Abstract
In almost all vertebrates, the downstream of the sox9 signaling axis is well conserved for testis differentiation. The upstream genes of this pathway vary from species to species during evolution. Yet, little is known about how these signaling cascades are regulated and what cellular processes are dominant in ovary–testis transformation in juvenile zebrafish. In this study, we find that the transforming gonads undergo activation of sox9a-expressing stromal cells with increased deposition of extracellular matrix and formation of degenerative compartments. This leads to follicle disassembly, oocyte degeneration, follicle cell-cyp19a1a-amh conversions, and, eventually, formation of the testis cord. In vitro primary culture of juvenile ovary tissue in gonadotropins increases cytoplasmic accumulation of sox9a and p-Erk1/2, and induces mesenchymal morphology. MAPK inhibitors (MKI), a mixture of PD98059 and U0216, eliminate the cytoplasmic distribution but do not eradicate nuclear localization of sox9a and p-Erk1/2. Nuclear p53 are relatively increased in MKI-treated cells that exhibit less spreading and reduced proliferation. Despite uniform nuclear condensation, only a fraction of cells displayed the apoptotic phenotype. These results suggest that high levels of cytoplasmic sox9a and p-Erk1/2 activity activate stromal cells and enhance the production of extracellular matrix required for testis cord formation, whereas deregulation and translocation of sox9a and p-Erk1/2 induce follicle disassembly and incomplete apoptosis associated with nuclear p53. Together with the established FSH/cAMP/MAPK/AMH pathway in mammalian granulosa and Sertoli cells, we demonstrated that the sox9 axis signaling that determines testis formation in mammals also induces zebrafish ovary–testis transition, and adds to its conserved role in sex reversal.
Keywords: zebrafish, sex reversal, incomplete apoptosis, MAPK pathway, sox9
In mammals, gonad development can be divided into two phases: formation of the indifferent genital ridge/bipotential gonad and sex differentiation.1 The indifferent gonads are initially developed from the mesothelial layer of the peritoneum. As embryos grow, some cells of mesonephric origin join the gonad ridge and form the primary sex cord. Thus, the early gonad is an undifferentiated primordium composed of bipotential somatic stem cells that are precursors for supporting cells and steroid-secreting cells. Primordial germ cells (PGCs) arise from the dorsal endoderm of the yolk sac near the allantois, migrate along the hindgut and dorsal mesentery, and eventually join the gonadal ridge.2
When the PGCs reach the gonadal ridge, the supporting cell precursors become either testis-specific Sertoli cells or ovary-specific follicle (granulosa) cells. Meanwhile, the immigrating mesonephric cells give rise to peritubular myoid (PM) cells (endothelial cells that form the male-specific vasculature) and fetal steroidogenic-Leydig cells (ovarian theca cells).1, 3 Parallel to somatic precursors, PGCs differentiate into oogonia in ovarian follicles or spermatogonia in Sertoli cells dominant testis cord.
Compared with mammals, fishes exhibit a striking plasticity in the sex determination process, and show a wide range of gonad differentiation mechanisms, such as hermaphroditism and gonochorism.4 Hermaphrodite fishes can have either functional male and female gonadal tissues simultaneously (synchronous) or sequentially change their sex (sequential) from male to female or from female to male. In differentiated gonochoristic species, an indifferent gonad directly differentiates into a testis or an ovary. Zebrafish are undifferentiated gonochoristic fishes: during the juvenile period,5 all individuals develop undifferentiated ovary-like gonads containing immature oocytes.6 Between 20 and 30 days post fertilization (d.p.f.), immature oocytes develop into ovaries in approximately half of the zebrafish population. In the remaining population, the immature oocytes degenerate and the gonads acquire testis morphology. Therefore, the zebrafish is an optimal model with which to investigate cell death and differentiation for sex reversal. Direct evidence for plasticity of germline stem cells comes from spermatogonial stem cell transplantation in zebrafish. Spermatogonial stem cells are able to colonize in the recipient ovaries and differentiate into female germ line cells.7
