Abstract
There is mounting evidence that the established paradigm of nitric oxide (NO) biochemistry, from formation through NO synthases, over interaction with soluble guanylyl cyclase, to eventual disposal as nitrite/nitrate, represents only part of a richer chemistry through which NO elicits biological signaling. Additional pathways have been suggested that include interaction of NO-derived metabolites with thiols and metals to form S-nitrosothiols (RSNOs) and metal nitrosyls. Despite the overwhelming attention paid in this regard to RSNOs, little is known about the stability of these species, their significance outside the circulation, and whether other nitros(yl)ation products are of equal importance. We here show that N-nitrosation and heme-nitrosylation are indeed as ubiquitous as S-nitrosation in vivo and that the products of these reactions are constitutively present throughout the organ system. Our study further reveals that all NO-derived products are highly dynamic, have fairly short lifetimes, and are linked to tissue oxygenation and redox state. Experimental evidence further suggests that nitroso formation occurs substantially by means of oxidative nitrosylation rather than NO autoxidation, explaining why S-nitrosation can compete effectively with nitrosylation. Moreover, tissue nitrite can serve as a significant extravascular pool of NO during brief periods of hypoxia, and tissue nitrate/nitrite ratios can serve as indicators of the balance between local oxidative and nitrosative stress. These findings vastly expand our understanding of the fate of NO in vivo and provide a framework for further exploration of the significance of nitrosative events in redox sensing and signaling. The findings also raise the intriguing possibility that N-nitrosation is directly involved in the modulation of protein function.
Keywords: nitrosothiols, nitrosamines, heme-nitrosyls, ascorbate, oxidative stress
The endogenous production of nitric oxide (NO) by NO synthase (NOS) has been established as playing an important role in vascular homeostasis, neurotransmission, and host defense mechanisms (1). Many of these actions are thought to be mediated by means of stimulation of soluble guanylyl cyclase (sGC) and formation of the second messenger, guanosine 3′,5′-cyclic monophosphate (cGMP), after which NO is disposed in the form of inactive products, such as nitrite and nitrate. There is mounting evidence, however, that the above scenario represents only part of a broader array of alternative biochemical pathways through which NO can trigger or modulate cell signaling, including interaction with thiols and metals (2). Of those pathways, the best known is the concept of thiol nitrosation, often referred to as “S-nitrosylation,”§ a posttranslational protein modification that occurs independent of the sGC/cGMP pathway and could play a critical role in health and disease (3). S-nitroso species (RSNOs) have been implicated in controlling oxygen delivery to tissues, modulating the function or activity of transcription factors, enzymes, membrane receptors and ion channels, and participating in transnitrosation reactions. S-nitrosation is thought to proceed predominantly by means of NO2 production, a second order reaction in NO, followed by an additional reaction with NO to generate the nitrosating species N2O3 (4). According to this scheme, S-nitrosation is effectively third order in NO. Alternative pathways have been proposed to include RSNO formation by means of peroxynitrite (ONOO-) (5), NO–thiol interactions in the presence of electron acceptors (6), and transnitrosation mechanisms (7).
From a perspective of biochemical kinetics, the assertion that second-order or third-order reactions necessary for S-nitrosation might compete for biological significance with a first-order reaction such as heme-nitrosylation seems counterintuitive, particularly when the rates for the latter are often characterized as rapid (typically in the range of 107 M-1·s-1). That S-nitrosation could be of significance under those conditions suggests that these species might be very stable, to the point where even trickle production eventually leads to accumulation of significant concentrations. Recent in vitro evidence for slow RSNO decay in lipopolysaccharide (LPS)-stimulated macrophages seems to support this contention (8). However, one is then faced with a paradox where signaling, a supposedly transient process, takes a form that is long-lived and thus nearly irreversible. Furthermore, if S-nitrosation does indeed compete favorably with heme-nitrosylation, one might also expect nitrosation of, e.g., amines to occur, which would consequently lead to formation of significant levels of potentially harmful N-nitrosamines (RNNOs). The latter have indeed been detected in plasma of healthy human individuals (9), inviting a reassessment of the obligatory carcinogenic role of this nitroso species in humans.
At a time when S-nitrosation is being advanced as a central biological signaling mechanism, and where first attempts are underway to identify potential targets of S-nitrosation by using proteomic approaches, it seems timely to ascertain whether these protein modifications can even begin to compete with heme-nitrosylation in mammalian tissues in vivo, and whether their lifetimes account for their detectability in those tissues. Other issues related to nitrosation include whether the same pathway that leads to S-nitrosation can also result in significant N-nitrosation, and how antioxidants such as glutathione and ascorbate affect the balance of nitrosated species. To this end, we characterized the presence of endogenous RSNOs, RNNOs, and metal nitrosyls, as well as nitrite and nitrate, in tissues of several organs and in blood, and tracked their concentrations after changes in NOS activity, oxygen availability, and redox status. Our results provide insight into the biochemistry of NO and its interaction with biological targets in vivo by demonstrating that nitrosation can indeed compete effectively with nitrosylation in different mammalian tissues, while casting doubts about our current view on the predominating mechanism of nitrosation.
