Abstract
Achillea millefolium L. is cultivated in Iran and widely used in traditional medicine for gastrointestinal disorders. The aim of this study was to determine the effect of hydroalcoholic extract of A. millefolium on the contraction and relaxation of isolated ileum in rat. In this experimental study, aerial parts of A. millefolium were extracted by maceration in ethanol 70% for 72h. Terminal portion of ileum in 100 male Wistar rats was dissected and its contractions were recorded isotonically in an organ bath containing Tyrode solution (37 °C, pH 7.4) under one gram tension. Acetylcholine (1mM) and KCl (60mM) were used to create isotonic contractions. Propranolol and Nω-Nitro-L-arginine methylester hydrochloride (L-NAME) were used to investigate the mechanisms of action prior to giving the extract to the relevant groups. Data were compared by ANOVA and Turkey's post hoc test.. The results showed that the ileum contraction was induced by KCl and acetylcholine induced contraction was significantly reduced by A. millefolium extract. The cumulative concentrations of A. millefolium relaxed the KCl and acetylcholine induced contractions (n=14, p<0.001). The inhibitory effect of extract on contraction induced by KCl and acetylcholine was not significantly affected neither by propranolol (1µM) nor by L-NAME (100 µM). There was no significant difference in the rate of relaxation by propranolol and L-NAME between the two groups. In conclusion, A. millefolium can inhibit contraction of smooth muscle of ileum in rat, and it can be used for eliminating intestinal spasms. These results suggest that the relaxatory effect of A. millefolium on ileum contractions can be due to the blockade of voltage dependent calcium channels. In addition, the β-adrenoceptors, cholinergic receptors and nitric oxide production are not powerful actors in inhibitory effect of A. millefolium. So, the nitric oxide and adrenergic systems may also be involved in the antispasmodic effect of A. millefolium.
Keywords: Achillea millefolium, ileum, rats, contraction and relaxation, L-NAME, propranolol, smooth muscle
Introduction
A common functional disorder of the intestine that affects many people is irritable bowel syndrome (IBS). The factors inducing IBS are not known. Patients suffer from IBS episodes with alternating constipation and diarrhea. Abdominal pain and cramps are among the most common symptoms of IBS. Nowadays, many people have resorted to the use of natural medicines for the treatment of intestinal disorders.
Medicinal plants have been used for more than 2000 years. Increasing attention has been paid to herbal products because of their capability and cheaper cost in recent years (World et al., 2002, Uprety et al., 2012). One of the common natural medicines used for the treatment of IBS is peppermint oil (Hills, and Aaronson, 1991) which has a spasmolytic effect. There are various herbal medicines traditionally used for intestinal disorders and diarrhea (Sadraei et al., 2003). A famous characteristic of these medicinal plants is their spasmolytic activity. Therefore, the selection of the most effective herbs in this field is of particular interest.
Achillea millefolium L. (Asteraceae) grows wildly in Asia, Europe, North Africa, and North America. Its different types have been used by local people as folk or traditional medicinal plants. Bumadaran is a known name for several species of Achillea in Iran. It is cultivated in Iran and widely used in traditional medicine for gastrointestinal disorders (Nemeth and Bernath, 2008).
Several effects such as antibacterial (Candan et al., 2003, Stojanovic et al., 2005), anti-inflammatory (Benedek et al., 2007), antihypertensive and antihyperlipidemia (Asgary et al., 2000), and antitumor (Tozyo et al., 1994, Csupor-Loffler et al., 2009) have been reported for Achillea. Also, effects on the gastrointestinal tract such as antiulcer (Cavalcanti et al., 2006), antispasmotic (Lemmens-Gruber et al., 2006, Yaeesh et al., 2006, Karamenderes and Apaydin, 2003), antibacterial (Helicobacter pylori) (Mahady et al., 2005), choleretic (Benedek et al., 2006), and hepatoprotective (Lemmens-Gruber et al., 2006) have been reported. Flavonoids, alkaloids (achilleine), cineol, borneol, α and β pinen, camphor, caryophllene, thujene, rutin, monoterpenoids and sesquiterpenoids (Dokhani et al., 2005, Afsharypuor et al., 1996, Gherase et al., 2003, Javidian K et al., 2004) are among its chemical compounds.
Propranolol is a non-selective beta-adrenergic receptor blocking agent (Dobarro et al., 2013). It has no other autonomic nervous system activity. Propranolol is a competitive antagonist which specifically competes with beta-adrenergic receptor stimulating agents for available beta-receptor sites (Pereira-Leite et al., 2013).