Two cellular mechanisms for sex reversal have been proposed: apoptosis and transdifferentiation. The apoptosis mechanism stipulates that a large amount of oocyte apoptosis results in a weakened oocyte-derived signal and reduced aromatase expression. Consequently, in this model, testosterone is not converted to estrogen, spermatogenesis ensues, and the gonad becomes a testis. According to the transdifferentiation mechanism, follicular cells in oocyte-depleted follicles survive, proliferate, and transdifferentiate into Sertoli cells.8 There is still no direct evidence to link oocyte apoptosis to cell transformation in juvenile zebrafish gonads. Much remains to be learned about the signals activating juvenile oocyte degeneration, and the extent to which the signals involved in ovary apoptosis also contribute to ovary–testis transition.
Results
Time course of gonadogenesis
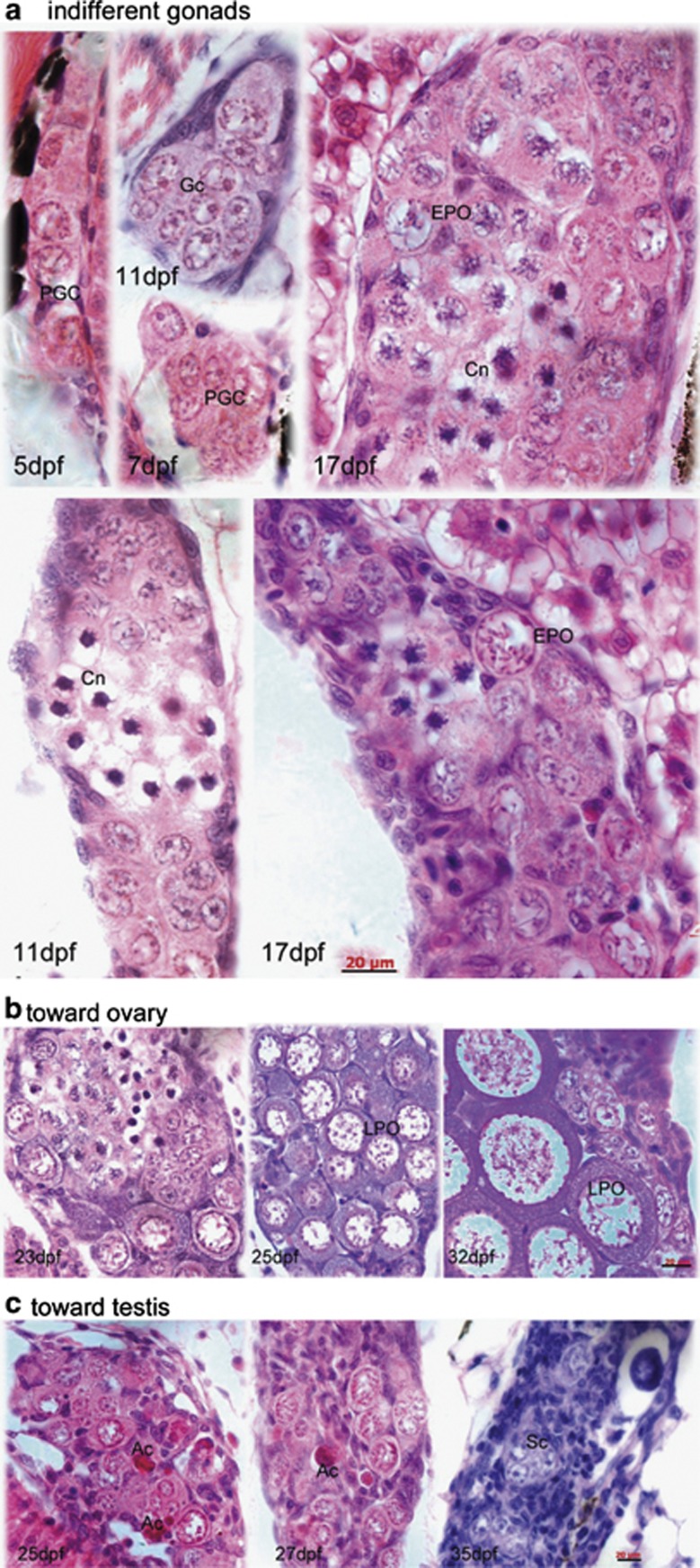
Gonadal development and sex differentiation were examined histologically. A minimum of six fish were sampled every 5 days, starting at 5 d.p.f. and continuing until 55 d.p.f. Serial sections were prepared to determine the developmental stage of the gonads (Figure 1 and Supplementary Figure 1). At 5 d.p.f., we observed PGCs clustered in groups of 3–5 cells (Figure 1a). Flattened and basophilic somatic cells surrounded the PGC clusters (Figure 1a). By 11 d.p.f., the number of germ cells increased, and some PGCs began to differentiate into gonocytes (Gc) that possess rather homogeneous, slightly basophilic karyoplasm, and contain one or more nucleoli in the large nucleus (Figure 1a). Almost at the same time, nucleus-condensed cells (Cn), Gc containing a nucleus with condensed chromatin, appeared. The morphological characteristics of Cn were recognized as entry into meiosis. At this stage, the gonads containing both Gc and Cn were categorized as indifferent gonads, because male- and female-specific Gc of fishes are difficult to differentiate solely based on cell morphological characteristics.
Figure 1.
Gonadal development and differentiation. Indifferent gonad (a), only primordial germ cells (PGCs) are found in the gonad at 5 d.p.f. PGCs contain a large nucleus and possess a distinct nuclear membrane, several big nucleoli, and a network of fine chromatin filaments distributed throughout the karyoplasm. Along the inner side of the nuclear membrane, deeply staining granulosa material is seen. The weakly stained cytoplasm accounts for only a small fraction of the cell volume. At 7 d.p.f., PGCs differentiate into gonocytes (Gc). At 11 d.p.f., more Gcs and meiotic gonocytes with condensed chromatin (Cn) are seen, in addition to PGCs. At 17 d.p.f., early perinucleolar oocytes (EPOs) appear. During juvenile gonadal differentiation toward the ovary (b), more meiotic germ cells are seen (23 d.p.f.), showing densely packed oocytes (27 d.p.f.) and tight connections between late perinucleolar oocytes (LPOs) at 35 d.p.f. In the presumptive testis (c), stromal cells increase and oocyte degenerations appear as acid staining cells (Ac). Sc, spermatogonia cyst. Scale bar=20 μm
Sex differentiation began as early as 17 d.p.f., as indicated by the appearance of perinucleolar oocytes (POs; Figure 1a). As the gonads grew into juvenile ovaries from 20 to 25 d.p.f., the number and size of POs increased and somatic cells were located mainly at the gonadal periphery. By quantifying the number of both germ and somatic cells in the gonads, we found that the ratio of meiotic Gc and POs to total gonadal cells was significantly different, such that individual fishes could be divided into two sex populations. At 20 d.p.f., the majority of the cells in the ‘presumptive ovary' group were meiotic germ cells that became condensed POs at 40 d.p.f. (Figure 1b and Supplementary Figure 1g). The early gonads showed normal (i.e., nontransforming) ovaries and progressed into maturation (Supplementary Figure 1j and 1). Asynchronous cell growth and delayed germ cell differentiation occurred in the ‘presumptive testis' group: fewer POs and more proliferating somatic cells were observed (Figure 1c and Supplementary Figure 1c and e). From 25 to 55 d.p.f., the gonads underwent ovary-to-testis transformation and testicular cord (Tc) morphogenesis (Supplementary Figure 1h and i). Although the time course of gonadal development (Figure 2) was generally consistent with previous descriptions,6 our histological analysis revealed biased sex differentiation toward either the presumptive testis or ovary at the very beginning of juvenile gonadal development.
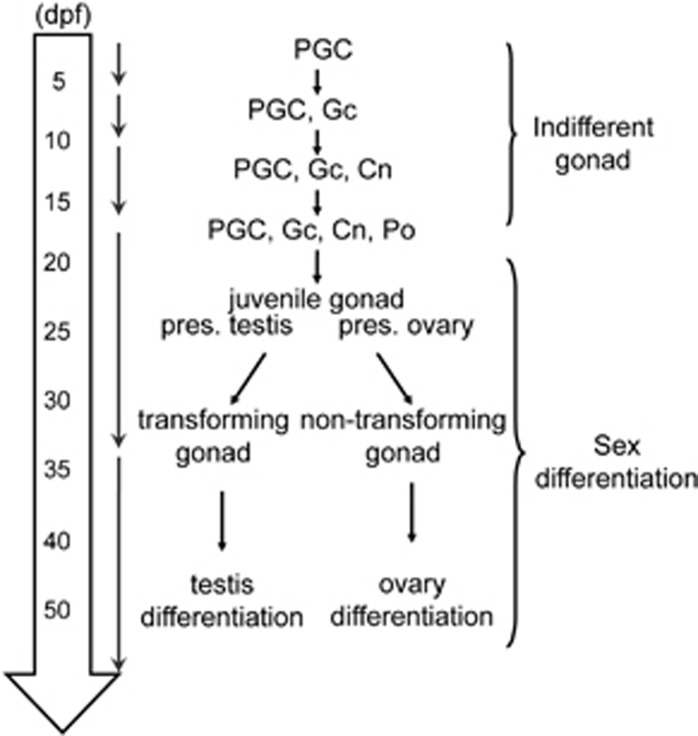
Figure 2.
