Abstract
The objective of this paper was to extract and purify lily polysaccharide and to study its anti-H22 hepatoma effect in mice. Orthogonal experimental method was used to analyze the factors influencing the extraction and purification of lily polysaccharide, and the anti-tumor effect of lily polysaccharide was studied by acting it on H22-bearing mice. The results showed that the size of influence of various factors on the extraction results of lily polysaccharide were extraction time, extraction times and extraction temperature in decreasing order. Lily polysaccharide can enhance the immune function of H22 tumor-bearing mice, and inhibit the growth of H22 tumor. The study concluded that the optimal conditions for the extraction and purification of lily polysaccharide should be extraction times of 3 times, an extraction time of 4 h each, and an extraction temperature of 60°C; lily polysaccharide has an anti-tumor effect.
Keywords: lily polysaccharide, orthogonal experiment, anti-tumor
Introduction
Bulbus Lilii is a perennial bulbiferous plant in the genus Lilium of the family Liliaceae (Dictionary, 1997). Its bulb resembles garlic, and it tastes like Chinese yam. So, in ancient times it was known as “Suan Nao Shu”. As one of both medicinal and edible plants, Bulbus Lilii is not only rich in protein, fat, starch, amino acids, dietary fiber and vitamins, with a high nutritional value, but it is also rich in selenium, calcium, magnesium, zinc and other trace elements, with the effects of easing mental anxiety, moistening lung dryness, regulating spleen and stomach, relieving cough and reducing sputum, invigorating blood circulation and relaxing bowels. Clinically, it is commonly used for anti-oxidation, anti-cancer, and immunity enhancement (Yang et al., 2002; Mimaki, 1999; Mimaki et al., 1998; Kokai, 2001). To further clarify the anticancer mechanism and principle of Bulbus Lilii, this paper studied the extraction and purification of lily polysaccharide and explored the anti-cancer effects of lily polysaccharide in vitro, laying the foundation for the in-depth development and utilization of Bulbus Lilii.
Materials and methods
Reagents and instruments
Bulbus Lilii was purchased from the market, which was identified as Bulbus Lilii in the genus Lilium of the family Liliaceae by Professor Wang Jianan (Shandong University of Chinese Medicine). 5-fluorouracil (5-FU) was purchased from Shanghai Xudong Haipu Pharmaceutical Co., Ltd.; its batch number was 20120512. Bluestar UV spectrophotometer, high-speed drug pulverizer, SHB-B95 circulating water multipurpose vacuum pump, all reagents were domestic products of analytical grade.
Experimental animals: Conventional male Kunming mice (body weight: 18∼22 g) and mouse H22 hepatoma cell lines were provided by the China Medical University. All experimental procedures were approved by the Animal Research Ethics Committee.
Extraction and purification of lily polysaccharide
Pretreatment
Bulbus Lilii was pulverized, passed through a 40-mesh sieve, added with a 3-fold amount of 95% ethanol, and extracted under reflux for 2 h. It was then filtered and the residue was collected and dried for later use.
Optimization of extraction and purification conditions of lily polysaccharide
Adequate amount of the above Bulbus Lilii powder was taken, added with water, extracted, and centrifuged. The supernatant was collected and concentrated under reduced pressure, added with 95% ethanol and allowed to stand overnight. It was then suction filtered. The precipitate was washed successively with anhydrous ethanol, acetone, and diethyl ether, and dried to obtain lily polysaccharide. On the basis of single factor experiment, L9(33) orthogonal test method was used to optimize factors such as extraction time, extraction times, and extraction temperature. The factor and level design is shown in Table 1.
Table 1.