NG-nitro-L-arginine methyl ester (L-NAME) is a non-selective inhibitor of nitric oxide synthesis (NOS) (Zhao et al., 2013). In this study, we investigated the effect of hydroalcoholic extract of floriated shoot of A. millefolium on the smooth muscle relaxation of isolated ileum in rats to find out their antispasmodic activity possibility.
Materials and Methods
Plant material
Aerial parts of A. millefolium were collected from plants in Tangesayad, Caharmahal va Bakhtiari province (Iran). The plant was identified by the staff of Medical Plants Research Center of Shahrekord University of Medical Sciences, (Iran). A voucher specimen was deposited at herbarium of the Medical Plants Research Center (voucher No. 304).
Extraction method
Hydroalcoholic extract was obtained by the Maceration method. The crude A. millefolium was reduced to pieces of suitable size, mixed thoroughly with the specified extracting solvent (ethanol 70%), and allowed to stand at room temperature in a closed container for an appropriate time (72h) with frequent agitation until soluble matter was dissolved. The mixture was filtered, the insoluble material was washed with the same solvent used for maceration, and the filtrates were combined and concentrated, usually under pressure reduced by the vacuum pump, to isolate the solvent from the extraction by lower temperature (40°C) to the desired consistency (Rashidi et al., 2005, Moreno-Loaiza and Paz-Aliaga, 2010).
Experimental procedure
This experimental study was conducted using 84 male Wistar rats (weighing 185–250 g). The animals were kept in a 20–24°C temperature with a 12h light/dark cycle and fed with standard diet, and drinking water was made easily accessible for them. The rats were deprived of food 24h before the experiment but they had free access to water. Animals were randomly assigned to six experimental groups: 1) KCl+saline; 2) KCl + A. millefolium; 3) acetylcholine (Ach) + saline; 4) Ach + A. millefolium; 5) L-NAME + KCl + A. millefolium; and 6) propranolol + KCl + A. millefolium. All procedures were in accordance with the guide for the care and use of laboratory animals adopted by Iran's National Institute of Health and federation of Iranian societies for experimental biology. This study was approved by the Ethics Committee of Shahrekord University of Medical Sciences, Sahrekord, Iran (approval number 89-1-3) and the study was conducted in accordance with the Ethical and Practical Principles of Laboratory Animal guidelines.
Animals were anesthetized by ether and 1.5–2cm of ileum terminal portion was isolated in post laparatomy and placed in oxygenated Tyrode's solution (Pickering et al., 2009, Sadraei et al., 2011, Ince et al., 2011, Brankovic et al., 2011) of the following composition (mM): NaCl 136.9, KCl 2.68, CaCl2 1.8, MgCl2 1.05, NaHCO3 11.9, NaH2PO4 0.42, and glucose 5.55. The ileum was mounted in a 10 ml organ bath containing Tyrode solution, which was maintained at 37°C and constantly aerated with oxygen (pH=7.4). The tissues were subjected to a resting tension of one gram. The period of adaptation was 60min and bath solutions were changed every 15 minutes. Isotonic contractions of tissue were recorded using isotonic transducer (Harvard Isotonic Transducer, UK) and displayed on a Harvard Universal Oscillograph pen recorder device. At the end of adaptation period, smooth muscle of ileum with KCl (60 mM) (Camilleri, 2001) or acetylcholine (1µM) ( Hasler and Owyang, 1999) was contracted. After reaching the plateau of contraction, cumulative concentrations of extract (1.5 mg/mL) was added to the organ bath. The percentage change in contractile force was compared with the plateau of contraction. The effects of concentrations of A. millefolium extracts on contraction induced by KCl and acetylcholine were measured. After 30min incubation of the tissue with 1µM propranolol at concentration (Vanner et al., 1999) and 20min with 100 µM L-NAME at concentration (Hamm et al., 1999) the previous steps were repeated. To investigate the role of extracellular calcium in the extracts, the tissues in calcium-free Tyrode's solution containing KCL concentration (120mM) were placed and the cumulative CaCl2 (0.225–3.6 mM) was added to the bathroom. After 5min presence of extracts in different tissues with 2–4 mg/ml concentrations, the previous steps were repeated.