Time course of gonadal development. Before 17 d.p.f., the gonad is indifferent and contains PGCs, Gc, Cn, and perinucleolar oocytes (POs). The gonad commits into sex differentiation at 17 d.p.f. and becomes mature at 35 d.p.f. The interval between 17 and 35 d.p.f. is referred to as the juvenile period, in which the gonad is identified as presumptive ovary or presumptive testis. The presumptive testis must undergo an ovary–testis transition before maturation
Cellular conversions from ovarian follicles to testis cords
In contrast to the nontransforming gonads, in which POs progress through meiosis and maturation, the transforming gonads showed activation of somatic stem cells (Figure 1c). The activated stromal cells and increased extracellular matrix (ECM) infiltrated the ovarian cavity and encapsulated the meiotic Gc (Figure 1c), accompanied by the development of spermatogonia cysts (Sc) and the degeneration of oocytes. Oocyte degeneration was evidenced by the appearance of red staining acidic body (presumably apoptotic body) and vacuolated oocytes (Figure 1c). The degenerating oocytes were surrounded by the increased gonadal matrix and mesenchymalized stromal cells and formed a degenerative structure, termed degenerative compartment hereafter (Figure 3a).
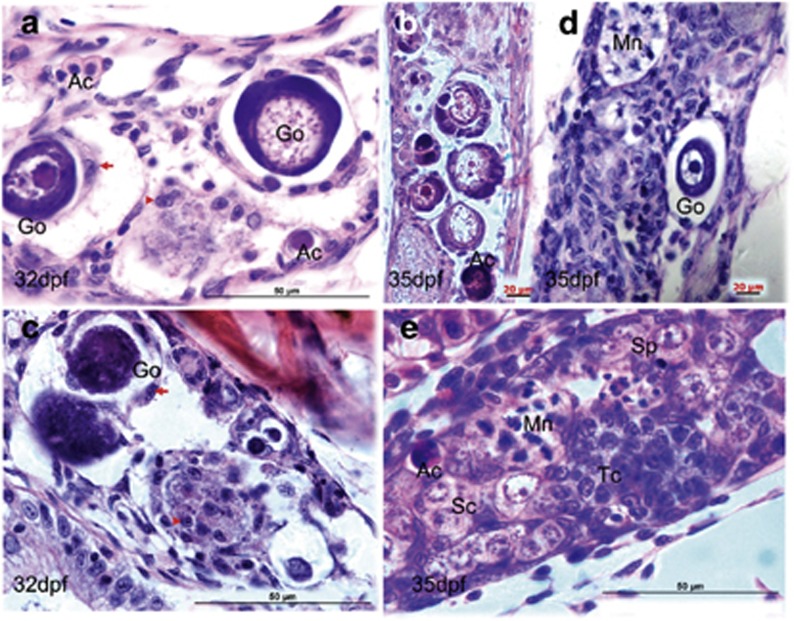
Figure 3.
Morphological alterations from ovarian follicle to testis cord. (a) Active stromal cells surround the giant oocytes (Go) and form degenerative compartment (a). Within the degenerative compartment, follicle cells dissociate from the oocytes (b) and acquire mesenchymal morphology (arrow, a). (c) Degeneration of perinucleolar oocytes (primordial follicles) with marked vacuolation and survival cells from the oocyte decomposition (arrowhead). (d) Coexistence of oocyte degeneration and multinucleated giant cells (Mn) that present many peripheral nuclei and annular chromatin. (e) Regeneration of testis cord tissues. Ac, acidic cells with red coloration; Sc, spermatogenic cysts; Sp, spermatogonia; Tc, testis cord-like structure. Scale bars= 20 μm (b, and d) and 50 μm (a, c and e)
Within the degenerative compartments, several cellular changes were observed. First, the follicle cells lost their tight junctions with adjacent cells and detached from the encapsulated oocyte, implying a preparation for eccentric migration and epithelial–mesenchymal transition (EMT) (Figures 3c and d). This extrapolation was based on the fact that granulosa cells are epithelial cells, and morphologically distinct from the stromal cells of mesenchymal origin.8 Second, the dissociated oocytes displayed apoptotic morphology with condensation of chromatin and a marked vacuolation. However, nuclear fragmentation and apoptotic bodies were not frequently observed (Figures 3a–e). There were no signs of phagocytosis from neighboring somatic cells, although a few lysosome-like structures with red coloration were seen (Figures 3b and e). Third, many cells retained intact morphology although the disassembled follicles exhibited various degrees of degeneration and decomposition (Figures 3a and c). In the vicinity of the degenerating oocytes, there were proliferating stromal cells, Sc, and a multinucleated giant cell (Mn), displaying gross morphological characteristics of Tc (Figures 3d and e). As degenerating oocytes and newly regenerating testis tissues coexisted in the transforming gonads, it was unclear whether the testis tissues were transformed from the degenerative follicles or regenerated from the reserved stem cells.
Molecular characterization of cellular changes throughout gonadal transition
To characterize the cellular phenotypes within the transforming gonads, we used sox9a, amh, and cyp19a1a to identify Sertoli and granulosa cells, and vasa to mark live germ stem cells. Relative to high expression of vasa in the nontransforming gonads (Figure 4a), the expression of vasa in the transforming gonads displayed weak and circular dot patterns in POs (Figure 4c). As previously reported in the vasa::egfp transgenic line,9 transforming gonads showed increased expression of sox9a and amh and decreased expression of cyp19a1a and vasa, compared with the nontransforming gonads (Figures 4a–d). To further understand the relationship between amh-expressing cells and cyp19a1a-expressing cells within the transforming gonads, we compared the expression of cyp19a1a and amh in adjacent sections of the same transforming gonads. It was frequently observed that both markers were coexpressed in the same ovarian follicle. Although cyp19a1a expression was downregulated, amh expression became increased (Figures 4e and f). Thus, ovary–testis transition was evidenced by switching cyp19a1a-expressing cells to amh-expressing cells.