Materials and Methods
Organ Harvest and Tissue Homogenization. Male Wistar or Sprague–Dawley rats (250–350 g) were obtained from Harlan Breeders (Indianapolis), housed in ventilated microisolator cages (three per cage) and kept on a 12/12 reverse light/dark cycle, with food (22/5 rodent diet, Harlan Breeders) and water ad libitum. A minimum of 10 days was allowed for acclimatization of rats to their new environment before use in experiments. Heparinized (0.07 units/g i.p.) rats were anesthetized by using diethylether (2 min) and killed by cervical dislocation. After thoracotomy, a catheter was inserted into the infrarenal part of the abdominal aorta, and organs were flushed free of blood by retrograde in situ perfusion with air-equilibrated PBS supplemented with N-ethylmaleimide (NEM)/EDTA (10/2.5 mM) at a rate of 10 ml/min. The superior vena cava was cut directly after the aortic cannulation to provide an exit port for blood and buffer. After 2 min of perfusion, the brain was removed, followed by the heart. Liver, kidneys, and lung were individually cannulated and perfused through the portal vein, renal artery, and pulmonary artery, respectively, and excised 2–3 min apart. At last, the thoracic aorta was harvested. This order of extraction was based on the relative metabolic rates of the organs and was strictly adhered to throughout the study to minimize tissue hypoxia. Excised tissues were blotted dry on filter paper, weighed, cut into small pieces, and homogenized immediately in ice-cold NEM/EDTA-containing perfusion buffer by using either a Teflon-glass Potter-Elvehjem or a Wheaton glass-glass homogenizer immersed in an ice/water bath. The addition of NEM/EDTA served the purpose of blocking SH-groups and inhibiting transition metal-catalyzed transnitrosation reactions, preventing artificial nitrosation, as well as thiolate- and ascorbate-mediated degradation of endogenous RSNOs (10). All of the above steps were carried out within 2 min of organ harvest under reduced ambient lighting conditions (<15 lux) to minimize photolytic decomposition of tissue nitroso products (11). Tissue homogenates were kept on ice in the dark and analyzed within 1–4 min. Due to the relatively high concentrations of nitroso species in rat RBCs, trapping of small quantities of blood during flushing of individual organs could conceivably have contributed to the apparent level of tissue nitrosation. However, the measured hemoglobin content of tissue homogenates (12) was minimal, indicating a <7% (lung) and <1% (all other tissues) contribution to overall nitroso levels.
Determination of Tissue Nitroso/Nitrosyl and Nitrite/Nitrate Content. The tissue content of S- and N-nitroso species was quantified by gas phase chemiluminescence as described (13). Tissue NO-heme adducts were determined in parallel by injection of aliquots of tissue homogenates into a solution of 0.05 M ferricyanide in PBS at pH 7.5 and 37°C. This method, which represents a modification of a recent assay used for determination of the iron nitrosyl content of RBCs (14), employs one-electron oxidation rather than reduction to achieve denitrosylation. Released NO is quantified by gas phase chemiluminescence as described for the iodine/iodide assay (13). Extensive validation experiments were performed with nitrosyl hemoglobin (NOHb), nitrosylated catalase, and a variety of biological samples spiked with NOHb standards. The denitrosylation mechanism presumably involves oxidation of the heme iron “underneath” the ligand, which, owing to the weaker NO affinity of ferric over ferrous heme (15), is associated with a release of NO into the gas phase. No crossreactivity was observed with nitrite, S-nitrosoglutathione (GSNO), S-nitrosoalbumin, and different RNNOs (all tested at 1–100 μM). Notably, the iron nitrosyl complex sodium nitroprusside did not produce a signal either. This method thus seems to be specific for NO-heme compounds. At the conditions specified above, which were optimized for ferricyanide concentration, pH, and reaction temperature, recoveries for NOHb in spiked tissue homogenates ranged from 96% to 101%. Pilot experiments performed with this technique indicated that tissue nitrosyl species are rather unstable, necessitating the analysis of samples to be carried out within 1 min after tissue homogenization. Nitrate and nitrite were determined by using HPLC (ENO20, Eicom) (9). Of the 98 (nitrite, nitrate, RSNO, RNNO, NO-heme) data sets collected from unstimulated Wistar rats, 5 were rejected by using Chauvenet's Criterion (16) on the basis of their unusually high nitrite content, which was likely due to contaminated glass-ware. In some experiments, diethylamine-NONOate (DEA/NO; 5 mg/kg) or GSNO (50 mg/kg) was administered i.p. 15 min before organ harvest. Tissue homogenates were separated by high-speed centrifugation (20,000 × g for 4 min at 4°C) into a soluble and particulate fraction. The former was further subjected to ultrafiltration over a 30-kDa membrane. In separate aliquots, proteins were removed by methanol precipitation (1:1 vol/vol, 4°C), followed by centrifugation.
NOS Inhibition. Blood pressure was monitored by using the tail cuff method (SC1000, Hatteras Instruments, Cary, NC) after administration of different doses of NG-iminoethyl-l-ornithine (l-NIO) along with other NOS-inhibitors to determine their maximal effective dose. Based on these preliminary studies, l-NIO was selected, and a set of animals were administered a dose of 100 mg/kg s.c. every 45 min over a period of 0.75–3.75 h. Blood and tissue from these animals were subjected to biochemical analysis as described above.
Asphyxia Model of Global Hypoxia. In a subset of ether-anesthetized animals, respiratory arrest was induced by cervical dislocation. Tissues were allowed to perfuse without external ventilatory support, gradually developing global hypoxia. Organs were flushed free of blood at defined periods of time (0.5–10 min) after respiratory arrest and processed as described above. Aliquots of blood were analyzed for pO2, pCO2, and pH by using an Omni AVL Modular System (Roche Diagnostics).
