Abstract
The objective of the study was to investigate the antioxidant effect of Chinese medicine Coriolus versicolor polysaccharide on brain tissue and its mechanism in rats. SOD, MDA and GSH-Px levels in rat brain tissues were determined with SD rats as the animal model. The results showed that Coriolus versicolor polysaccharide can reduce the lipid peroxidation level in brain tissues during exhaustive exercise in rats, and can accelerate the removal of free radicals. The study concluded that its antioxidant effect is relatively apparent.
Keywords: Coriolus versicolor polysaccharide, antioxidation, SOD, MDA and GSH-Px
Introduction
Yun Zhi, also known as Coriolus versicolor, is the fruiting body of Polyporaceae fungus Coriolus versicolor, which is in the genus Coriolus, family Polyporaceae, order Polyporales, division Basidiomycotina. It is widely distributed in more than 20 provinces and autonomous regions in China, including Heilongjiang, Jilin, Liaoning and Yunnan.
It is agreed in Chinese medicine that Coriolus versicolor is sweet in nature, mild in taste, slightly cold, and enters the liver, spleen and lung meridians. It has the spleen-nourishing and dampness-eliminating, cough-and asthma-relieving, heat-clearing and detoxifying, and antitumor effects. It is mainly used in the treatment of chronic active hepatitis, cirrhosis, chronic bronchitis, pediatric spastic bronchitis, sore throat rheumatoid arthritis, leukemia, etc.
Coriolus versicolor intracellular polysaccharide is a polysaccharide substance extracted from mycelium of Coriolus versicolor which has a variety of pharmacological effects. Modern medicine has proved that Coriolus versicolor polysaccharide is a good immune activator, which has the effects of immune regulation (LU et al., 2011), anti-oxidation, anti-tumor (YOSHINO et al., 2010), and promotion of the recovery of damaged liver cells. It contains a variety of chemical constituents, including glucose, fucose, mannose, galactose, rhamnose, etc. (Qin et al., 2012); and has a wide range of biological activities. Its free radical eliminating (Kobayashi et al., 1994), immunity enhancing, anti-myocardial, anti-cerebral ischemia and hypoxia effects have been somewhat studied.
In this study, Coriolus versicolor polysaccharide was investigated in accordance with the requirements of antioxidant activity testing method in the “Technical Standards for Testing & Assessment of Health Food” (2003 Edition). Manifestation of its antioxidant activity in animal testing was preliminarily studied, and antioxidant mechanism of action of Coriolus versicolor polysaccharide was explored, in order to provide theoretical and experimental evidence for development and mass production of Coriolus versicolor polysaccharide related products.
Materials and methods
Drugs
Preparation of Coriolus versicolor polysaccharide: Large and full Coriolus versicolor fruiting bodies were picked, purified, dried, crushed, and sieved for later use. 8 g of Coriolus versicolor powder was taken, added with a 60-fold volume of distilled water, heated in an 80°C water bath for 3 h while stirring. It was then cooled, centrifuged, and the supernatant was concentrated under reduced pressure to about 1/5 of total amount. Anhydrous ethanol was added to the concentrated solution. Supernatant was discarded, and precipitate was centrifuged. The precipitate was taken and freeze-dried, and the resulting solid was the Coriolus versicolor crude polysaccharide.
Main instruments and reagents
Electronic balance, electric treadmill for rats, constant temperature water bath, 722 spectrophotometer, centrifuge, malondialdehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) assay kits were provided by Nanjing Jiancheng Bioengineering Institute.
Animals
Healthy male SD rats, weighing (170–220)g, were fed in separate cages ad libitum. The approval for the experimental use of the animals with batch number 201303564-A6 was obtained from the experimental animal ethics society of China.