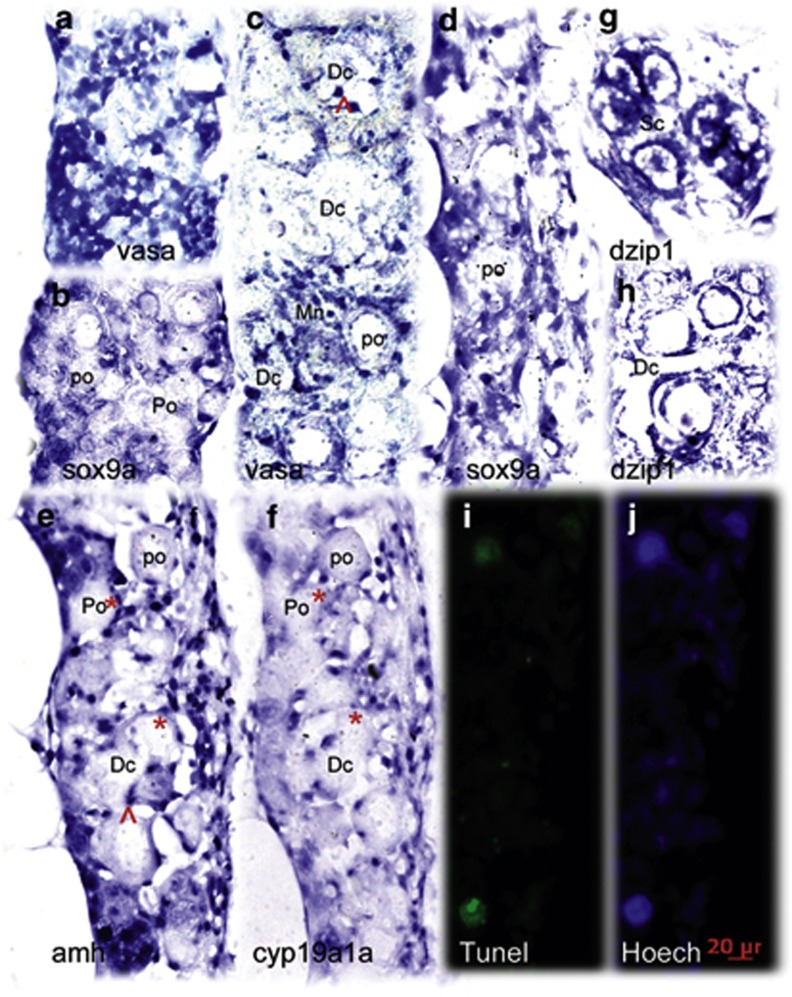
Figure 4.
Comparative analysis of the expression pattern of multiple sex determinant genes between transforming and nontransforming gonads at 35 d.p.f. In the nontransforming gonads, vasa (a) was highly expressed whereas sox9a (b) expression was restricted in the two sides of the gonadal somatic cells. The transforming gonads revealed distinct expression patterns for vasa (c), sox9a (d), amh (e), cyp19a1a (f), and dzip1 (g and h). amh, cyp19a, and sox9a were expressed in somatic cells (supporting cells) located in the gonadal matrix and surrounding the oocytes. dzip1 was highly expressed in both spermatogonia and supporting cells. (e and f) Two adjacent sections with low levels of cyp19a1a and high levels of amh expressed with partial overlap in the same follicles (asterisk). Abnormally expressing aggregates in degenerative compartment (Dc) are indicated by the arrow. TUNEL staining assay showed the larger TUNEL-positive cells were consistent with degenerative oocytes (i). Hoechst staining (j) was used to count total number of gonadal cells. Go, giant oocyte; Mn, multinucleated giant cells; PO, perinucleolar oocyte. Scale bars=20 μm
Cell transdifferentiation during follicle ovarian–testis transition
As other studies of cellular plasticity have suggested, the ability of follicular cells to switch to their testis counterparts may reflect the similar embryonic origin of these two cell types.8 There is evidence that pre-Sertoli cells, Sertoli cells, and granulosa cells share a common precursor in mice.10, 11 To test this possibility in zebrafish, we selected 19 genes associated with sex-differentiation and examined their expression pattern during gonad development (Supplementary Tables 1–3). In comparison, the transcript levels of amh and dzip1 were relatively higher in the testis, whereas the expressions of pou5f1, nanos3, dazap2, na5a2, nr5a1b, and cyp19a1a were predominant in the ovary.
We then analyzed the location of RNA expression in testis and ovary. In contrast to amh and cyp19a1a for which sexually dimorphic expression patterns are confined to somatic cells (Supplementary Figure 3a), dzip1 and nanos3 were expressed in both gonadal somatic cells and germ cells. Using vasa expression as a control, nanos3 was predominantly expressed in the oocyte proper as well as the granulosa cells, but also expressed specifically by germ cells at varying stages of spermatogenesis (Supplementary Figure 3b). Dzip1 colocalized with dazl in oocytes and granulosa cells in the follicles, but was predominantly expressed in testis cord-localized premeiotic spermatogonia (Supplementary Figure 3c). Based on their expression bias in both testis and ovary, and known stem cell-association properties,12 we consider dzip1 and nona3 to be the common markers of germ cells and supporting cells that preferentially develop into testis and ovary.
We then extended our analysis retrospectively to determine whether these genes are expressed in earlier developing embryos. As expected, vasa-expressing cells migrated and resided in the presumptive gonads. Pou5f1, nanos3, and dzip1 were highly expressed in developing tissues (Supplementary Figure 3d). In the juvenile transforming gonads, dzip1 was strongly expressed in the Sc and the surrounding supporting cells (Figure 4g), even in the degenerative compartment (Dc) (Figure 4h). Nanos3 expression was detected in the degenerated oocyte (data not shown). The upregulation of dzip1, a stem cell-associated marker, in the supporting cells of the degenerating follicles implies that the follicle cell transition from cyp19a1a to amh may go through the transdifferentiation process.
Sox9 expression precedes oocyte apoptosis and degeneration
We defined ‘stromal cell activation' as the combinatory effect of cell proliferation (such as increase in the number of stromal cells), and migration with mesenchymal morphology. Having determined that stromal cell activation was the first step in initiating ovary–testis transformation, we then compared the activation of stromal cells with the expression of sox9a. In the transforming gonads, sox9a-expressing cells were no longer restricted to the gonadal periphery, but infiltrated into the interior gonadal stroma and surrounded the oocytes. Unlike the dot expression pattern of vasa, amh, and cyp19a1a, sox9a-expression showed increased cytoplasmic distribution with mesenchymal morphology (Figure 4d). As the sox9-expressing cells and the active stromal cells showed similar mesenchymal morphology and topographic distribution in the transforming gonads (Figure 3c vs 4d), we conclude that sox9a-expressing stromal cells or pre-Sertoli cells were first activated to trigger ovary–testis transformation.
To determine whether and to what degree the degenerating follicles undergo apoptosis, we examined and quantified the degeneration of gonadal cells from 20 to 35 d.p.f. using the terminal dUTP nick end-labeling (TUNEL) method (Supplementary Figure 2 and Figures 4i and j). Of all DAPI staining gonadal cells, ∼10% were TUNEL-positive cells in the presumptive male group by 32 d.p.f., whereas only a small proportion of TUNEL-positive cells contained the typical apoptotic bodies (Supplementary Figure 2). TUNEL-positive somatic cells were observed. Although TUNEL-positive cells were more numerous than the morphologically degenerative oocytes, many degenerative follicles were TUNEL negative. We also saw substantial nuclear staining with irregular shapes of vasa expression (Figure 4c) and amh expression within Dc (Figure 4e). This abnormal staining shape with a high level of vasa- and amh-RNA expression was in accordance with the histological observations that many cells survived the degeneration. This finding further suggests that many cells are at the pre-apoptotic stage, a transient state of cell morphology for subsequent cell transformation or complete apoptosis. Thereafter, we referred to this as partial apoptosis and referred to partial cellular transformation as incomplete apoptosis. Immunohistochemical analysis revealed that weak expressions of Mek1/2, phosphorylated Mek1/2 (p-Mek1/2), sox9a, and p53 were widely detected. Only a fraction of oocytes and the surrounding follicle cells (presumably transforming follicles) showed an increase of staining intensity (Supplementary Figure 4). These results suggest a functional link between p53 and MAPK components and sox9a-amh/cyp19a1a pathways during gonadal transitions from protogenic ovary to early testis.