Tissue Ascorbate and Glutathione (GSH)/Glutathione Disulfide (GSSG) Determination. Tissue ascorbate content was assayed essentially as described by Carr et al. (17) and read at 524 nm by using a tunable 96-well plate reader (μQuant, Bio-Tek, Burlington, VT). An additional blank was run in which (2,4-dinitrophenyl) hydrazine (DNPH) was not added until after the addition of H2SO4 to account for tissue-specific background coloration of samples (18). Total ascorbate concentration in tissues was determined by the difference in readings of the 2,6-dichlorophenol-indophenol (DCIP)-treated and control samples by comparison with a standard curve with authentic ascorbic acid. Ascorbate recovery in spiked organ homogenates amounted to 87–95%. The concentration of GSH and GSSG in the organ homogenates was determined essentially as devised by Tietze (19). Tissues were homogenized in 100 mM sodium phosphate buffer supplemented with 5 mM EDTA (pH 7.4) in a weight to volume ratio of 1:5 for brain, heart, liver, kidney, and lung tissues, and 1:25 for aorta.
Results
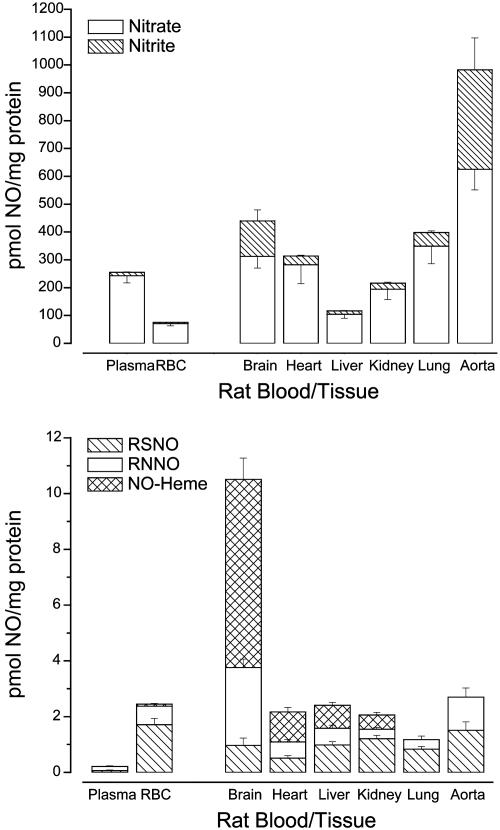
Profiles of RSNOs, RNNOs, and NO-Heme Species Present in Blood and Tissues. The results of this study have uncovered the existence of RSNOs, RNNOs, and NO-hemes constitutively present in blood and tissue homogenates of various rat organs. Our findings, summarized in Table 1 and Fig. 1, provide a detailed insight into the distribution and nature of cellular nitros(yl)ation products throughout the organ system and the circulation of a mammalian species under basal conditions. The results demonstrate that nitros(yl)ation is a ubiquitous phenomenon in all tissues, i.e., that it extends beyond the circulation, where most studies have focused their attention up until recently. In fact, our results show that the nitros(yl)ation level in all tissues examined corresponding to 1–10 pmol NO/mg protein matches or even exceeds that found in plasma (high vs. low nanomolar). The data further reveal that a large fraction of nitros(yl)ation products comprises RNNOs and nitrosyl hemes. A particularly high level of NO-hemes was found in the brain. Moreover, whereas the steady-state levels of nitrate are in the range of 3–20 μM in most tissues (and thus comparable to the levels in blood) those of nitrite are typically below 1 μM, except in organs rich in constitutive NOS such as the brain [neuronal NOS (nNOS)] and aorta [endothelial NOS (eNOS)]. A qualitatively similar tissue pattern of nitroso/nitrosyl species was found in Sprague–Dawley rats, guinea pigs and select tissues of mice (data not shown).
Table 1. Concentrations of NO-related products, glutathione concentration, and redox status (GSH/GSSG ratio), as well as distribution of the total nitroso signal between soluble and particulate cell fractions in blood and tissues of male Wistar rats.
| NO distribution, %
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Nitrite, μM | Nitrate, μM | RSNO, nM | RNNO, nM | NO-heme, nM | GSH, mM | GSH/GSSG ratio | Soluble | Particulate | |
| Blood | |||||||||
| Plasma | 0.29 ± 0.05 | 5.70 ± 0.6 | 1.35 ± 0.46 | 3.52 ± 0.4 | <1.0 | ND | ND | ND | ND |
| Erythrocytes | 0.68 ± 0.06 | 10.2 ± 1.2 | 246 ± 32 | 94.8 ± 14 | 10.8 ± 1.8 | ND | ND | ND | ND |
| Tissues | |||||||||
| Brain | 1.68 ± 0.31 | 6.07 ± 1.07 | 22.4 ± 5.5 | 60.8 ± 7.7 | 157 ± 28 | 1.28 ± 0.06 | 23.6 | 26 ± 5 | 74 ± 5 |
| Heart | 0.77 ± 0.08 | 5.93 ± 1.72 | 13.1 ± 2.0 | 14.2 ± 2.2 | 14.6 ± 1.0 | 0.93 ± 0.12 | 13.9 | 66 ± 7 | 34 ± 7 |
| Liver | 0.46 ± 0.06 | 3.49 ± 0.56 | 36.3 ± 4.4 | 23.6 ± 4.0 | 12.0 ± 0.8 | 3.52 ± 0.09 | 143.6 | 25 ± 4 | 75 ± 4 |
| Kidney | 0.61 ± 0.09 | 4.43 ± 1.03 | 32.9 ± 3.3 | 9.3 ± 1.5 | 5.8 ± 0.9 | 0.36 ± 0.01 | 131.1 | 32 ± 4 | 68 ± 4 |
| Lung | 0.45 ± 0.06 | 3.14 ± 0.63 | 7.90 ± 0.97 | 3.4 ± 1.3 | <1.0 | 0.94 ± 0.19 | 29.3 | 78 ± 7 | 22 ± 7 |
| Aorta | 22.5 ± 9.2 | 48.6 ± 7.2 | 96.3 ± 24.1 | 18.6 ± 11 | <1.0 | 0.34 ± 0.08 | 6.8 | ND | ND |
Absolute amounts of NO-related metabolites were converted into concentrations, taking into account the individual densities (quotient of weight over displaced volume). Means ± SEM from 5 (GSH/GSSG) and 10-14 (all other data) animals. ND, not determined.