Statistical analysis
Mean and standard error of mean (SEM) values were calculated for each group of results and the significant difference between the means was calculated by one way analysis of variance (ANOVA) and Turkey's post hoc test. Differences were considered statistically significant when P<0.05.
Results
In all experiments, the addition of KCl solution (60mM) and Acetylcholine (1mM) increased the initial sudden contraction in the ileum rapidly. The contractions returned to the plateau state immediately. At this point, the contractions of the ileum were considered 100%. Adding the extracts to bath tissue decreased KCl- and acetylcholine-induced contractions.
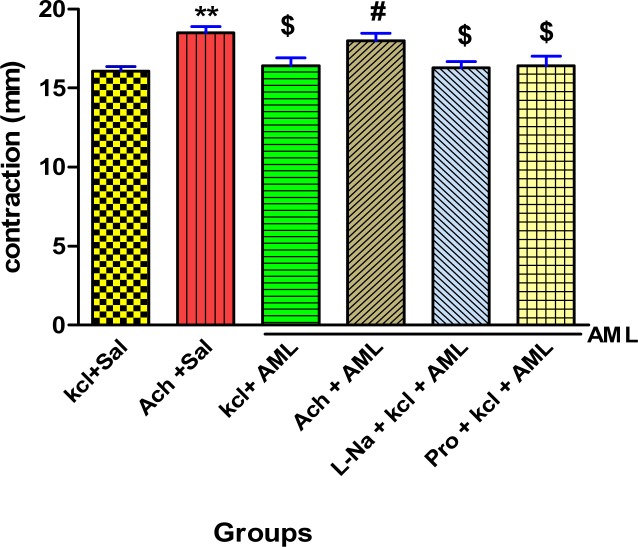
According to Figure 2, significant differences between groups Ach + saline with KCl + saline were seen (P<0.01). Acetylcholine caused more contraction of ileum compared to KCl. Significant difference was observed between groups Ach + saline, KCl + A. millefolium, L-NAME + KCl + A. millefolium, and propranolol + KCl + A. millefolium (P <0.05). In the acetylcholine group, contraction rate was higher compared to those of others.
Figure 2.
Effect of A. millefolium on ileum contractions of rats in different groups
Values are expressed as mean ± S.E.M. (n = 14). **p < 0.01 as compared to kcl+Sal group; #p<0.05 as compared to kcl+Sal group; $p<0.05 as compared to Ach +Sal group; (One-way ANOVA followed by Tukey's test) Sal= saline, AML= A. millefolium L., Ach= acetylcholine, L-Na= L-NAME, Pro= propranolol
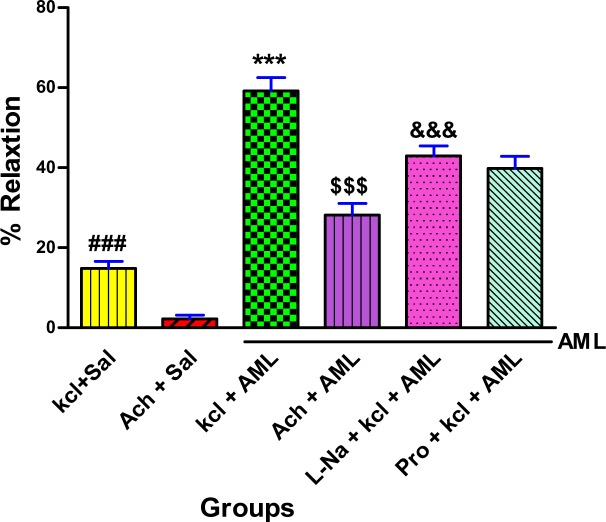
According to Figure 3 antispasmodic effect of A. millefolium extract was greater than those of the other groups (P<0.001).
Figure 3.
Effect of A. millefolium on ileum relaxation of rats in different groups
Values are expressed as mean ± S.E.M. (n = 14). ***p < 0.001 as compared to all other groups; ###p<0.001 as compared to Ach +Sal group; $$$p<0.001as compared to Ach +Sal and kcl + Sal groups; &&& p<0.001 as compared to Ach +Sal, kcl + Sal and Ach+ A. millefolium groups (one-way ANOVA followed by Tukey's test) Sal= saline, AML= A. millefolium L., Ach= acetylcholine, L-Na= L-NAME, Pro= propranolol.