Sox9 pathways induced mesenchymal morphology changes in primary culture of gonadal tissues
Although histomorphological and histo-in situ hybridization analyses indicated that degeneration and transformation were likely induced by MAPK-sox9a/p53 pathways, it is still unclear how these pathways are regulated during the juvenile ovary–testis transformation. To this end, we used a mammalian growth factor, human chorionic gonadotropin (HCG), to mimic follicle-stimulating hormone (FSH) activity, and cultured the gonad tissues in the modified culture system. Rather than performing in vitro culture of entire gonad,13 we sliced the gonad tissue into small pieces. On the fourth day, a monolayer of adherent fibroblast-like cells grew from the periphery of the primary explants. We then added activator-dcAMP and/or MKI (MAPK (mitogen-activated protein kinase) inhibitor) and continued the culture for 48 h. Gonadotropin and cAMP induced mesenchymal morphology, cell spreading, and proliferation of the cultured primary cells. In contrast, MKI inhibited proliferation and cell spreading, and repressed sox9a and amh transcriptions (Supplementary Figure 5, data not shown). These results were consistent with the in vivo histological observations that the sox9a axis pathways increase ECM production and mesenchymal morphological transformation of gonadal cells.
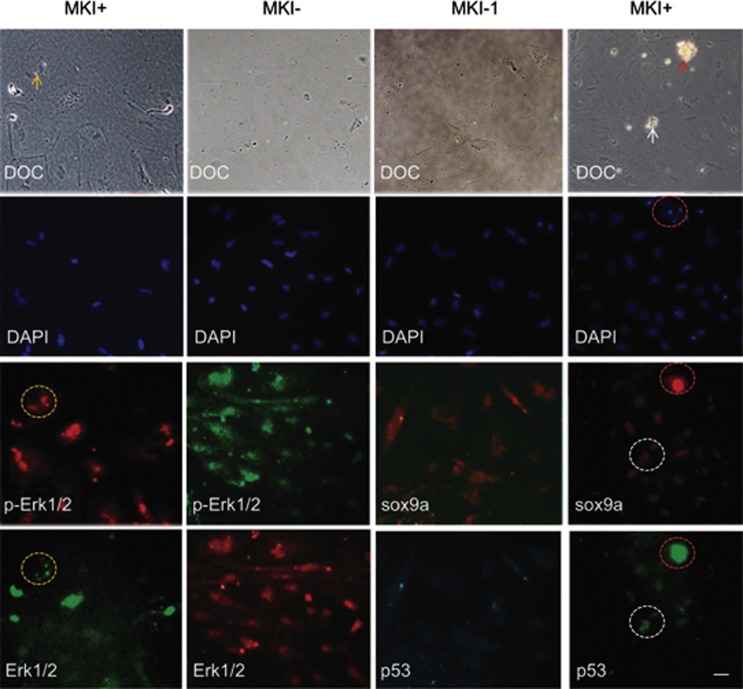
We next examined how the sox9a pathways linked oocyte apoptosis to cellular transformation. Without MKI treatment, the cultured cells exhibited mesenchymal morphology with abundant extracellular matrix. Sox9a and phosphorylated Erk1/2 (p-Erk1/2) were highly expressed in the cytoplasm. Similar phenotypes were observed in sox9-activated stromal cells of the transforming gonads (Figures 3a and 4d). After MKI treatment, levels of sox9a and p-Erk1/2 were dramatically decreased in the cytoplasm, whereas nuclear sox9a and p-Erk1/2 showed little reduction. In contrast, levels of p53 increased in the nuclei, and cell number decreased. Deregulation and translocation of Erk1/2, p-Erk1/2, sox9a, and p53 were indicated by changes of signal intensity (mean values) and size measurement (pixel values) of immunostaining images (Figure 5 and Table 1). The MKI-treated cells became uniformly round with nuclear condensation, and partially apoptotic with fragmented nuclei (Figure 5). These results suggest that cytoplasmic p-Erk1/2 and sox9a activate stromal cells and promote ECM production, whereas reduced p-Erk1/2 activity deregulate expression and translocation of sox9a and p53, and induce partial apoptosis and cellular transformation (Figure 6).
Figure 5.
Involvement of MAPK-sox9a and p53 pathways in morphological alterations of cultured gonadal tissue. Ovary tissues were sliced and cultured in vitro. After 2–3 days, MKI or DMSO control were added and tissues were incubated for 48 h. The cultures were costained with Erk1/2 and p-Erk1/2 antibodies (left two columns) or costained with sox9a and p53 antibodies (right two columns). DAPI staining and DOC were seen. Apoptotic or degenerating cells (arrow) and related signal staining (dotted circle) are shown. Scale bars=20 μm
Table 1. Quantitative analysis of immunostaining signals in Figure 5.
| Antibody | Parameter | MKI− | MKI+ | t-test |
|---|---|---|---|---|
| p-Erk1/2 | Mean | 42.3±2.0a | 23.1±4.2 | <0.001b |
| Pixels | 2858.6±954.9 | 1803±496.6 | 0.06 | |
| Erk1/2 | Mean | 22.8±5.9 | 15.7±4.8 | 0.07 |
| Pixels | 2404.2±1463.0 | 1681.2±659.1 | 0.343 | |
| sox9a | Mean | 7.4±1.1 | 12.4±1.9 | 0.001 |
| Pixels | 2754.6±1292.1 | 558.4±145.5 | 0.019 | |
| p53 | Mean | 9.6±3.5 | 25.4±4.1 | <0.001 |
| Pixels | 2345.4±550.6 | 713.6±241.0 | <0.001 |
Average values±S.D.