Factors and levels of lily polysaccharide extraction
| Level | Factor | ||
| Extraction times (times) | Extraction time (h) | Extraction temperature (°C) |
|
| 1 | 1 | 1 | 50 |
| 2 | 2 | 2 | 60 |
| 3 | 3 | 4 | 70 |
Experiment of anti-tumor effect of lily polysaccharide in H22-bearing mice
Preparation of subcutaneous xenograft model of H22
In accordance with the conventional operation method, mouse H22 cells were thawed. 0.2 mL of cell suspension was injected intraperitoneally into male mice, and passaged continuously for two generations. The 2nd generation ascites was diluted with sterile saline to adjust the cell density to 1×107 cells / mL. 0.3 mL of H22 cell suspension was inoculated subcutaneously into the right armpit of mice (Li et al., 2009).
Animal grouping and treatment
24 h after inoculation, the mice were randomly divided into model group, lily polysaccharide low-, medium-, high-dose (75, 150, 225 mg / (kg·d)) groups and 5-Fu group (n=10 each). Each lily polysaccharide treatment group was administered intragastrically with lily polysaccharide once daily for 7 consecutive days; 5-Fu was administered intraperitoneally at 25 mg/kg dose every other day for a total of 5 times in 5-Fu group; while normal saline was administered intragastrically instead of the drug in model group. Drug was withdrawn on the 8th day of administration, and animals in each group were fasted with water only. Another 10 normal mice were selected and served as the control group, which were administered intragastrically with normal saline daily for a total of 7 days.
Determination of subcutaneously transplanted mouse H22 hepatoma inhibition rate, immune organ index, and serum indicators
Body weight and tumor weight of mice were weighed separately to calculate the tumor inhibition rate. Thymus and spleen were weighed to calculate the thymus and spleen indices. Blood was collected from the eye socket, serum was separated, serum TNF-α and IL-2 were detected by ELISA assay, and serum T-AOC and MDA levels were detected by colorimetric assay.
Tumor inhibition rate% = (tumor weight of model group − tumor weight of treatment group) / tumor weight of model group × 100% Thymus (spleen) index = thymus (spleen) mass / body mass
Effect on serum hemolysin in mice
The experimental method in the literature (Liu et al., 1994) was referred to. Half maximal hemolytic concentration (HC50) in each experimental group was calculated as: HC50 = OD value of sample / OD value of SRBC at half hemolytic concentration × serum dilution ratio.
Statistical analysis
Results were expressed as x± s; experimental data were statistically analyzed using SPSS 11.5 software.
Results
Orthogonal experimental results for extraction and purification of lily polysaccharide
As can be seen from Tab. 2, the degree of influence of various lily polysaccharide extraction and purification factors on experimental results were A>B>C. The results indicated that the optimal process for extraction and purification of lily polysaccharide was A3 B3 C3, i.e., extraction times of 3 times, extraction time of 4 h each, and extraction temperature of 70°C.
Table 2.
Orthogonal experimental results for extraction and purification of lily polysaccharide
| Experiment No. |
A | B | C | Lily polysaccharide yield (%) |
| 1 | 1 | 1 | 1 | 2.13 |
| 2 | 1 | 2 | 2 | 3.46 |
| 3 | 1 | 3 | 3 | 5.26 |
| 4 | 2 | 1 | 2 | 4.61 |
| 5 | 2 | 2 | 3 | 6.33 |
| 6 | 2 | 3 | 1 | 7.21 |
| 7 | 3 | 1 | 3 | 7.57 |
| 8 | 3 | 2 | 1 | 6.01 |
| 9 | 3 | 3 | 2 | 8.93 |
| K1 | 10.85 | 14.31 | 15.35 | |
| K2 | 18.15 | 15.8 | 17 | |
| K3 | 22.51 | 21.4 | 19.16 | |
| R | 3.88 | 2.36 | 1.27 |
Anti-tumor effect of lily polysaccharide
Effects of lily polysaccharide on tumor inhibition and weight of immune organs in H22-bearing mice
The experimental results showed that the purified lily polysaccharide had no significant effect on survival and body weight of the host, while it had an inhibitory effect on the growth of mouse H22. Thymus and spleen are important immune organs of animals. The size of thymus and spleen indices of mice determines the body's immune level. The experimental results showed that lily polysaccharide can significantly increase the thymus and spleen weights of mice, compared with the 5-Fu control group. Anti-tumor level of lily polysaccharide was lower.