Discussion
The purpose of this study was to investigate the pharmacological effects of A. millefolium on rat ileum contractions. As obtained in the results, the alcoholic extract of A. millefolium reduced KCl and Acetylcholine-induced contraction of ileum. Smooth muscle membrane contains more voltage-dependent calcium channels compared to skeletal muscle, but much less voltage-dependent sodium channels. Therefore, calcium ions flow into fiber through slow calcium-sodium channels thanks to KCl effect is responsible for the contraction (Ratz et al., 2005). Substances that inhibit KCl-induced contraction of the smooth muscle are referred to as voltage-dependent calcium channel blockers (Berridge, 2008). Acetylcholine causes the contraction through the effect on the receptors of muscarinic M4, decrease in cAMP (Cyclic adenosine monophosphate), increase in potassium, the activation of muscarinic M3 receptors and calcium channels, increase in intracellular calcium, or by affecting nicotinic receptor and opening the ion channels and depolarization (Nausch et al., 2010, Wright and Huddart, 2002). The inhibitory effects of A. millefolium extract stimulated by KCl and acetylcholine are not fully reversible. These effects only decline and will not be completely eliminated through washing or replacing the tissue. The relative decline of the effects of extract after washing the tissue is probably attributable to the removal of extract from surface receptors that were reversibly bound to extract. Also, the results of long term ileum contraction by KCl and acetylcholine without using extracts revealed that ileum can stay contracted in long term with no decline in contractile force. These results indicated that the observed decrease in contractile force was due to the performance of extract rather than muscle fatigue. Since the nitric oxide synthase inhibitor drug that is normally responsible for the production of nitric oxide in the body could not block the antispasmodic effects of the extract, the extract could not act by affecting the body's levels of nitric oxide. Also, contractile effect is not completely inhibited with the presence of propranolol that is an inhibitor of adrenergic system. So, the extract can affect through adrenergic as well as nitric oxide receptors. Increasing the intracellular calcium and potassium is the controlling factor of tension of gastrointestinal smooth muscle (Wells et al., 2008). So, the existence of extract may have been inhibiting the normal function of calcium and potassium, through which the present extract could have inhibited the contraction.
Apigenin flavonoid has been reportedly present in the extract (Yaeesh et al., 2006) and can take part in an antispasmodic activity (Takzare et al., 2011). As a result, antispasmodic effects observed in the present work could be attributed to the apigenin flavonoid of A. millefolium. However, more research is needed in this regard. By methods such as immunohistochemistry and binding, the precise mechanisms of A. millefolium antispasmodic effects could be more clarified. Decrease in small intestinal contractions could suggest that A. millefolium may be helpful in the treatment of diarrhea predominant irritable bowel syndrome (IBS).
Conclusion
A. millefolium can inhibit contraction of smooth muscle of ileum in rat, and it can be used for eliminating intestinal spasms. These results suggest that the relaxatory effect of A. millefolium on ileum contractions can be due to the blockade of voltage dependent calcium channels. In addition, the β-adrenoceptors, cholinergic receptors and nitric oxide production are not powerful actors in inhibitory effect of A. millefolium. So, the nitric oxide and adrenergic systems may also be involved in the antispasmodic effect of A. millefolium.
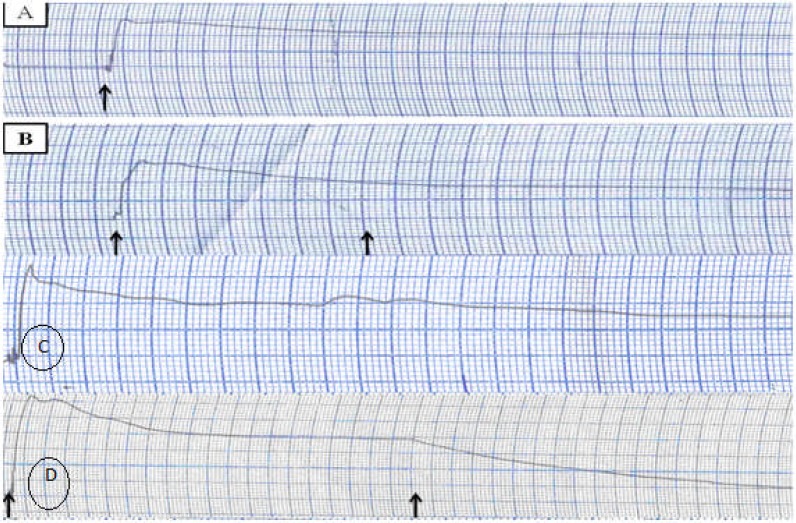
Figure 1.