Fig. 1.
Relative blood and tissue concentrations of nitrate and nitrite (Upper) and individual nitros(yl)ation products normalized to protein concentration (Lower) within each compartment. RSNOs and RNNOs are found in every compartment whereas NO-heme compounds are only found in a select few tissues. Data are means ± SEM of 10–14 animals.
Attempts aimed at further characterizing different cell types within the liver using classical elutriation techniques, as well as identifying specific cellular compartments subject to nitrosation, proved to be a formidable challenge due to poor product stability and artefactual transitrosation reactions as a result of increased sample processing times. Nevertheless, initial experimental evidence revealed that the majority of nitrosation occurs at the protein level because the entire nitroso/nitrosyl signal was associated with the pellet by using methanol precipitation and with the high molecular weight fraction by using ultrafiltration. Further analysis revealed that the pattern of protein nitrosation is unique to each organ. For instance, in brain, kidney, and liver, the majority of NO is associated with the particulate cell fraction whereas, in heart and lung, highest signals were found in the soluble fraction (see Table 1 for details).
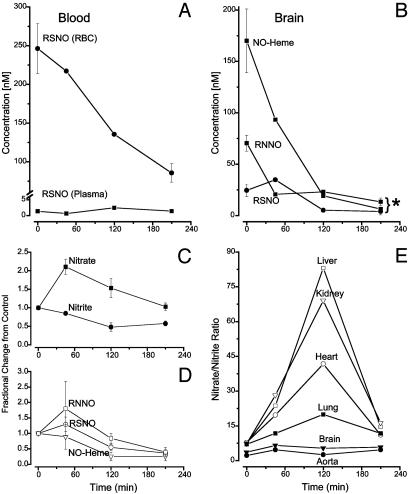
Stability of Endogenous Nitroso and Nitrosyl Compounds After NOS Inhibition. To determine the relation between products of tissue nitros(yl)ation and enzymatic NO production, and to characterize their stability, a separate set of experiments was performed in which tissues were analyzed after NOS inhibition. Fig. 2 A–D depicts the time-dependent changes in concentration of NO-related products in the blood and in extravascular compartments. Evidently, acute NOS inhibition leads to a marked depletion of nitros(yl)ated products, as well as significant changes in nitrite and nitrate, indicating that all of these species are largely linked to enzymatically generated NO. Whereas the limited stability of NO-hemes is not unexpected, that of the RNNO component is rather surprising, given their traditionally higher stability in vitro. Another interesting feature is the transient rise exhibited by many of the NO-related products in response to NOS inhibition (Fig. 2 C and D). This transient rise is particularly salient when the ratio of nitrate and nitrite are plotted for different organs (Fig. 2E). The amplitudes of those transients also correlate well with the GSH/GSSG ratios obtained for those tissues (see Table 1).
Fig. 2.
Kinetic changes in the concentration of different nitros(yl)ation products, as well as nitrate/nitrite in blood and tissues after NOS inhibition by l-NIO. (A) Changes in RSNO concentration in plasma and RBCs. (B) Change in individulal nitros(yl)ation products in the brain. *, P < 0.05. (C) Fractional changes in nitrate/nitrite across all tissues and blood. (D) Fractional changes in nitros(yl)ation products across all tissues and blood. (E) Changes in nitrate/nitrite ratio in different tissues. Depicted data are the means from 2–3 animals per time point for NOS inhibition and 14 animals for the controls. *, P < 0.05.
Tissue Nitros(yl)ation Elicited by NO Donor Administration. To further characterize tissue nitroso/nitrosyl products and to determine whether the variations displayed in Table 1 are due to differences in organ-specific trapping efficiencies or differences in local formation of nitrosating species, we performed additional experiments under conditions during which NO levels were elevated systemically by application of NO-donors. A striking feature noted under these conditions was a massive increase in RNNO and NO-heme products across all organs, particularly in red blood cells (N.S.B. and M.F., unpublished results). The major difference between diethylamine-NONOate (DEA/NO) and S-nitrosoglutathione application was that, with the latter, only moderate increases in tissue nitrite/nitrate levels were observed, suggesting direct transfer of NO+ equivalents without involvement of free NO (S-N transnitrosation).