Effect of lily polysaccharide on serum hemolysin in H22-bearing mice
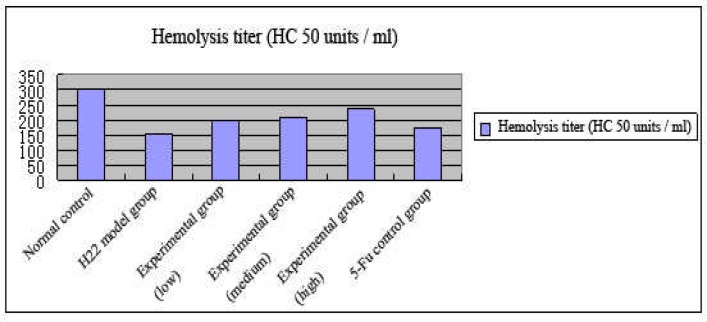
HC50 of three experimental groups were all higher compared with the control group. The hemolysin formation capacity of each tumor-bearing mice group was significantly lower than the normal control group. Hemolysin formation capacity of 5-Fu group was lower than each polysaccharide administration group and higher than the tumor model group. The data are shown in Figure 1.
Figure 1.
Effect of lily polysaccharide on serum hemolysin level in H22-bearing mice (x ±s, n=10)
Discussion
It can be seen from the experimental results that the optimal extraction process for lily polysaccharide was extraction times of 3 times, extraction time of 4 h each, and extraction temperature of 70°C. But the polysaccharide can easily be depolymerized, and the starch can easily be gelatinized and denaturalized. So, the extraction temperature was set as 60°C. The optimum extraction process was thus: extraction times of 3 times, extraction temperature of 60°C, and extraction time of 4 h.
The growth of solid tumors relies on supply of nutrition by new blood vessels; therefore in recent years, the majority of measures for control of tumor growth, invasion and metastasis are achieved by inhibition of tumor cell-induced angiogenesis (Oehler et al., 2000). Experimental data have proved that the xenograft tumors can cause serious thymus atrophy, and abnormal spleen index (Robins et al., 1985) in mice, and significantly lower the anti-tumor effect.
In the present study, the lily neutral polysaccharide has a significant anti-tumor effect, which was consistent with the anti-tumor effect of many purified Chinese medicine polysaccharides (Kakutani et al., 2007).
Immune regulation is one of the currently recognized mechanisms of the anti-tumor effect of polysaccharides. As an immunomodulator, polysaccharide stimulates immune cells to improve immune function through a variety of mechanisms, and thus exerts anti-tumor effect (WAN et al., 2006; Zhao et al., 2002; Miao, 2001).
It can be seen from the results of this experiment that lily polysaccharide can increase the thymus and spleen weights of tumor-bearing mice, at the same time increase the serum levels of specific antibodies of mice, suggesting that lily polysaccharide can enhance the specific humoral immune function. The experiment demonstrated that lily polysaccharide can inhibit the growth of H22 mouse hepatoma. At the same time it can increase the weight of immune organs of tumor-bearing mice, and significantly elevate serum hemolysin level of tumor-bearing mice. In addition, it can be seen from the experimental results that compared with 5-Fu, polysaccharide can significantly reduce toxic and side effects.
Table 3.