The recorded samples taken from the ileum of rats Response to (A) added acetylcholine, (B) KCl (left arrow) or saline (right arrow), (C) KCl + extracts and (D) acetylcholine (left arrow) + extract (right arrow)
Acknowledgements
This study was conducted with the assistance of Medical Plants Research Center of Shahrekord University of Medical Science and was approved by the Ethics Committee of Shahrekord University of Medical Sciences. Authors also would like to thank Research and Technology Deputy of Shahrekord University of Medical Sciences for their financial support.
References
- 1.Afsharypuor S, Asgary S, Lockwood G B. Constituents of the essential oil of Achillea wilhelmsii from Iran. Planta medica. 1996;62:77–78. doi: 10.1055/s-2006-957810. [DOI] [PubMed] [Google Scholar]
- 2.Asgary S, Naderi G H, Sarrafzadegan N, Mohammadifard N, Mostafavi S, Vakili R. Antihypertensive and antihyperlipidemic effects of Achillea wilhelmsii. Drugs under experimental and clinical research. 2000;26:89–93. [PubMed] [Google Scholar]
- 3.Benedek B, Geisz N, Jager W, Thalhammer T, Kopp B. Choleretic effects of yarrow (Achillea millefolium s.l.) in the isolated perfused rat liver. Phytomedicine. 2006;13:702–706. doi: 10.1016/j.phymed.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Enedek B, Kopp B, Melzig M F. Achillea millefolium L. s.l. --is the anti-inflammatory activity mediated by protease inhibition? Journal of Ethnopharmacology. 2007;113:312–317. doi: 10.1016/j.jep.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 5.Berridge M J. Smooth muscle cell calcium activation mechanisms. The Journal of physiology. 2008;586:5047–5061. doi: 10.1113/jphysiol.2008.160440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brankovic S, Kitic D, Radenkovic M, Veljkovic S, Jankovic T, Savikin K, Zdunic G. Spasmolytic activity of the ethanol extract of Sideritis raeseri spp. Raeseri Boiss. & Heldr. on the isolated rat ileum contractions. Journal of medicinal food. 2011;14:495–498. doi: 10.1089/jmf.2010.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camilleri M. Review article: tegaserod. Alimentary pharmacology and therapeutics. 2001;15:277–289. doi: 10.1046/j.1365-2036.2001.00925.x. [DOI] [PubMed] [Google Scholar]
- 8.Candan F, Unlu M, Tepe B, Daferera D, Polissiou M, Sokmen A, Akpulat H A. Antioxidant and antimicrobial activity of the essential oil and methanol extracts of Achillea millefolium subsp. millefolium Afan. (Asteraceae. J Ethnopharmacol. 2003;87:215–220. doi: 10.1016/s0378-8741(03)00149-1. [DOI] [PubMed] [Google Scholar]
- 9.Cavalcanti A M, Baggio C H, Freitas C S, Rieck L, De sousa R S, Da silva-santos J E, Mesia-vela S, Marques M C. Safety and antiulcer efficacy studies of Achillea millefolium L. after chronic treatment in Wistar rats. J Ethnopharmacol. 2006;107:277–284. doi: 10.1016/j.jep.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Csupor-loffler B, Hajdu Z, Zupko I, Rethy B, Falkay G, Forgo P, Hohmann J. Antiproliferative effect of flavonoids and sesquiterpenoids from Achillea millefolium s.l. on cultured human tumour cell lines. Phytother Res. 2009;23:672–676. doi: 10.1002/ptr.2697. [DOI] [PubMed] [Google Scholar]
- 11.Dobarro M, Orejana L, Aguirre N, Ramirez M J. Propranolol restores cognitive deficits and improves amyloid and Tau pathologies in a senescence-accelerated mouse model. Neuropharmacology. 2013;64:137–144. doi: 10.1016/j.neuropharm.2012.06.047. [DOI] [PubMed] [Google Scholar]
- 12.Dokhani S, Cottrell T, Khajeddin J, Mazza G. Analysis of aroma and phenolic components of selected Achillea species. Plant foods for human nutrition. 2005;60:55–62. doi: 10.1007/s11130-005-5100-9. [DOI] [PubMed] [Google Scholar]