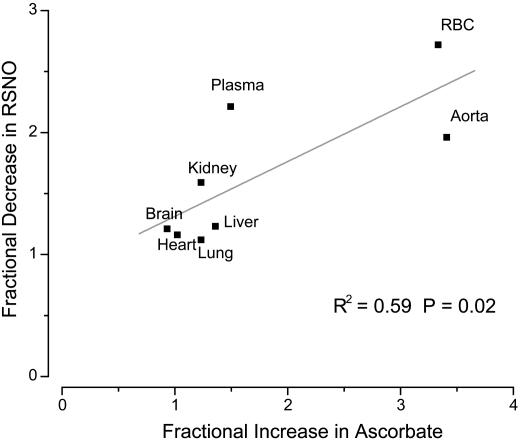
Cellular Redox Modulation of Nitroso/Nitrosyl Products. Natural tissue antioxidants such as glutathione and ascorbate are instrumental in scavenging nitrosating agents, including N2O3. Regulation of antioxidant levels, coupled with measurements of nitros(yl)lated products, can therefore provide some insight into nitrosation pathways in vivo. Preliminary experiments conducted with buthionine sulfoximine (BSO, 6.7 g/liter for 3 days with the drinking water) indicated that inhibition of glutathione synthesis caused dramatic changes in the concentration of NO-related products. Although this finding suggests that tissue nitros(yl)ation is under redox-control, one feature that potentially complicates interpretation of these data is that GSH itself can be a target of nitrosation and/or transnitrosation. Therefore, we opted to verify these findings by modulating the concentration of another redox-active cell constituent. Given the considerable literature on the effects of ascorbate on inhibition of N-nitrosamine formation (20) and decomposition of RSNOs (21), we sought to investigate whether these endogenous nitrosation products are affected by an increased availability of ascorbate. Whereas only modest changes in RNNO concentration were observed after 1 week of ascorbate feeding with the drinking water (data not shown), RSNO concentrations were noticeably reduced at higher tissue ascorbate levels (Fig. 3).
Fig. 3.
Fractional decrease in RSNO (RSNOfinal/RSNObasal) vs. fractional increase in total ascorbate (Ascbasal/Ascfinal) in different rat tissues after administration of ascorbate with the drinking water (10 g/liter for 7 days). The results show that the changes in RSNO content in the various organs are correlated to the changes in intracellular ascorbate concentrations.
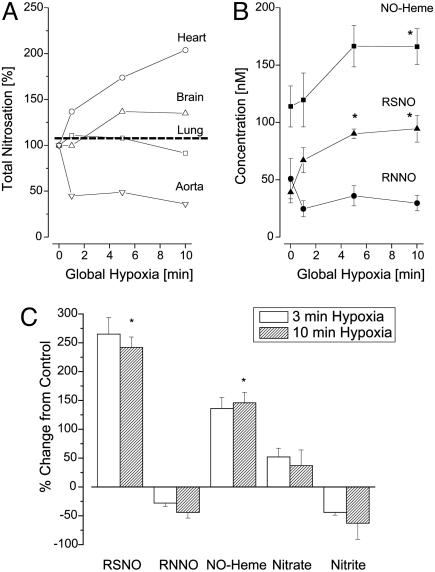
Changes in Tissue Nitros(yl)ation Elicited by Systemic Hypoxia. During earlier phases of this investigation, it became apparent that the values obtained for basal levels of nitroso compounds varied considerably depending on how quickly the aorta was cannulated and how efficiently organs were perfused. Given these observations, and the enormous attention received by recently proposed models of NO activity by red blood cells (22, 23), we sought to investigate what concentration changes, if any, these species undergo during controlled hypoxic episodes. To this end, blood and tissues of rats were analyzed after 1–10 min of global hypoxia produced by respiratory arrest. Blood gas analysis confirmed that pH and pO2 were decreased and pCO2 markedly increased already after 1 min of hypoxia (venous blood pH = 7.1, pO2 = 17 mmHg, pCO2 = 61 mmHg). The effects on tissue nitros(yl)ation are depicted in Fig. 4 and reveal that 10 min of hypoxia (by which the pH of venous blood had reached a value of 6.8) leads to a consistently sharp drop in nitrite across all organs and to significant increases in RSNO and NO-heme concentrations whereas RNNO concentrations decreased. The pattern of nitros(yl)ation seems to be particularly dynamic within the first 5 min of hypoxia.
Fig. 4.
Dynamics of NO-related products during global hypoxia. (A and B) Representative time courses obtained for different products and tissues (n = 3–5). (A) Changes in total nitrosation in select tissues. B focuses on changes in individual nitros(yl)ation products in the brain. Total nitrosation in the brain increases by 40–50% but reveals that RSNOs and NO-hemes account for the increase whereas RNNOs actually decrease, suggestive of a crosstalk between species. (C) Changes in concentration of NO-related products in the brain observed after 3 and 10 min of global hypoxia (means ± SEM; n = 3–5).
Discussion
The results of the present study demonstrate that S-nitrosation, a process increasingly implicated in cellular signaling, is indeed a ubiquitous phenomenon largely localized to cellular proteins. In most organs, this product was found at concentrations that rivaled those of heme nitrosyl species, e.g., the chemical form associated with activation of soluble guanylyl cyclase, and inhibition of cytochrome c oxidase and cytochrome P450 activities (24). Our NOS inhibition experiments also demonstrate that S-nitrosated species seem equally reversible as those associated with heme-nitrosylation, and thus theoretically capable of performing regulatory functions over the same physiologically relevant timescales. Finally, we observed that levels of tissue RSNOs are significantly changed with antioxidant and oxygen availability, suggesting that S-nitrosation is tightly linked to the intracellular redox status. Thus, S-nitrosation does indeed have many of the features that might be expected of redox-sensitive regulatory functions, operating through posttranslational protein modifications and at levels comparable to heme-nitrosylation.
Our studies further reveal that N-nitrosation is as pervasive as S-nitrosation and heme-nitrosylation reactions, and that the reaction products are equally unstable. These results raise the question as to whether N-nitrosation is an active mechanism involved in the modulation of thiol nitrosation, either by S-N transnitrosation reactions or simply by competing with thiols for NO. The results from our experiments with S-nitrosoglutathione, which found that a significant portion of the resulting nitrosation products were RNNOs, would suggest a role for the former mechanism. In addition, N-nitrosation could be involved directly in the regulation of protein function and activity through posttranslational protein modification. The latter is particularly relevant in light of our NOS inhibition experiments, which revealed that N-nitrosation is also a reversible phenomenon that takes place on a time scale relevant to regulatory systems. Although there is an abundant body of literature on the mutagenic and carcinogenic potential of low-molecular-weight RNNOs (25), to our knowledge nothing is known about the potential role of N-nitrosation in posttranslational protein modification, a possibility that deserves further investigation.
