: 10.1371/journal.pone.0025009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun HZ, Xu L, Zhou B, Zang WJ, Wu SF. Depletion of insulin receptor substrate 2 reverses oncogenic transformation induced by v-src. Acta Pharmacol Sin. 2011;32:611–618. doi: 10.1038/aps.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CP, Hsieh CH, Wu YS. The emergence of drug transporter-mediated multidrug resistance to cancer chemotherapy. Mol Pharm. 2011;8:1996–2011. doi: 10.1021/mp200261n. [DOI] [PubMed] [Google Scholar]
- Beumer JH, Franke NE, Tolboom R, Buckle T, Rosing H, Lopez-Lazaro L, et al. Disposition and toxicity of trabectedin (ET-743) in wild-type and mdr1 gene (P-gp) knock-out mice. Invest New Drugs. 2010;28:145–155. doi: 10.1007/s10637-009-9234-8. [DOI] [PubMed] [Google Scholar]
- Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol. 2008;4:682–690. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- Wang Z, Liu J, Yu Y, Chen Y, Wang Y. Modular pharmacology: the next paradigm in drug discovery. Expert Opin Drug Discov. 2012;7:667–677. doi: 10.1517/17460441.2012.692673. [DOI] [PubMed] [Google Scholar]
- Sun QM, Miao ZH, Lin LP, Gui M, Zhu CH, Xie H, et al. BB, a new EGFR inhibitor, exhibits prominent anti-angiogenesis and antitumor activities. Cancer Biol Ther. 2009;8:1640–1647. doi: 10.4161/cbt.8.17.9205. [DOI] [PubMed] [Google Scholar]
- Manzo SG, Zhou ZL, Wang YQ, Marinello J, He JX, Li YC, et al. Natural product triptolide mediates cancer cell death by triggering CDK7-dependent degradation of RNA polymerase II. Cancer Res. 2012;72:5363–5373. doi: 10.1158/0008-5472.CAN-12-1006. [DOI] [PubMed] [Google Scholar]
- Smit LA, Bende RJ, Aten J, Guikema JE, Aarts WM, van Noesel CJ. Expression of activation-induced cytidine deaminase is confined to B-cell non-Hodgkin's lymphomas of germinal-center phenotype. Cancer Res. 2003;63:3894–3898. [PubMed] [Google Scholar]
- Chen J, Yu WM, Daino H, Broxmeyer HE, Druker BJ, Qu CK. SHP-2 phosphatase is required for hematopoietic cell transformation by Bcr-Abl. Blood. 2007;109:778–785. doi: 10.1182/blood-2006-04-019141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng H, Liu KW, Guo P, Zhang P, Cheng T, McNiven MA, et al. Dynamin 2 mediates PDGFRalpha-SHP-2-promoted glioblastoma growth and invasion. Oncogene. 2012;31:2691–2702. doi: 10.1038/onc.2011.436. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Eden ER, White IJ, Tsapara A, Futter CE. Membrane contacts between endosomes and ER provide sites for PTP1B-epidermal growth factor receptor interaction. Nat Cell Biol. 2010;12:267–272. doi: 10.1038/ncb2026. [DOI] [PubMed] [Google Scholar]
- Huang M, Gao H, Chen Y, Zhu H, Cai Y, Zhang X, et al. Chimmitecan, a novel 9-substituted camptothecin, with improved anticancer pharmacologic profiles in vitro and in vivo. Clin Cancer Res. 2007;13:10. doi: 10.1158/1078-0432.CCR-06-1277. [DOI] [PubMed] [Google Scholar]
- Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, et al. Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- Lee H, Herrmann A, Deng JH, Kujawski M, Niu G, Li Z, et al. Persistently activated Stat3 maintains constitutive NF-kappaB activity in tumors. Cancer Cell. 2009;15:283–293. doi: 10.1016/j.ccr.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.