- 13.Gherase F, Spac A, Dorneanu V, Stanescu U, Grigorescu E. [Pharmacognostic research of some species of Achillea. Note 1. Volatile oils analysis] Revista medico-chirurgicala a Societatii de Medici si Naturalisti din Iasi. 2003;107:188–191. [PubMed] [Google Scholar]
- 14.Hamm L R, Sorrells S C, Harding J P, Northcutt A R, Heath A T, Kapke G F, Hunt C M, Mangel A W. Additional investigations fail to alter the diagnosis of irritable bowel syndrome in subjects fulfilling the Rome criteria. The American journal of gastroenterology. 1999;94:1279–1282. doi: 10.1111/j.1572-0241.1999.01077.x. [DOI] [PubMed] [Google Scholar]
- 15.Hasler W L, Owyang C. Irritable bowel syndrome. In: Yamada T, Alpers D H, Laine L, Owyang C, Powell D W, editors. Text book of gastroenterology. 3rd ed. Philadelphia: Lippincott Williams and Wilkins; 1999. [Google Scholar]
- 16.Hills J M, Aaronson PI. The mechanism of action of peppermint oil on gastrointestinal smooth muscle. Gastroenterology. 1991;101:55–65. doi: 10.1016/0016-5085(91)90459-x. [DOI] [PubMed] [Google Scholar]
- 17.Ince S, Turkmen R, Yavuz H. The effect of boric acid on acethylcholine, bethanechol and potasssium-evoked responses on ileum of rat. Autonomic and autacoid pharmacology. 2011;31:50–56. doi: 10.1111/j.1474-8673.2011.00466.x. [DOI] [PubMed] [Google Scholar]
- 18.Javidian K, Miri R, H S Composition of the volatile oil of Achillea wilhelmsii C. Koch from Iran. Daru. 2004;12:63–66. [Google Scholar]
- 19.Karamenderes C, Apaydin S. Antispasmodic effect of Achillea nobilis L. subsp. sipylea (O. Schwarz) Bassler on the rat isolated duodenum. Journal of Ethnopharmacology. 2003;84:175–179. doi: 10.1016/s0378-8741(02)00296-9. [DOI] [PubMed] [Google Scholar]
- 20.Lemmens-gruber R, Marchart E, Rawnduzi P, Engel N, Benedek B, Kopp B. Investigation of the spasmolytic activity of the flavonoid fraction of Achillea millefolium s.l. on isolated guinea-pig ilea. Arzneimittelforschung. 2006;56:582–588. doi: 10.1055/s-0031-1296755. [DOI] [PubMed] [Google Scholar]
- 21.Mahady G B, Pendland S L, Stoia A, HamilL F A, Fabricant D, Dietz B M, Chadwick L R. In vitro susceptibility of Helicobacter pylori to botanical extracts used traditionally for the treatment of gastrointestinal disorders. Phytotherapy Research: PTR. 2005;19:988–991. doi: 10.1002/ptr.1776. [DOI] [PubMed] [Google Scholar]
- 22.Moreno-loaiza O, Paz-aliaga A. [Vasodilator effect mediated by nitric oxide of the Zea mays L. (andean purple corn) hydroalcoholic extract in aortic rings of rat] Revista peruana de medicina experimental y salud publica. 2010;27:527–531. doi: 10.1590/s1726-46342010000400006. [DOI] [PubMed] [Google Scholar]
- 23.Nausch B, Heppner T J, Nelson M T. Nerve-released acetylcholine contracts urinary bladder smooth muscle by inducing action potentials independently of IP3-mediated calcium release. American journal of physiology Regulatory, integrative and comparative physiology. 2010;299:R878–R888. doi: 10.1152/ajpregu.00180.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nemeth E, Bernath J. Biological activities of yarrow species (Achillea spp.) Current pharmaceutical design. 2008;14:3151–3167. doi: 10.2174/138161208786404281. [DOI] [PubMed] [Google Scholar]
- 25.Pereira-leite C, Carneiro C, Soares J X, Afonso C, Nunes C, Lucio M, Reis S. Biophysical characterization of the drugs-membrane interaction: the case of propranolol and acebutolol. European journal of pharmaceutics and biopharmaceutics : official Journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2013 doi: 10.1016/j.ejpb.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Pickering J D, White E, Duke A M, Steele D S. DHPR activation underlies SR Ca2+ release induced by osmotic stress in isolated rat skeletal muscle fibers. The Journal of general physiology. 2009;133:511–524. doi: 10.1085/jgp.200910191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rashidi I, TMM, Ar M. Study of anti-inflammatory and healing effects of Achillea Millefolium in the treatment of Indomethacininduced gastric ulcer in rat. Journal of Ghazvin University of Medical Sciences and Health Services. 2005;33:9–13. [Google Scholar]