It is worth pointing out that the steady-state concentrations for the nitroso and nitrosyl species reported in the present paper represent global tissue values. Considering the lack of knowledge about specific cellular targets and associated chemical pathways, it is conceivable that the nanomolar concentrations reported here could translate into micromolar or even millimolar concentrations at localized cellular sites.
In addition to providing evidence for the reversibility of S- and N-nitrosation, our experiments revealed a surprising transient increase in these products within 45 min of NOS inhibition. This transient rise was also evident in the nitrate kinetics, but not in nitrite or heme-nitrosyls. The dynamic differences among these products are clearly exemplified by the ratios of nitrate to nitrite plotted as a function of time (Fig. 2E). These plots demonstrate vividly how the nitrate-yielding pathway is temporarily enhanced on NOS inhibition. An important clue to the origin of this phenomenon can be gleaned from the magnitude of its peak activity for the different organs examined in this study. This magnitude is strongly correlated to the magnitude of the GSH/GSSG ratios shown in table 1. This link indicates that the phenomenon is somehow related to the level of oxidative stress in that particular organ and thus likely to originate from an interplay between reactive oxygen species and NO produced within the tissue.
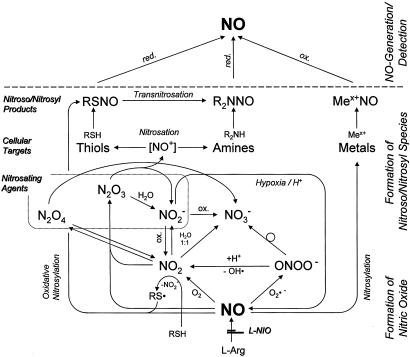
The generation of nitrate, nitrite, and nitros(yl)ation products reported here can be rationalized with the aid of Scheme 1. Enzymatically produced NO in the presence of low O2- levels should react more readily with O2, leading to the formation of NO2. Some of this product can dimerize to form the nitrosating species N2O4, which is hydrolyzed rapidly to produce equimolar amounts of nitrite and nitrate. A more probable fate of NO2 is to react with another NO molecule to form the nitrosating species N2O3, which is rapidly hydrolyzed to nitrite. Under elevated O2- levels, NO reactivity is shifted toward the formation of peroxynitrite, which can decompose to nitrate or lead to formation of NO2 and hydroxyl radicals. The latter is a very rapid first-order reaction and therefore more likely to generate nitrosating species than NO autoxidation under the same conditions. Another feature of the ONOO-/NO2 pathway is its capacity to generate reactive intermediates that can oxidize thiols to thiyl radicals (RS·), which may directly react with NO, a process referred to as oxidative nitrosylation (26, 27). The effectiveness of the O2- pathways for nitrosation has been shown to increase dramatically in chemical systems and in cell cultures whenever NO and O2- are produced at comparable fluxes (26).
Scheme 1.
Biochemical pathways of NO–target interactions in vivo.
According to this architecture of chemical pathways, the reaction of NO with O2 favors the production of nitrite by means of decomposition of NO2 whereas the reaction with O2- favors the production of nitrate by means of decomposition of ONOO-. Our finding that nitrate production is comparable to that of nitrite therefore indicates that the reaction of NO with superoxide is a major pathway for NO chemistry, even under basal conditions where NO production should significantly exceed the flux of O2-. This finding also allows us to rationalize the spikes in nitrate/nitrite ratio (Fig. 2E) seen under NOS inhibition. Assuming that, under physiological conditions, NO serves to counteract increased oxidative stress produced by O2- (28, 29) and NO fluxes exceed those of O2- under these conditions, then as the flux of NO drops due to NOS inhibition, it temporarily passes through the point where its value matches that of O2-, and thus to a level where production of ONOO- is optimal. This conjecture would account not only for the transient increases in NO-derived products but also for the apparent correlation between the amplitude of the nitrate/nitrite ratios shown in Fig. 2E and the reciprocal of the GSH/GSSG values reported in Table 1. That is, tissues with the lowest levels of oxidative stress (highest GSH/GSSG ratio) are those likely to have NO fluxes that far exceed those of O2- and thus are the least likely to produce ONOO-. As the NO flux drops with NOS inhibition, those tissues with greater NO/O2- ratios are the ones expected to exhibit the greatest change in nitrate production when NO/O2- fluxes are matched and thus the greatest nitrate/nitrite peak amplitudes. The notion that matched NO/O2- production enhances peroxynitrite generation may also explain the temporary increases we observe for nitroso species but not for heme-NO species (Fig. 2D): peroxynitrite formation is linked to NO and O2- synthesis whereas heme-nitrosylation should depend only on NO availability. Overall, the evidence gathered here may provide a compelling clue as to the in vivo relevance of superoxide for nitrosation chemistry in mammalian tissues.