P-values
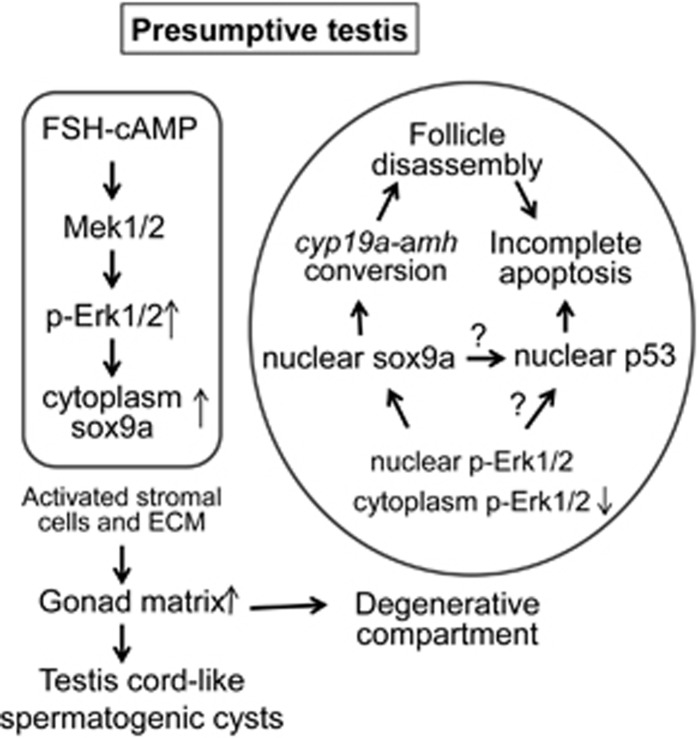
Figure 6.
A proposed model that FSH-MAPK-sox9a/p53 signaling pathways regulate juvenile ovary–testis transition in zebrafish. In the stromal cells of the presumptive testis group, juvenile FSH upregulates sox9a expression and cytoplasmic distribution through phosphorylation of Mek1/2–Erk1/2 signaling. Activated stromal cells and increased ECM form gonad matrix that grows as testis cord-like spermatogenic cysts or becomes degenerative compartments that surround the ovarian follicles. Within the degenerative compartments, p-Erk1/2 and sox9a are reduced, particularly in cytoplasm whereas sox9a and p53 in nucleus are relatively increased. Misexpression and translocation of sox9a deregulates the expression of amh/cyp19a1a and induces the follicle disassembly. Meanwhile, nuclear p53 promotes degeneration of the disassociated follicle cells, leading to incomplete apoptosis. Finally, these incompletely apoptotic cells are either transformed to testis cord or die. It is uncertain how p53 is regulated by sox9a and p-Erk1/2 are interplayed in the transforming gonads
Discussion
In juvenile zebrafish, the gonadal transition from ovary-like tissue to testis is reminiscent of the sex-specific timing of meiosis entry in mammals. Female PGCs (oogonia) first enter meiosis at birth and undergo oogenesis during juvenile development. In contrast, male PGCs (spermatogonia) do not enter meiosis and spermatogenesis until puberty. It has been proposed that germ stem cells are driven to enter meiosis by an intrinsic clock. Signals produced by the male-specific somatic cells inhibit Gc from entering meiosis, arresting Gc in G1/G0 and directing them toward spermatogenesis. Like many other species, zebrafish do not have obvious sexually dimorphic chromosomes; instead, multiple sex determination loci14, 15, 16 have been identified to be responsible for sex-associated effects. This study presents compelling evidence that sox9a axis pathways orchestrate the supporting cell-dominated transition from ovary to testis.
In the testes, sox9a is an upstream positive regulator of amh that, in turn, is an upstream negative regulator of cyp19a1a.17 In the ovary, AMH inhibits primordial follicle assembly.13 Although the regulatory interactions of sox9a-amh/cyp19a1a have been extensively investigated, it is unclear whether and how this pathway is regulated to implement the juvenile ovary–testis transformation in zebrafish. The present study showed that upregulation of sox9a activated stromal cells, increased extracellular matrix production, proceeded follicle cell-cyp19a1a-amh conversions, and induced ovarian follicle degeneration and testis cord generation. This somatic cell-dominated sex differentiation model expands the previous hypothesis that ovarian fate is passively determined by a threshold number of developing oocytes;18 that is, a number of oocytes exceeding the threshold activate the female pathway, whereas a number of oocytes under the threshold induce the male pathway.18 The loss of female germ cells, by either injecting dnd antisense morpholino or mutating sox9b,19 induces female-to-male sex reversal. Loss of germ cells is not an adequate mechanism to explain zebrafish juvenile ovary–testis transition, however. We demonstrated that this transition is regulated by FSH/cAMP/MAPK-sox9 pathway.
In humans and mice, FSH induces upregulation of AMH expression in Sertoli cells and granulosa cells via its second messenger cAMP.20, 21 FSH induces an adenylyl cyclase-dependent increase in cAMP levels that activate several kinases in the PKA, PI3K/PKB, p38 MAPK, and MEK1/2-ERK1/2 pathways.9, 13, 20 Here, we propose a novel mechanism to explain how the degenerative oocytes undergo incomplete apoptosis and transform into testis cords during juvenile ovary–testis transition (Figure 6). According to this model, juvenile FSH upregulates sox9a expression and distribution in presumptive testis stromal cells via Mek1/2-Erk1/2 pathways. Cytoplasmic sox9a activates stromal cells to form degenerative compartments that surround the ovarian follicles. Because of a blocked blood supply, or other unknown factors within the degenerative compartments, Erk1/2 activity and cytoplasmic sox9a are dramatically reduced with relative increases of nuclear sox9a and p53. Deregulated expression of sox9a and p53 coordinately induce follicle disassembly, apoptotic processes, and cellular transformations.
Germ cell development is unique in that the cells progress through meiosis and break double-strand DNA for homologous recombination. Therefore, maintenance of genome stability in germ cells is required for normal gonad development.18, 22 Tp53 (p53) has been reported to mediate germ cell apoptosis and regulate oocyte survival through meiosis.18, 22 On this basis, we theorized that preapoptotic cells might undergo DNA repair through meiotic homologous recombination or mitotic recombination. The recombination provides an opportunity for cells to undergo either transformation or apoptosis. As meiosis is a ‘one-way' process for germ cell division and cannot engage in a cell cycle as mitosis does, oocyte degeneration and programmed cell death are regarded as their final fate.
In summary, gene expression analyses in zebrafish have already revealed the conservation of structure, function, and expression of a large number of mammalian genes at the downstream of the sex determination pathway, including cyp19a1a, sox9a, and amh.9, 13, 20 Our results support the idea that sox9 is a fundamental testis-differentiating gene common to all vertebrates.23, 24 We demonstrated that sox9a expression pattern closely correlates with the behavior of the stromal cells in gonadal tissue of juvenile zebrafish. High level of cytoplasmic sox9a expression promotes cellular proliferation, mesenchymal transformation, and ECM deposition, whereas misexpression of sox9a induces p53-associated follicle degeneration and cell transformation. Obviously, the expression and nuclear translocation of sox9a and p53 are regulated by Mek1/2–Erk1/2 MAPK signaling. The exact regulatory mechanisms in MAPK-sox9/p53 pathway remain for further investigations. Therefore, the manipulation of sex determination might be possible via indirectly disrupting upstream regulatory pathways of Sox9a.