Model establishment
After 10 days of adaptive feeding, the experimental animals were screened (amount of training: 15 m/min, 5 min/d, 3 d). The rats which met the requirements were randomly divided into three groups: sedentary control group (n=10), trained control group (n=10), and trained dosing group (n=30) (divided into low, medium, and high dose groups). Rats in the sedentary control group were fed normally, while the rats in the trained control group and trained dosing group were treadmill trained 6 times per week. In the last training, the rats were made to undergo exhaustive exercise. The criteria for determination of exhaustion were thus: rats with decreased movement speed of rats, with buttocks close to the back wall of cages, with weakened hind limbs, unable to motivate by brush stimulus, and drag along conveyor belt reaches 40 s. The training period was 8 weeks.
Experimental methods
Coriolus versicolor polysaccharide was dissolved in 0.3% sodium carboxymethyl cellulose to prepare the test solution. The experimental group was divided into low, medium and high dose groups. Doses were 75, 150 and 225 mg / (kg·d) (in body mass), respectively. Control groups were intragastrically administered with equal volume of solvent for 8 consecutive weeks. On the last day of the 8-week period, rats in the sedentary control group were weighed. Post-exercise exhaustion time of rats in the trained control group and the experimental groups were recorded. Brain tissues were removed from rats in each experimental group, homogenized and placed at −20°C for later use. Blood was sampled from tails of rats in each experimental group, centrifuged, and the supernatant and serum were taken and refrigerated for later use.
SOD activity was determined by xanthine oxidase assay. MDA level was determined by thiobarbituric acid assay, and GSH-Px activity by glutathione peroxides assay. The assays were performed according to the kit instructions.
Statistical analysis
Analysis was performed using SPSS 11.0 software.
Results
Effect of Coriolus versicolor polysaccharide on serum lipid peroxidase in rats
After administering different doses of Coriolus versicolor polysaccharide for 8 weeks, blood MDA level of each dose group showed decreasing trends compared with the sedentary control group, of which the difference between the high-dose group and the trained control group was statistically significant (P<0.05) (Figure 1). The difference was not statistically significant (P>0.05) between the low-, medium-dose groups and the aged control group.
Figure 1.
Effect of Coriolus versicolor polysaccharide on blood MDA level in rats
Effect of Coriolus versicolor polysaccharide on serum superoxide dismutase (SOD) activity in rats
As can be seen from Fig. 2, compared with the sedentary control group, SOD activity in the brain tissues of each experimental group decreased to some extent. The SOD activity decreased quite significantly in the trained control group compared with the sedentary control group, and the difference was highly significant (P<0.01). Change in SOD activity in the high-dose experimental group was also relatively significant.