The results of our ascorbate experiments provide additional support for the notion that tissue nitrosation in vivo is, at least in part, driven by a mechanism other than by means of N2O3. At the concentrations commonly present in most tissues, ascorbate is an effective scavenger of nitrosating species including N2O3 (20). Consequently, it should suppress the formation of all nitroso species formed through N2O3. Our results indicate that steady state levels of RSNOs in those tissues that readily take up exogenous ascorbate are indeed decreased on increased antioxidant availability. The correlation between the increase in intracellular ascorbate and the decrease in tissue RSNOs confirms our suspicion from the GSH measurements and the experiments with buthionine sulfoximine that S-nitrosation is under redox control. However, the absence of a positive correlation between ascorbate and RNNO levels suggests that the antioxidant is not operating on targets of nitrosation by means of N2O3 scavenging. Ascorbate may rather act by destabilizing existing RSNOs (21) but not RNNOs. Alternatively, it may inhibit S-nitrosation by reducing the concentration of thiyl radicals (27). This finding, combined with those derived from NOS inhibition, would argue that the main pathway for S-nitrosation in mammalian tissues proceeds by means of oxidative nitrosylation.
Finally, the ischemia experiments reported in this study indicate that nitros(yl)ation is acutely perturbed by changes in oxygen availability. In general, we observe a transient rise within the first 5 min of hypoxia (Fig. 4 A and B) that is reminiscent of the peaks seen on NOS inhibition, which were attributed to the enhancement brought about by activation of the O2- pathway. Although a similar effect may be anticipated when NO fluxes fall secondary to depletion in oxygen and critical cofactors required for the NO synthesis from l-arginine, here an alternate source of NO may be activated. During the first couple of minutes, we observe a statistically significant decline in nitrite, which may be decomposing due to tissue acidification (30) or to the nitrite reductase activity of heme-proteins (23, 24). The resulting generation of NO from nitrite could make up for the deficiency in NO from NOS, which would account for the increased levels of NO-heme concentrations we observe during hypoxia but not during NOS inhibition. Formation of nitrosoheme species has been observed in mice subjected to 3 h of cardiopulmonary arrest, but only after prior application of high doses of nitrite (31). In contrast, our results demonstrate that the amounts of endogenous nitrite are sufficient to serve as alternative NO source during brief periods of hypoxia. The depletion of nitrite during hypoxia is particularly noteworthy because it occurs at rates on the order of hundreds of nM per minute, i.e., several nM/s. Regardless as to whether NO autoxidation or oxidative nitrosylation dominate NO chemistry during hypoxia, our results demonstrate that heme-nitrosylation and S-nitrosation are increasing significantly. The biological consequence of the rise in these nitros(yl)ation products is still unclear, but our findings may offer new insights into physiological phenomena previously associated with NO but yet poorly understood, such as hypoxic vasodilation (32) and ischemic preconditioning (33).
In summary, ours is a comprehensive study whereby all major metabolites and breakdown products of NO have been investigated, along with their relative stabilities and redox modulation throughout the organ system in vivo. Our results demonstrate that S-nitrosation is indeed a ubiquitous phenomenon in mammalian biology, of comparable magnitude to heme-nitrosylation. Our studies further reveal that N-nitrosation reactions are equally pervasive. Moreover, evidence is provided that the process of S-nitrosation is likely to proceed by means of oxidative nitrosylation, making it kinetically competitive with heme-nitrosylation. A significant portion of the cellular targets of nitrosation seem to reside in proteins. Furthermore, the nitrosation of mammalian tissues seems to be as readily reversible as heme-nitrosylation, consistent with a role in posttranslational protein modification for signaling purposes. Increases in heme-nitrosylation seem to indicate increased NO production whereas increases in nitrosation products point to increased oxidative stress. Consequently, the ability to distinguish between different nitros(yl)ation products in vivo may ultimately help unravel the reasons for the often dichotomous biological actions of NO by allowing to correlate specific cellular mechanisms with changes in the concentration of individual nitros(yl)ation products, and providing a fingerprint of NO signaling in health and disease.
Acknowledgments
We thank V. Specian, R. Kraemer, and Dr. S. Mnaimneh for skillful technical assistance, L. Coe and Dr. T. Y. Aw for help with the elutriation experiments, and Dr. J. Loscalzo for helpful comments. This work was supported in part by National Institutes of Health Grant R01 HL 69029 (to M.F.). T.R. is a research fellow sponsored by the Deutsche Forschungsgemeinschaft.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: NOS, NO synthase; RSNO, S-nitrosothiol; RNNO, N-nitrosamine; ONOO-, peroxynitrite; GSH, glutathione; GSSG, GSH disulfide.
Footnotes
We prefer to stay with the classical chemical nomenclature whereby “nitrosation” is defined as addition of an NO+ equivalent and “nitrosylation” as addition of an NO radical to another reactant to form a nitroso or nitrosyl species. Under circumstances where the mechanism is either unknown or includes both pathways, the chimera “nitros(yl)ation” is used here to indicate the involvement of nitrosation and/or nitrosylation.