Materials and methods
Zebrafish
Zebrafish TAB lines (hybrid from AB and Tubingen lines) were maintained under an ambient temperature of 27 °C with a timer-controlled light period of 14 h light and 10 h dark. All experiments were performed in accordance with the Animal Care and Use Committee guidelines of the Shanghai Ocean University.
Histological analysis and TUNEL staining
Fish were anesthetized in 0.1% tricaine (3-aminobenzoic acid ethyl-ester methanesulphonate salt; Sigma, Poole, UK) in filtered system water. Gonads were fixed with 4% paraformaldehyde, sectioned, and stained with hematoxylin and eosin (HE).25 For TUNEL staining, paraffin-embedded sections were created following the manufacturer's protocol and subsequently stained in DAPI (Invitrogen Molecular Probes, Carlsbad, CA, USA) for 5 min, followed by two rinses in phosphate-buffered saline (PBS) for 10 min. After photography, the sections were counterstained with HE, and photographed.
In situ hybridization
Whole-mount in situ hybridization and histological in situ hybridization experiments were conducted as described previously.25 Complementary DNAs corresponding to the target genes were used to generate riboprobes.
Immunohistochemistry and quantitative analysis
Immunohistochemistry was performed as described previously.26 Antibodies used for the study include sox9a antibody (ab82578), anti-Erk1+Erk2 antibody (ab54230), phosphorylated Erk1+Erk2 (Phospho T202+Y204) antibody (ab76165), and p53 antibody (ab77813). Fluorochrome-conjugated secondary antibodies, goat anti-rabbit IG-FITC (ab6717), goat anti-mouse IgG-TR (ab6787), Dylight 594 rabbit anti-goat IgG (RAG5942), Dylight488 goat anti-mouse IgG (GAM4882), Dylight549, and goat anti-rabbit (GAR5492), were purchased from Abcam (Hong Kong) Ltd (Hong Kong) or Beijing CoWin Biotech Co., Ltd (Beijing, China). The sections were counterstained with Hoechst 33258 (Sigma) and then mounted with prolong antifade reagent (Invitrogen P7481).
To localize and quantify gene expression, color intensity of the immunostaining signals were quantified by using Adobe Photoshop 7.0 (Adobe Systems Inc., San Jose, CA, USA). The image was first converted as grayscale mode. The lasso tool palette was then used to draw a line around the edges of the cell. After opening the image-histogram window, the values for the selection were recorded as mean, S.D., median and pixels. The same procedure was repeated to collect background intensity. After subtracting the background signals, the remaining signal values represented the immunostaining intensity for the specific antibody in the selected cell. For each image, we collected histogram information from five cells and input the values in Excel spreadsheet. To perform comparison and data analyses we selected two parameters: the mean to represent the average gray value for the area inside the selection, and the pixel values to measure the image size.
RNA isolation and quantitative real-time PCR (RT-PCR)
Total RNA was isolated using Trizol reagent (Invitrogen) and reverse transcribed to cDNA with Transcript II two-step RT-PCR supermix (Transgen Biotech, Beijing, China) in the presence of oligo (dT) 20 primer. Quantitative RT-PCR was carried out using 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) and data analyses were performed as previously described.25, 27 Oligonucleotide primers (listed in Supplementary Table 2) were synthesized by Shanghai Sangon Biological Engineering Technology and Service Co. Ltd (Shanghai, China).
Gonadal tissue culture
Basic gonadal tissue culture medium (GTCM) was based on the zebrafish testis and ovary growth medium in the previous reports.23 L-15 (Leibovitz's L-15 medium, Gibco, Life Technologies, Carlsbad, CA, USA) was supplemented with 2 mM L-glutamine (Gibco), 50 U/ml penicillin and 50 μg/ml streptomycin (Gibco), 100 μg/ml kanamycin sulfate (Gibco), and 20% AmnioPAN complete medium (PAN Biotech GmbH, Aidenbach, Germany) with and without 10 IU/ml HCG (Sigma). After anesthesia with 0.1% tricaine, testes or ovary were removed from 2–3-month-old adult fish and sliced into 1.5 mm sections.3 The gonadal fragments were then plated on gelatin-coated Labteck dishes and incubated at 28 °C in air. After culture, the cells were fixed in 2% buffered paraformaldehyde for 2 h, and permeabilized with 0.2% PBT (0.2% Triton X-100 in PBS) for 10 min. The slides were ready for RNA in situ hybridization, HE staining, or immunostaining. Monoethanolate (U0126), 2-2-amino-3-methoxyphenyl (PD098), and cAMP (D0627) were purchased from Sigma and used at final concentrations of 20 μm, 10 μm, and 1 mM, respectively.
Acknowledgments
We thank Qingyi Liang, Xinyi Wang, and others in the Yan Lab for their technical assistance and helpful discussions. We also thank two anonymous reviewers for their constructive critics to improve our manuscript. We gratefully acknowledge the support of Xin Yang, Xianyi Wang, Bolin Li, Jiale Li, Weidong Zhen, and Yingjie Pan. This work was supported in part by the international Cooperation Initiative Program of Shanghai Ocean University to JY (A0209-13-0808), the Innovation Program of Shanghai Municipal Education Commission to JY (13ZZ126), and Shanghai Universities First-class Disciplines Project of Fisheries. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Glossary
- MKI
MAPK (mitogen-activated protein kinase) inhibitor
- p-Erk1/2
phosphorylated Erk1/2
- p-Mek1/2
phosphorylated Mek1/2
- HCG
human chorionic gonadotropin
- FSH
follicle-stimulating hormone
- PGC
primordial germ cell
- d.p.f.