Figure 2.
Effect of Coriolus versicolor polysaccharide on serum superoxide dismutase (SOD) activity in rats
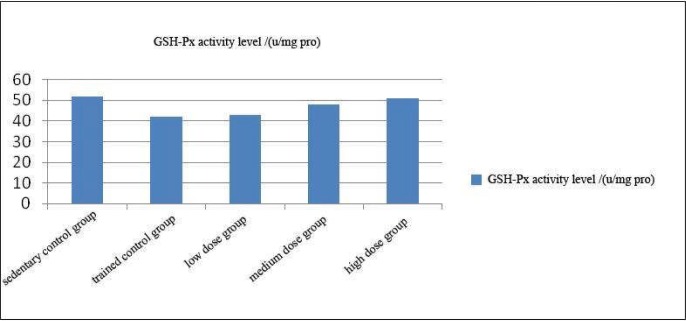
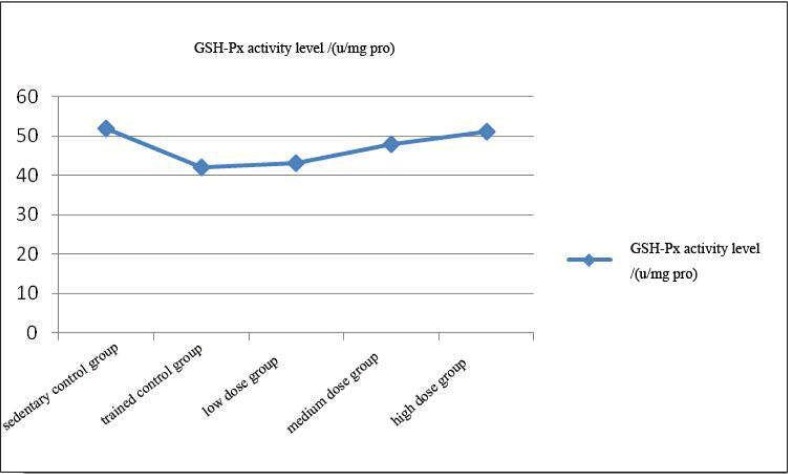
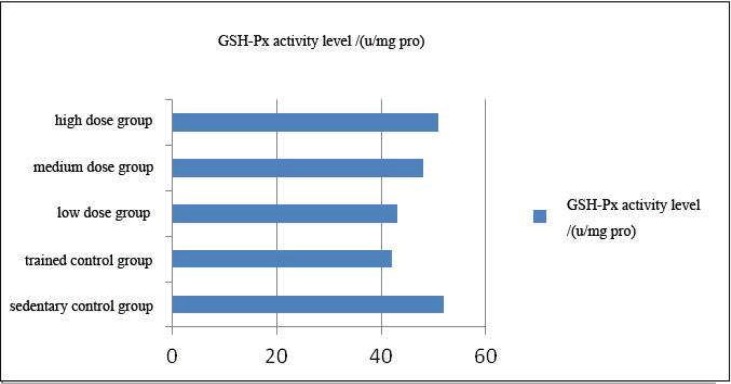
Effect of Coriolus versicolor polysaccharide on serum glutathione peroxidase (GSH-Px) activity in rats
As can be seen from Figure 3, trend of the change in GSH-Px activity level of each experimental group was similar as the SOD.
Figure 3.
Effect of Coriolus versicolor polysaccharide on serum glutathione peroxidase (GSH-Px) activity in rats
Discussion
Oxygen free radical reactions and lipid peroxidation play an important role in the body's metabolic processes, under normal conditions. The two are in the state of coordination and dynamic equilibrium, maintaining various physiological and biochemical reactions and immune responses within the body (Wei et al., 1993; Wei et al., 1995). Once such coordination and dynamic equilibrium is disturbed and disordered, a range of metabolic disorders and immune function decline will occur. Oxygen free radical chain reactions will be formed, as well as the damage of biological membranes and their functions (Siddhuraju et al., 2002; Huang et al., 2005), resulting in the hyaline change and fibrosis in cells; a large area of cell damage will lead to the damage of nerves, tissues, organs, etc.
MDA is the end product of lipid peroxidation chain reaction, which reflects the state of lipid peroxidation within the body, and it is an important marker of lipid peroxidation (Mo et al., 2001, Pang et al., 2000). The level of its content can indirectly reflect the severity of free radical attack to body cells. GSH-Px is an important peroxide decomposition enzyme widely distributed in the body and is particularly important in preventing free radicals from causing membrane lipid peroxidation. Its activity is presented by the reaction speed for catalysis of GSH oxidation and the amount of GSH decrease within unit time. SOD is an important antioxidant enzyme in the body; it is regarded as the most magical enzyme in the life sciences. SOD can induce superoxide anion radicals to become hydrogen oxide and oxygen molecules and thus be removed. Therefore, it has the reputation of the body's waste scavenger. It reflects the body's ability to scavenge oxygen free radicals, which is the natural enemy of oxygen free radicals, the number-one killer of oxygen free radicals in the body, and it is essential to life and health. In this experiment, exhaustive exercise made the brain tissues trigger a variety of free radical chain reactions in cell membranes through mediating N-methyl-D-aspartate receptor and other pathways. Large amounts of reactive oxygen free radicals were produced, leading to the decline of brain tissue's ROS scavenging capacity.