- 28.Ratz P H, Berg K M, Urban N H, Miner A S. Regulation of smooth muscle calcium sensitivity: KCl as a calcium-sensitizing stimulus. American journal of physiology Cell physiology. 2005;288:C769–C783. doi: 10.1152/ajpcell.00529.2004. [DOI] [PubMed] [Google Scholar]
- 29.Sadrae I H, Asghari G, Poorkhosravi R. Spasmolytic effect of root and aerial parts extract of Pycnocycla spinosa on neural stimulation of rat ileum. Research in pharmaceutical sciences. 2011;6:43–50. [PMC free article] [PubMed] [Google Scholar]
- 30.Sadraei H, Ghannadi A, Malekshahi K. Relaxant effect of essential oil of Melissa officinalis and citral on rat ileum contractions. Fitoterapia. 2003;74:445–452. doi: 10.1016/s0367-326x(03)00109-6. [DOI] [PubMed] [Google Scholar]
- 31.Stojanovic G, Radulovic N, Hashimoto T, Palic R. In vitro antimicrobial activity of extracts of four Achillea species: the composition of Achillea clavennae L. (Asteraceae) extract. Journal of Ethnopharmacology. 2005;101:185–190. doi: 10.1016/j.jep.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 32.Takzare N, Hosseini M J, Hamideh M S, Safaie S, Moradi R. The effect of Achillea millefolium extract on spermatogenesis of male Wistar rats. Human and Experimental Toxicology. 2011;30:328–334. doi: 10.1177/0960327110372401. [DOI] [PubMed] [Google Scholar]
- 33.Tozyo T, Yoshimura Y, Sakurai K, Uchida N, Takeda Y, Nakai H, Ishii H. Novel antitumor sesquiterpenoids in Achillea millefolium. Chem Pharm Bull (Tokyo) 1994;42:1096–100. doi: 10.1248/cpb.42.1096. [DOI] [PubMed] [Google Scholar]
- 34.Uprety Y, Asselin H, Dhakai A, Julien N. Traditional use of medicinal plants in the boreal forest of Canada: review and perspectives. Journal of Ethnobiology and Ethnomedicine. 2012;8:7. doi: 10.1186/1746-4269-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanner S J, Depew W T, Paterson W G, Dacosta L R, Groll A G, Simon J B, Djurfeldt M. Predictive value of the Rome criteria for diagnosing the irritable bowel syndrome. The American Journal of Gastroenterology. 1999;94:2912–2917. doi: 10.1111/j.1572-0241.1999.01437.x. [DOI] [PubMed] [Google Scholar]
- 36.Wells R W, Lourenssen S, Blennerhassett M G. Impaired acetylcholine-induced smooth muscle contraction in colitis involves altered calcium mobilization and AKT phosphorylation. Pflugers Archiv : European journal of physiology. 2008;456:507–517. doi: 10.1007/s00424-007-0415-z. [DOI] [PubMed] [Google Scholar]
- 37.World, Health Organization, author. Traditional medicine growing needs and potential. Geneva: World Health Organization; 2002. p. 2. [Google Scholar]
- 39.Wright T J, Huddart H. The nature of the acetylcholine and 5-hydroxytryptamine receptors in buccal smooth muscle of the pest slug deroceras reticulatum. Journal of comparative physiology B, Biochemical, systemic, and environmental physiology. 2002;172:237–249. doi: 10.1007/s00360-001-0248-6. [DOI] [PubMed] [Google Scholar]
- 40.Yaeesh S, Jamal Q, Khan A U, Gilani A H. Studies on hepatoprotective, antispasmodic and calcium antagonist activities of the aqueous-methanol extract of Achillea millefolium. Phytother Research. 2006;20:546–551. doi: 10.1002/ptr.1897. [DOI] [PubMed] [Google Scholar]
- 41.Zhao Y, Vanhoutte P M, Leung S W. Endothelial nitric oxide synthase-independent release of nitric oxide in the aorta of the spontaneously hypertensive rat. The Journal of Pharmacology and Experimental Therapeutics. 2013;344:15–22. doi: 10.1124/jpet.112.198721. [DOI] [PubMed] [Google Scholar]