days post fertilization
- Gc
gonocytes
- Cn
nucleus-condensed cells
- PO
perinucleolar oocyte
- Tc
testicular cord
- Sc
spermatogonia cysts
- Mn
multinucleated giant cell
- Dc
degenerative compartment
- GTCM
basic gonadal tissue culture medium
- EMT
epithelial–mesenchymal transition
- ECM
extracellular matrix
- HE
hematoxylin and eosin
- TUNEL
terminal dUTP nick end-labeling
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by A Stephanou
Supplementary Material
References
- Wilhelm D, Palmer S, Koopman P. Sex determination and gonadal development in mammals. Physiol Rev. 2007;87:1–28. doi: 10.1152/physrev.00009.2006. [DOI] [PubMed] [Google Scholar]
- De Felici M.Origin, migration, and proliferation of human primordial germ cellsIn: Santis GCDFALD (ed)Oogenesis Vol. XII.Springer: London; 201319–37. [Google Scholar]
- McClelland K, Bowles J, Koopman P. Male sex determination: insights into molecular mechanisms. Asian J Androl. 2012;14:164–171. doi: 10.1038/aja.2011.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Nagahama Y, Nakamura M. Diversity and plasticity of sex determination and differentiation in fishes. Sex Dev. 2013;7:115–125. doi: 10.1159/000342009. [DOI] [PubMed] [Google Scholar]
- Uchida D, Yamashita M, Kitano T, Iguchi T. Oocyte apoptosis during the transition from ovary-like tissue to testes during sex differentiation of juvenile zebrafish. J Exp Biol. 2002;205 (Pt 6:711–718. doi: 10.1242/jeb.205.6.711. [DOI] [PubMed] [Google Scholar]
- Maack G, Segner H. Morphological development of the gonads in zebrafish. J Fish Biol. 2003;62:895–906. [Google Scholar]
- Nobrega RH, Greebe CD, van de Kant H, Bogerd J, de Franca LR, Schulz RW. Spermatogonial stem cell niche and spermatogonial stem cell transplantation in zebrafish. PLoS One. 2010;5:9. doi: 10.1371/journal.pone.0012808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guigon CJ, Coudouel N, Mazaud-Guittot S, Forest MG, Magre S. Follicular cells acquire sertoli cell characteristics after oocyte loss. Endocrinology. 2005;146:2992–3004. doi: 10.1210/en.2005-0045. [DOI] [PubMed] [Google Scholar]
- Wang XG, Orban L. Anti-Mullerian hormone and 11 beta-hydroxylase show reciprocal expression to that of aromatase in the transforming gonad of zebrafish males. Dev Dyn. 2007;236:1329–1338. doi: 10.1002/dvdy.21129. [DOI] [PubMed] [Google Scholar]
- Albrecht KH, Eicher EM. Evidence that Sry is expressed in pre-Sertoli cells and Sertoli and granulosa cells have a common precursor. Dev Biol. 2001;240:92–107. doi: 10.1006/dbio.2001.0438. [DOI] [PubMed] [Google Scholar]
- Jameson SA, Natarajan A, Cool J, De Falco T, Maatouk DM, Mork L, et al. Temporal transcriptional profiling of somatic and germ cells reveals biased lineage priming of sexual fate in the fetal mouse gonad. PLoS Genet. 2012;8:e1002575. doi: 10.1371/journal.pgen.1002575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore FL, Jaruzelska J, Dorfman DM, Reijo-Pera RA. Identification of a novel gene, DZIP (DAZ-interacting protein), that encodes a protein that interacts with DAZ (deleted in azoospermia) and is expressed in embryonic stem cells and germ cells. Genomics. 2004;83:834–843. doi: 10.1016/j.ygeno.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Nilsson EE, Schindler R, Savenkova MI, Skinner MK. Inhibitory actions of Anti-Mullerian Hormone (AMH) on ovarian primordial follicle assembly. PLoS One. 2011;6:e20087. doi: 10.1371/journal.pone.0020087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JL, Rodriguez Mari A, Braasch I, Amores A, Hohenlohe P, Batzel P, et al. Multiple sex-associated regions and a putative sex chromosome in zebrafish revealed by RAD mapping and population genomics. PLoS One. 2012;7:e40701. doi: 10.1371/journal.pone.0040701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley KM, Breyer JP, Melville DB, Broman KW, Knapik EW, Smith JR. An SNP-based linkage map for zebrafish reveals sex determination loci. G3 (Bethesda) 2011;1:3–9. doi: 10.1534/g3.111.000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew WC, Bartfai R, Lim Z, Sreenivasan R, Siegfried KR, Orban L. Polygenic sex determination system in zebrafish. PLoS One. 2012;7:e34397. doi: 10.1371/journal.pone.0034397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Mari A, Yan YL, Bremiller RA, Wilson C, Canestro C, Postlethwait JH. Characterization and expression pattern of zebrafish Anti-Mullerian hormone (Amh) relative to sox9a, sox9b, and cyp19a1a, during gonad development. Gene Expr Patterns. 2005;5:655–667. doi: 10.1016/j.modgep.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Mari A, Postlethwait JH. The role of Fanconi anemia/BRCA genes in zebrafish sex determination. Methods Cell Biol. 2011;105:461–490. doi: 10.1016/B978-0-12-381320-6.00020-5. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Watakabe I, Nishimura T, Toyoda A, Taniguchi Y, Tanaka M. Analysis of medaka sox9 orthologue reveals a conserved role in germ cell maintenance. PLoS One. 2012;7:e29982. doi: 10.1371/journal.pone.0029982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasala C, Schteingart HF, Arouche N, Bedecarras P, Grinspon RP, Picard JY, et al. SOX9 and SF1 are involved in cyclic AMP-mediated upregulation of anti-Mullerian gene expression in the testicular prepubertal Sertoli cell line SMAT1. Am J Physiol Endocrinol Metab. 2011;301:E539–E547. doi: 10.1152/ajpendo.00187.2011. [DOI] [PubMed] [Google Scholar]
- Taieb J, Grynberg M, Pierre A, Arouche N, Massart P, Belville C, et al. FSH and its second messenger cAMP stimulate the transcription of human anti-Mullerian hormone in cultured granulosa cells. Mol Endocrinol. 2011;25:645–655. doi: 10.1210/me.2010-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Mari A, Wilson C, Titus TA, Canestro C, BreMiller RA, Yan YL, et al. Roles of brca2 (fancd1) in oocyte nuclear architecture, gametogenesis, gonad tumors, and genome stability in zebrafish. PLoS Genet. 2011;7:e1001357. doi: 10.1371/journal.pgen.1001357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A, Chang H, Chaboissier MC, Schedl A, Behringer RR. Sox9 in testis determination. Ann N Y Acad Sci. 2005;1061:9–17. doi: 10.1196/annals.1336.003. [DOI] [PubMed] [Google Scholar]
- von Hofsten J, Olsson PE. Zebrafish sex determination and differentiation: involvement of FTZ-F1 genes. Reprod Biol Endocrinol. 2005;3:63. doi: 10.1186/1477-7827-3-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, He H, Tang W, Zhang XA, Hua X, Yan J. Two origins of blastemal progenitors define blastemal regeneration of zebrafish lower jaw. PLoS One. 2012;7:e45380. doi: 10.1371/journal.pone.0045380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Yang Y, Zhang H, King C, Kan HM, Cai Y, et al. Menin interacts with IQGAP1 to enhance intercellular adhesion of beta-cells. Oncogene. 2009;28:973–982. doi: 10.1038/onc.2008.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Chen YX, Desmond A, Silva A, Yang Y, Wang H, et al. Cdx4 and menin co-regulate hoxa9 expression in hematopoietic cells. PLoS One. 2006;1:e47. doi: 10.1371/journal.pone.0000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.