The results of this experiment showed that the SOD and GSH-Px activities in the brain tissues of rats in the trained control group were significantly or very significantly lower than the sedentary control group (P<0.05 or P<0.01), while the MDA level was significantly higher than the sedentary control group (P<0.05). SOD and GSH-Px activities significantly increased (P<0.05) in the brain tissues of rats in the trained dosing group compared with the trained control group, suggesting that Coriolus versicolor polysaccharide can reduce the level of lipid peroxidation in the brain tissues of rats during exhaustive exercise, and accelerate the removal of free radicals. Its antioxidant effect was relatively apparent (Hu., 2003; Pang et al., 1999). Its mechanism of action may be associated with the increased mRNA expression of SOD, GSH-Px in cells by Coriolus versicolor polysaccharide at the transcriptional level (Pang et al., 1999).
References
- 1.Hu W P. Effect of Coridus versicolar polysaccharide on stress-induced learning and memory impairment in rats. Chinese Journal of Traditional Medical Science and Technology. 2003;10(1):32–33. [Google Scholar]
- 2.Huang D J, Huang E J, Ou B X. The Chemistry behind Antiox-idant Capacity Assays. Agric Food Chem. 2005;(53):1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi Y, Kariya K, Saigenji K. Enhancement of anti-cancer activity of cisdia minedichloroplatinum by the protein-bound polysaccharide of Coriolus versicolor QUEL (PS-K) in vitro. Cancer Biother. 1994;9(4):351–353. doi: 10.1089/cbr.1994.9.351. [DOI] [PubMed] [Google Scholar]
- 4.Lu H L, Yang Y, Gad E, Cynthia A W, Amy C, Emily R L, Yu S D, Mark M, Leanna J S, Mary L Ds. Polysaccharide krestin is a novel TLR2 agonist that mediates inhibition of tumor growth via stimulation of CD8 Tcells and NK cells. Clin Cancer Res. 2011;17(1):67–76. doi: 10.1158/1078-0432.CCR-10-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mo Y Y, Chen Y, Zhou M, Jiang Y. Studies on anti-oxidant effects of Coriolus versicolor polysaccharide on brain and liver tissues. Chinese Pharmacological Bulletin. 2001;17(6):628–631. [Google Scholar]
- 6.Pang U, Chen Y, Zhou M. Polysaccharide Krestin enhances manganese superoxide dismutase activity and mRNA expression in mouse peritoneal macrophase. Am J clin Med. 2000;28(6):331–334. doi: 10.1142/S0192415X00000398. [DOI] [PubMed] [Google Scholar]
- 7.Pang Z J, Chen Y, Zhou M. The effect of polysaccharide krestin on GPx gene expression in macrophages. Acta Biochem BiophysSin. 1999;31(3):284–288. [PubMed] [Google Scholar]
- 8.Qin X D, Liu JK. Chemical constituents of Coriolus versicolor. Journal of Yunnan Agricultural University. 2012;27(5):774–776. [Google Scholar]
- 9.Siddhuraju P, Mohan P S, Becker K. Studies on the anti-oxidant activity of Indian Labumun (Cassiafistulal): apreliminaryassessment of crude extracts from stembark, leaves, flowers and fruitpulp. Food Chem. 2002;(79):61–63. [Google Scholar]
- 10.Wei W S, Guo F, Tan J Q. Correlation between lymphocyte-mediated immune response and erythrocyte immune function. Chinese Journal of Immunology. 1995;11(S):187–187. [Google Scholar]
- 11.Wei W S, Tan J Q, Guo F. Effects of Coriolus versicolor polysaccharide on red blood cell immunity, superoxide dismutase, and lipid peroxidase. In: Guo Feng, Luo Yong-zhen., editors. New Exploration on Red Blood Cell Immunology (Vol. 2) Vol. 2. Nanjing: Nanjing University Press; 1993. pp. 195–198. [Google Scholar]
- 12.Yoshino S, Yoshimura K, Suzuki N, Iida M, Yoshida S, Maeda Y, Mae da K, Hazama S, Oka M. Immunoregulatory effects of PSK on the Th1/Th2 balance and regulatory T-cells in patients with colorectal cancer. Gan To Kagaku Ryoho. 2010;37(12):2234–2236. [PubMed] [Google Scholar]