References
- 1.Moncada, S., Palmer, R. M. J. &. Higgs, E. A. (1991) Pharmacol. Rev. 43, 109-142. [PubMed] [Google Scholar]
- 2.Stamler, J. S., Singel, D. J. & Loscalzo, J. (1992) Science 258, 1898-1902. [DOI] [PubMed] [Google Scholar]
- 3.Foster, M. W., McMahon, T. J. & Stamler, J. S. (2003) Trends Mol. Med. 9, 160-168. [DOI] [PubMed] [Google Scholar]
- 4.Espey, M. G., Miranda, K. M., Thomas, D. D. & Wink, D. A. (2001) J. Biol. Chem. 276, 30085-30091. [DOI] [PubMed] [Google Scholar]
- 5.Moro, M. A., Darley-Usmar, V. M., Goodwin, D. A., Read, N. G., Zamora-Pino, R., Feelisch, M., Radomski, M. W. & Moncada, S. (1994) Proc. Natl. Acad. Sci. USA 91, 6702-6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gow, A. J., Buerk, D. G. & Ischiropoulos, H. (1997) J. Biol. Chem. 272, 2841-2845. [DOI] [PubMed] [Google Scholar]
- 7.Liu, Z., Rudd, M. A., Freedman, J. E. & Loscalzo, J. (1998) J. Pharmacol. Exp. Ther. 284, 526-534. [PubMed] [Google Scholar]
- 8.Zhang, Y. & Hogg, N. (2003) Am. J. Physiol. Lung Cell Mol. Physiol., in press. [DOI] [PubMed]
- 9.Rassaf, T., Bryan, N. S., Kelm, M & Feelisch, M. (2002) Free Radical Biol. Med. 33, 1590-1596. [DOI] [PubMed] [Google Scholar]
- 10.Marley, R., Feelisch, M., Holt, S. & Moore, K. (2000) Free Radical Res. 32, 1-9. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez, J., Maloney, R., Rassaf, T., Bryan, N. S. & Feelisch, M. (2003) Proc. Natl. Acad. Sci. USA. 100, 336-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marklund, S. (1979) Clin. Chim. Acta 92, 229-234. [DOI] [PubMed] [Google Scholar]
- 13.Feelisch, M., Rassaf, T., Mnaimneh, S., Singh, N., Bryan, N. S., Jourd'Heuil, D. & Kelm, M. (2002) FASEB J. 16, 1775-1785. [DOI] [PubMed] [Google Scholar]
- 14.Gladwin, M. T., Wang, X., Reiter, C. D., Yang, B. K., Vivas, E. X., Bonaventura, C. & Schechter, A. N. (2002) J. Biol. Chem. 277, 27818-27828. [DOI] [PubMed] [Google Scholar]
- 15.Sharma, V. S., Traylor, T. G., Gardiner, R. & Mizukami, H. (1987) Biochemistry 26, 3837-3843. [DOI] [PubMed] [Google Scholar]
- 16.J. R. Taylor. (1982) in An Introduction to Error Analysis. (Univ. Sci. Books, Mill Valley, CA), p. 168.
- 17.Carr, R. S., Bally, M. B., Thomas, P. & Neff, J. M. (1983) Anal. Chem. 55, 1229-1232. [DOI] [PubMed] [Google Scholar]
- 18.Terada, M., Watanabe, Y., Kunitomo, M. & Hayashi, E. (1978) Anal. Biochem. 84, 604-608. [DOI] [PubMed] [Google Scholar]
- 19.Tietze, F. (1969) Anal. Biochem., 27, 502-522. [DOI] [PubMed] [Google Scholar]
- 20.Tannenbaum, S. R., Wishnok, J. S. & Leaf, C. D. (1991) Am. J. Clin. Nutr. 53, 247S-250S. [DOI] [PubMed] [Google Scholar]
- 21.Kashiba-Iwatsuki, M., Kitoh, K., Kasahara, E., Yu, H., Nisikawa, M., Matsuo, M. & Inoue, M. (1997) J. Biochem. (Tokyo) 122, 1208-1214. [DOI] [PubMed] [Google Scholar]
- 22.Jia, L., Bonaventura, C., Bonaventura, J. & Stamler, J. S. (1996) Nature 380, 221-226. [DOI] [PubMed] [Google Scholar]
- 23.Cosby, K., Partovi, K. S., Crawford, J. H., Patel, R. P., Reiter, C. D., Martyr, S., Yang, B. K., Waclawiw, M. A., Zalos, G., Xu, X., et al. (2003) Nat. Med. 9, 1498-1505. [DOI] [PubMed] [Google Scholar]
- 24.Thomas, D. D., Miranda, K. M., Colton, C. A., Citrin, D., Espey, M. G. & Wink, D. A. (2003) Antioxid. Redox Signal. 5, 307-317. [DOI] [PubMed] [Google Scholar]
- 25.Mirvish, S. S. (1995) Cancer Lett. 93, 17-48. [DOI] [PubMed] [Google Scholar]
- 26.Espey, M. G., Thomas, D. D., Miranda, K. M. & Wink, D. A. (2002) Proc. Natl. Acad. Sci. USA 99, 11127-11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jourd'Heuil, D., Jourd'heuil, F. L. & Feelisch, M. (2003) J. Biol. Chem. 278, 15720-15726. [DOI] [PubMed] [Google Scholar]
- 28.Wink, D. A., Miranda, K. M., Espey, M. G., Pluta, R. M., Hewett, S. J., Colton, C., Vitek, M., Feelisch, M. & Grisham, M. B. (2001) Antioxid. Redox Signal. 3, 203-213. [DOI] [PubMed] [Google Scholar]
- 29.Tsukahazu, H., Hiraoka, M., Kobata, R., Hata, I., Ohshima, Y., Jiang, M. Z., Moiri, E. & Mayumi, M. (2000) Redox Report 5, 23-28. [DOI] [PubMed] [Google Scholar]
- 30.Zweier, J. L., Wang, P., Samouilov, A. &. Kuppusamy, P. (1995) Nat. Med. 1, 804-809. [DOI] [PubMed] [Google Scholar]
- 31.Kuppusamy, P., Shankar, R. A., Roubaud, V. M. & Zweier, J. L. (2001) Magn. Reson. Med. 45, 700-707. [DOI] [PubMed] [Google Scholar]
- 32.Modin, A., Bjorne, H., Herulf, M., Alving, K., Weitzberg, E. & Lundberg, J. O. (2001) Acta Physiol. Scand. 171, 9-16. [DOI] [PubMed] [Google Scholar]
- 33.Ferdinandy, P. & Schulz, R. (2003) Br. J. Pharmacol. 138, 532-543. [DOI] [PMC free article] [PubMed] [Google Scholar]