Effects of lily polysaccharide on tumor inhibition and weight of immune organs in H22-bearing mice (X ±s, n=10)
| Group | Thymus index | Spleen index | Tumor weight (g) | Tumor inhibition rate (%) |
| Normal control | 3.542±0.573 | 4.874±0.965 | ||
| H22 model group | 1.92±0.532 | 5.541±0.898 | 0.753±0.211 | |
| Experimentalgroup (low) |
2.434±0.327 | 6.432±0.943 | 0.623±0.352 | 17.26 |
| Experimental group (medium) |
2.574±0.497 | 6.736±0.875 | 0.531±0.587 | 29.48 |
| Experimental group (high) |
2.784±0.533 | 6.564±1.311 | 0.54±0.243 | 28.29 |
| 5-Fu control group | 0.712±0.345 | 2.867±0.987 | 0.349±0.127 | 53.65 |
Acknowledgement
The work was supported by the integration and demonstration of repair protection technology in Qilian wetlands ecosystem, 2012BAC08B04. The research on hypolipidemic material base of Rhubard 2013-z-053.
References
- 1.Dictionary of Traditional Chinese Medicines. Vol. 1. Shanghai: Shanghai Renmin Press; 1997. pp. 856–857. [Google Scholar]
- 2.Kakutani R, Adachi Y, Kajiura H, Takata H, Kuriki T, Ohno N. Relationship between structure and immunostimulating activity of enzymatically synthesized glycogen. Carbohydr Res. 2007;342(16):2371–2379. doi: 10.1016/j.carres.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Kokai Tokkyo Koho, author. Isolation of antitumor steroidal glycosides from liliaceae. Japan C07J71/00. 2001;02:698–699. [Google Scholar]
- 4.Li Kun-xing, Zhu Xue-ping, Zhang Hai-lin, Ni Ke, Guo Ye. Anti-tumor Effect of Phellinus Linteus and Coriolus Versicolor Capsule on S180 sarcoma and H22 hepatoma in mice. Chinese Journal of Experimental Traditional Medical Formulae. 2009;15(7):83–84. [Google Scholar]
- 5.Liu Hai-feng, Yuan Ai-li, Liu Si-de, Zhou Dian-yuan. The anti-tumor effect by the combination of tumor necrosis factor and cyclophosphamide in H22 hepatic carcinoma bearing mice. Chinese Journal of Cancer. 1994;13(6):509–513. [Google Scholar]
- 6.Miao Ming-san. Study on antioxidant effect of lily polysaccharide. Pharmacology and Clinics of Chinese Materia Medica. 2001;17(2):12–13. [Google Scholar]
- 8.Mimaki Y. Steroidal saponins from the bulbs of lilium candidum. Phytochemistry. 1999;51(4):567–573. doi: 10.1016/s0031-9422(99)00022-9. [DOI] [PubMed] [Google Scholar]
- 9.Mimaki Y, Tadaaki S, Minpei K, Sashida Y, Hatakeyama Y. New steroidal constituents from the bulbs of lilium candidum. Chem Pharm Bul. 1998;46(11):1829–1832. doi: 10.1248/cpb.46.1829. [DOI] [PubMed] [Google Scholar]
- 10.Oehler M K, Bicknell R. The promise of anti-angiogenic cancer therapy. B J Cancer. 2000;82(4):2749–2750. doi: 10.1054/bjoc.1999.0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robins R A, Baldwin R W. T-cell subsets in tumor rejection responses. Immunol Today. 1985;6(2):55–58. doi: 10.1016/0167-5699(85)90048-9. [DOI] [PubMed] [Google Scholar]
- 12.Wan Y Y, Du Y M, Yang F X, Ying X, Chen R Z, Kennedy J F. Purification and characterization of hydrosoluble components from the sap of Chinese lacquer tree Rhus vernicifera. Int J Biol Macromol. 2006;38(3–5):232–240. doi: 10.1016/j.ijbiomac.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 13.Yang Lin-sha, Sun Yan-hong, Fang Xiao-yan. Research progress in Chinese medicine Bulbus Lilii. Henan Journal of Traditional Chinese Medicine and Pharmacy. 2002;17(1):74–75. [Google Scholar]
- 14.Zhao Guo-hua, Li Zhi-xiao, Chen Zong-dao. Chemical Structure and Antitumor Activity of Polysaccharide from Lilium brownii. Journal of Food Science and Biotechnology. 2002;21(1):62–66. [Google Scholar]